Abstract
Introduction
The galactose analogue 2-[18F]fluoro-2-deoxy-D-galactose (FDGal) is a promising positron emission tomography (PET) tracer for studies of regional differences in liver metabolic function and for clinical evaluation of patients with liver cirrhosis and patients undergoing treatment of liver diseases. However, there is an unmet need for routine production of FDGal from readily available starting material. In this study, we present the preparation of FDGal with high radiochemical purity and in amounts sufficient for clinical investigations from commercially available Talose triflate (1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-talopyranose). In addition, the biodistribution of FDGal in the pig is presented.
Methods
FDGal was prepared by nucleophilic fluorination of Talose triflate followed by basic hydrolysis. The entire synthesis was performed using the GE TRACERlab MX 2-[18F]fluoro-2-deoxy-D-glucose (FDG) synthesizer and existing methods for quality control of FDG were applied. Biodistribution of FDGal was studied by successive whole-body PET recordings of two anaesthetized 37-kg pigs.
Results
Up to 3.7 GBq sterile, pyrogen-free and no-carrier-added FDGal was produced with a radiochemical yield of 3.8±1.2% and a radiochemical purity of 98±1% (42 productions; yield is decay corrected). The adopted quality control methods for FDG were directly applicable for FDGal. Biodistribution studies in the pig revealed the liver and the urinary bladder as critical organs in terms of radiation dose.
Conclusion
Commercially available Talose triflate is a suitable starting material for routine productions of FDGal. The presented radiosynthesis and quality control methods allow for the production of pure, no-carrier-added FDGal in sufficient amounts for clinical PET-investigations of the liver.
Keywords: Radiopharmaceutical, Galactose, Positron emission tomography, Nuclear hepatology, Liver metabolism
1. Introduction
Galactose is metabolised almost exclusively in the liver, where it is converted to glucose 1-phosphate through the Leloir pathway [1,2]. The complete transformation involves four enzymes with the rate-limiting step being 1-phosphorylation of galactose catalysed by galactokinase [3]. The [18F]-labelled galactose analogue 2-[18F]fluoro-2-deoxy-D-galactose (FDGal) is also a substrate for galactokinase and has been shown to be a potential PET-tracer for studies of regional metabolic liver function [4–12]. Variations in regional metabolic liver function are important for the clinical evaluation of patients with liver cirrhosis and patients undergoing local treatment of liver diseases [13]. Furthermore, the tracer may also be able to detect liver cancer (hepatocellular carcinoma) [14], which is often difficult to diagnose at an early and curable stage by PET using the common tracer for glucose metabolism, 2-[18F] fluoro-2-deoxy-D-glucose (FDG).
Both radiosyntheses of FDGal involving electrophilic and nucleophilic fluorination have been reported. However, we found that existing methods were inadequate for routine productions of the tracer for clinical evaluations of patients with liver diseases. Electrophilic fluorinations, which usually involve reaction of D-galactal with gaseous [18F]F2 [4,15] or [18F]acetylhypofluorite [16,17], suffer from low specific activity (e.g., 57 MBq/μmol was reported in reference [17]) due to the unavoidable presence of unlabelled fluorine in the production of the [18F]-reagents. Alternative methods for the production of [18F]F2 with higher specific activity have been reported, but these are not yet commonly implemented in PET-laboratories [18].
Haradahira et al. reported on the synthesis of FDGal by nucleophilic fluorination using aqueous [18F]fluoride [19]. In line with previously reported preparations of FDG, Haradahira et al. prepared no-carrier-added FDGal from a protected talose triflate with an overall radiochemical yield of 36–39% (without correction for decay) and a radiochemical purity of about 95% after purification. Thus, nucleophilic fluorination seems to be the methodology of choice for preparation of FDGal with a radiochemical purity and specific activity useful for clinical investigations. However, Haradahira et al. performed the radiosynthesis of FDGal with very low starting activity (19–52 MBq); less than what is required for high contrast PET images in human liver studies [12,14]. Furthermore, the talose triflate precursor, which contains three different protecting groups (see reference [19] for structure), requires several synthetic steps to prepare and its transformation into FDGal is relatively time consuming (total preparation time: 90 min); partly due to a lengthy deprotection step (ca. 30 min). Both of these issues most be considered when the tracer is prepared routinely and in sequence with other PET-tracers for clinical evaluations. In addition, the relatively harsh acidic conditions (6 N HCl at 115 °C) required to remove all the protective groups cause dehydration of FDGal and formation of 1,6-anhydro-2-[18F] fluoro-2-deoxy-β-D-galactopyranose (ca. 5% of the total isolated activity). It is therefore likely that additional purification will be necessary frequently to obtain a radiochemical purity of the tracer >95% as required for clinical use.
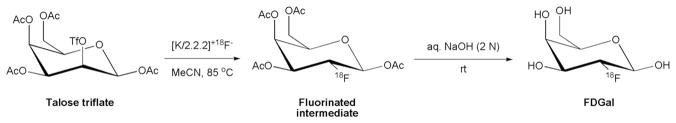
Overall, there is a need for an easily adoptable method for routine productions of FDGal suitable for human studies. The lack of such a method may explain why the use of this PET-tracer has remained as preclinical evaluations in rodents [4,5] and isolated pilot studies in humans [6]. For FDGal to be of common interest to the clinic, the tracer must be simple to produce in high quality from readily available starting material. In this paper, we report on the radiosynthesis of no-carrier-added FDGal suitable for clinical evaluations using the commercially available Talose triflate as starting material (Scheme 1).
Scheme 1.
Radiosynthesis of FDGal by nucleophilic fluorination of Talose triflate.
2. Materials and methods
2.1. Chemicals
The precursor Talose triflate (1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-talopyranose; Scheme 1) was purchased from ABX (Radeberg, Germany) with a stated chemical purity >95%. It was supplied in glass vials containing 25 mg under argon atmosphere and was stable at −20°C for a minimum of 18 months. The precursor was allowed to warm to room temperature before use. The reference compounds 2-[19F]fluoro-2-deoxy-D-galactose and D-galactose were purchased from ABX and Sigma-Aldrich, respectively, and used as received. All other chemicals [i.e., buffers, solvents, phase transfer catalysts, bases (all part of the standard FDG reagent kit) and acids] were either obtained in pharmaceutical grade from ABX or were custom manufactured in pharmaceutical grade at the pharmacy of Aarhus University Hospital (Aarhus, Denmark).
2.2. Radiosynthesis
[18F]Fluoride was produced using the Aarhus PET Centre’s GE Medical Systems PETtrace cyclotron (GEMS, Uppsala, Sweden) by irradiation of 98% enriched 18O-water (Rotem industries, Beer Sheva, Israel). The radiofluorination was performed in the commercially available system TRACERlab MX (GEMS, Uppsala, Sweden) including basic hydrolysis. The synthesis could also be performed in the FDG Microlab (GEMS, Uppsala, Sweden) with acidic hydrolysis. However, we prefer the TRACERlab MX system, because it is faster than the FDG Microlab system both with regards to total synthesis time (28 min vs. 52 min) and set-up. We use the FDG Microlab system only for test purposes. In general, the factory default reaction conditions of both systems for the synthesis of FDG were successfully adopted with the only difference being the use of Talose triflate instead of Mannose triflate as starting material. TRACERlab MX and Microlab cassettes were used without modification. For each synthesis, the amount of Talose triflate was 25 mg and the compound was dissolved in acetonitrile just before delivery of the [18F]fluoride to the reaction set-up and start of the synthesis. Reaction parameters such as temperature, duration and phase transfer catalyst were experimentally varied.
In case of radiochemical purity <95%, FDGal was purified by preparative high-performance liquid chromatography (HPLC) using a Phenomenex Spherisorb ODS 10 μ 250×10 mm column (Phenomenex, Torrance, CA, USA) connected to a Perkin Elmer LC 200 isocratic pump (Perkin Elmer Life science Denmark, Hvidovre, Denmark) with 100% sterile saline as eluent at a flow rate of 5 ml/min. Fractions were collected according to the signal obtained with a radiodetector of in-house design. The fraction containing FDGal eluted first.
2.3. Quality control
Quality control of the final tracer formulation was performed similar to the standard conditions for FDG as described in the monograph of the European Pharmacopoeia [20] using radio-HPLC with a strongly basic anion-exchange column for determination of radiochemical purity and product identification, thin layer chromatography (TLC) for Kryptofix 2.2.2 analysis, and gas chromatography (GC) for determining residual solvents (i.e., acetonitrile and ethanol). In variation to the European Pharmacopoeia monograph, the fraction of unhydrolysed FDGal was not determined by radio-TLC, but employing a validated method using radio-HPLC and an amino sugar column. We apply this method for routine quality control of FDG formulations as well.
For the determination of radiochemical purity and product identity, we used a radio-HPLC system consisting of a Perkin Elmer LC 200 titanium isocratic pump (Perkin Elmer Life Science Denmark, Hvidovre, Denmark), a Rheodyne 7025 injector, a Dionex Carbopac PA 1 250×4 mm column (Dionex Denmark, Rødovre, Denmark), a Dionex PED II electrochemical detector and a radio-detector of in-house design. The eluent was 0.2 M sodium hydroxide at flow rate 0.7 ml/min. For analysis of unhydrolysed FDGal, a Dionex P680 quaternary pump, a Phenomenex Luna NH2 10 μ 250×4.6 mm column (Phenomenex), and a radiodetector of in-house design were used. The eluent was 70% acetonitrile and 30% water at flow rate 1 ml/min. Residual solvents were determined using a Perkin Elmer AutoSys GC, a Perkin Elmer PE 624 capillary column, and a flame ionization detector (FID). All chromatographic data were analyzed using Dionex Chromeleon software (version 6.50).
For stability testing, additional quality control was performed on three independent batches of FDGal 6 h after production, both in formulated pH (approx. pH 6) and in solutions adjusted to pH 4.5 and 8.5.
Endotoxin content was determined using a Charles River Endosafe system (Charles River, Charleston, SC, USA). Sterility was tested out-of-house on a retrospective basis.
2.4. Biodistribution studies
Biodistribution studies were performed in two 37 kg female pigs (Yorkshire×Danish Landrace crossbreed) in accordance with the Danish Animal Experimentation Act on a license granted by the Danish Ministry of Justice. The animals were deprived of food for 16 h prior to the experiment, but had free access to water. Anaesthesia was induced with an intramuscular injection of midazolam and ketamine-HCl. The animal was intubated and anaesthesia was maintained by isoflurane and O2/N2O. A catheter was placed in the urinary bladder via the urethra. Catheters were placed surgically in a femoral artery and vein. Blood pressure, heart rate, body temperature and arterial blood pO2, pCO2, pH and glucose were monitored continuously and disturbances corrected for by appropriate procedures [7]. At the end of the experiment the pig was killed by an overdose of phenobarbital.
The biodistribution studies were performed by means of successive whole-body PET scans. The pigs were placed in supine position in a Siemens ECAT Exact HR scanner (Siemens, Knoxville, TN, USA) and were given an intravenous injection of 155 MBq (#1) and 159 MBq (#2) FDGal, respectively, followed by a sequence of five whole-body PET recordings starting 5 min after tracer injection and ending after 270 min. Regions of interest (ROIs) were manually defined in central parts of organs with elevated concentrations of radioactivity relative to the surrounding tissue (liver, kidney and brain), and average radioactivity concentrations in each time-frame were retrieved. The concentrations of radioactivity in the organs were normalized with respect to the injected dose. The reported values, in units of percentage of injected dose per organ, were all extrapolated from pig (37 kg) to human (74 kg) data. The radioactivity content in the urinary bladder was determined as the radioactivity drained from the bladder at the midpoint of each time frame.
3. Results and discussion
We have prepared no-carrier-added (15±6 GBq/μmol; 17 productions) FDGal by nucleophilic fluorination of commercially available Talose triflate (Scheme 1). In analogy to the production of FDG [21,22], the triflate was reacted with a potassium Kryptofix 2.2.2 complex of [18F]fluoride and the fluorinated intermediate was subsequently hydrolysed under basic conditions to give FDGal. The tracer was produced with a total synthesis time of 28 min (from the delivery of the [18F]fluoride to the end of synthesis) with high radiochemical purity and in amounts suitable for clinical evaluations: From a typical 60-min irradiation at 40 μA, 2.0±0.8 GBq of sterile pyrogen-free FDGal was produced with a radiochemical purity of 98±1% (without additional purification; 42 productions). It should be noted that the deprotective hydrolysis step could also be performed under acidic conditions (1 N HCl at 100°C) providing FDGal in comparable radiochemical yield and purity, but at the expense of time (2 min were required for complete basic hydrolysis and 20 min for complete acidic hydrolysis).
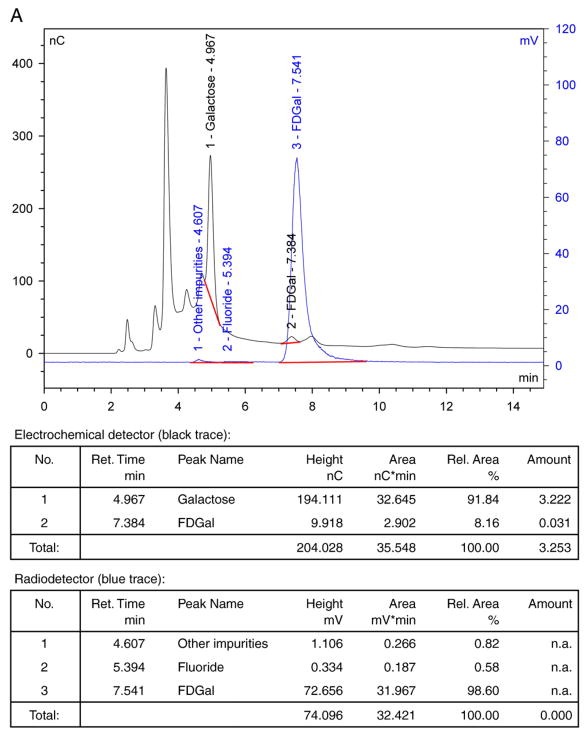
Once isolated, the FDGal product was stable for at least 6 h when stored at a pH range of 4.5–8.5, meaning that no degradation was observed in any of the quality assurance tests performed (see section 2.3). Fig. 1A–C illustrates typical analytical HPLC chromatograms of the final product solution (without purification by preparative HPLC). The amount of nonradioactive FDGal (typically around 20 μg per entire batch volume of 18 ml) was determined from the chromatogram in Fig. 1A (the black curve) by comparison with a standard solution of authentic 2-[19F]fluoro-2-deoxy-D-galactose. In this chromatogram, galactose was also identified by comparison with a sample of authentic galactose. The remaining peaks were not identified in this work.
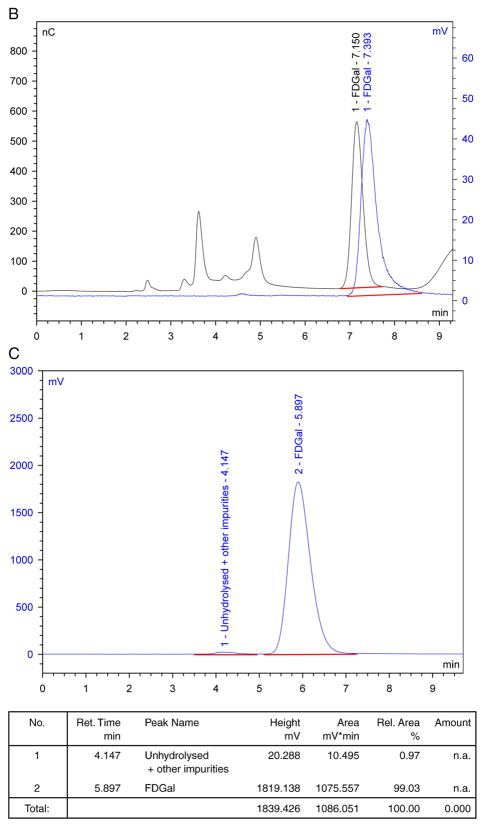
Fig. 1.
Typical quality control HPLC chromatograms for FDGal. Black traces are signals from the electrochemical detector, while blue traces are signals from the radio-detector: (A) Chromatograms of final solution on Dionex Carbopac PA1. (B) Chromatograms of final solution spiked with authentic FDGal on Dionex Carbopac PA1. (C) Radio-chromatogram of final solution on amino sugar column.
The radiochemical purity of FDGal was determined from the chromatograms illustrated in Fig. 1A and C (blue curves). Positive identification of FDGal in the chromatograms was assured by spiking a sample of the product solution with authentic 2-[19F]fluoro-2-deoxy-D-galactose. A typical example is illustrated in Fig. 1B. The difference in time, ranging from 0.2 to 0.3 min, of the signals collected from the electrochemical and radio-detectors (black and blue curve, respectively) corresponds to the difference in path length between the two detectors. [18F]Fluoride and unhydrolysed FDGal were identified from the chromatograms in Fig. 1A and C, respectively. These were usually present only in trace amounts along with other unidentifiable 18F-impurities (each typically <1%).
For one specific batch of the commercially obtained Talose triflate, we observed the formation of an unidentified, slightly lipophilic, radiochemical impurity in productions with low radiochemical yields. With this batch, which fulfilled quality control release criteria both upon initial product release and under retest conditions, the impurity comprised up to 10% of the final product. Applying additional C-18 Seppak cartridges did not remove the impurity. However, both the impurity and traces of unhydrolysed FDGal were efficiently removed by preparative HPLC (see Section 2.2), yielding the FDGal product with radiochemical purity higher than 99%.
After end of synthesis, up to 75% of the starting 18F-radioactivity was found as [18F]fluoride in the waste container and approximately 20% was lost during the labeling procedure as volatile fluorinated compounds. The radiochemical yield of the transformation of Talose triflate into FDGal by the present method was hence limited to 3.8±1.2% (decay corrected; 42 productions). Experimental variations in reaction temperature (60–85°C), duration (2–10 min), phase transfer catalyst (Kryptofix 2.2.2./K2CO3, tetrabutylammoiumhydrogen carbonate), hydrolysis conditions (1 M HCl, 20 min, 100°C or 2 M NaOH, 2 min, room temperature), or synthesizer (TRACERlab MX or FDG Microlab) did not affect the radiochemical yield. The relatively low radiochemical yield obtained may be due to chemical instability of the acetyl-protected triflate. Unlike the more stable mannose triflate used for FDG productions, a 1,3-diaxial interaction between the 2-trifluoromethanesulfonyl group and the 4-acetoxy group is present in the talose derivative. This interaction produces significant ring strain and hence lowers the chemical stability of the talose derived starting material. Despite the low radiochemical yield obtained, we consider our method to be an improvement to that reported by Haradahira et al. [19], because FDGal is produced with significantly reduced synthesis time (28 vs. 90 min), higher amounts of radioactivity (2.0±0.8 GBq vs. 19–52 MBq), higher radiochemical purity (98±1% without additional purification vs. ca. 95%), and from the readily available starting material Talose triflate.
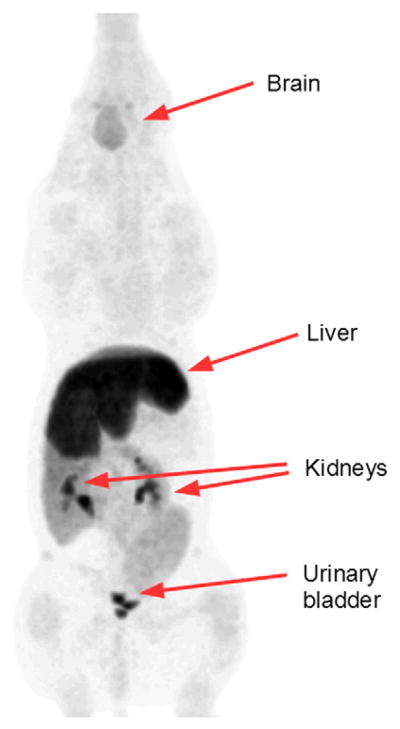
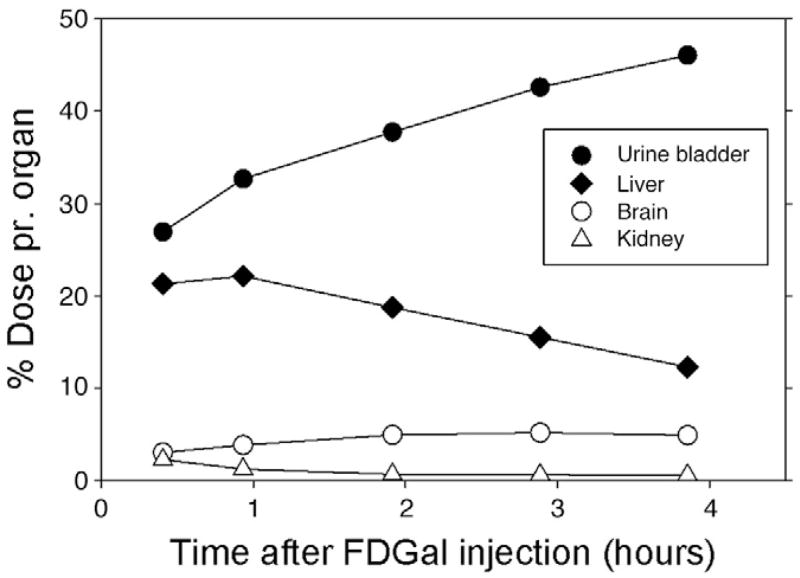
As preparation for human studies and clinical evaluations of patients, we investigated the biodistribution of the produced FDGal in a porcine model with subsequent extrapolation from pig (37 kg) to human (74 kg) data. The studies revealed that FDGal is efficiently taken up by the liver with a maximum radioactivity concentration after approximately 1 h (Figs. 2 and 3). Hereafter, the activity decreases in the liver. The drained urine contained considerable amounts of radioactivity; increasing throughout the experimental period. No significant radioactivity was observed in other organs such as brain, kidneys and intestines. As such, the final accumulation in the brain was less than 5% of the injected dose. From the biodistribution results, we calculated the effective doses of critical organs. Overall, the effective dose for FDGal was observed to be comparable to that for FDG (0.032 vs. 0.019 mSv/MBq, respectively) [23]. However, considerably higher organ doses were found as expected for the liver (FDGal: 0.55 mSv/MBq; FDG: 0.011 mSv/MBq) and the urinary bladder (FDGal: 0.46 mSv/MBq; FDG: 0.16 mSv/MBq). These values reflect the higher liver retention and increased urinary excretion due to the lower up-take of FDGal in other organs.
Fig. 2.
FDGal PET image of mean radioactivity concentration recorded 52–115 min after injection of 155 MBq FDGal to Pig #1 (37 kg; anaesthesia was induced with midazolam and ketamine-HCl and maintained by isoflurane and O2/N2O). The image shows maximum intensity projections. The activities in the liver, kidneys and urine bladder were high compared with other organs.
Fig. 3.
Radioactivity concentration time courses in various organs as indicated after intravenous administration of FDGal. Data was obtained from the experiment illustrated in Fig. 2 (without correction for decay) and extrapolated from pig (37 kg) to human (74 kg) data.
The present formulation of FDGal is considered to be toxicologically safe. The LD50 value for 2-fluoro-2-deoxy-D-galactose for rats and mice is approximately 800 mg/kg [24]. As such, an injection containing a maximum of 20 μg FDGal per dose (i.e., the typical amount in a full production) corresponds to a factor of 4×104 less than the LD50-value for rodents. In agreement with this (and additional quality assessments), the Danish Medicines Agency (DKMA) has accepted the presented production procedure for FDGal and the tracer’s use in humans. We are now routinely producing FDGal by this procedure for PET/computed tomographic clinical evaluation of regional differences in metabolic liver function in patients with liver disease and patients with primary liver cancer and we have found that, due to the high specificity of the tracer, doses as low as 100–120 MBq are sufficient to obtain high contrast PET images.
4. Conclusion
In conclusion, we have successfully prepared no-carrier-added (15±6 GBq/μmol; 17 productions) 2-[18F]fluoro-2-deoxy-D-galactose (FDGal) by nucleophilic fluorination of commercially available Talose triflate (1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-talopyranose) using reaction conditions similar to those for standard FDG productions. The FDGal tracer was produced with a total synthesis time of only 28 min., with high radiochemical purity (98%±1%; 42 productions) without the need of purification by preparative HPLC and in clinically useful radioactivity amounts (2.0±0.8 GBq; 42 productions). Quality control of the tracer was performed exactly as for FDG and, if needed, impurities were easily removed by preparative HPLC. The present production procedure and quality control for FDGal has been accepted by the Danish Medicines Agency (DKMA) for clinical use.
Footnotes
Financial support: The National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-074419), the Danish Medical Research Council (09-073658) and the Danish Cancer Society (DP08024; DP06114).
References
- 1.Caputto R, Leloir LF, Trucco RE, Cardini CE, Paladini AC. The enzymatic transformation of galactose into glucose derivatives. J Biol Chem. 1949;179:497–8. [PubMed] [Google Scholar]
- 2.Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 2003;45:43885–8. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 3.Keiding S, Johansen S, Winkler K, Tønnesen K, Tygstrup N. Michaelis-Menten kinetics of galactose elimination by the isolated perfused pig liver. Am J Physiol. 1976;230:1302–13. doi: 10.1152/ajplegacy.1976.230.5.1302. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda H, Matsuzawa T, Tada M, Takahashi T, Ishiwata K, Yamada K, et al. 2-Deoxy-2-[18F]fluoro-D-galactose: a new tracer for the measurement of galactose metabolism in the liver by positron emission tomography. Eur J Nucl Med. 1986;11:444–8. doi: 10.1007/BF00261007. [DOI] [PubMed] [Google Scholar]
- 5.Grün B, Berger U, Oberdorfer F, Hull WE, Ostertag H, Friedrich E, et al. Metabolism and actions of 2-deoxy-2-fluoro-D-galactose in vivo. Eur J Biochem. 1990;190:11–9. doi: 10.1111/j.1432-1033.1990.tb15539.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda H, Yamaguchi K, Matsuzawa T, Itoh M, Abe Y, Fujiwara T, et al. 2-Deoxy-2-[18F]fluoro-D-galactose: a new probe for the evaluation of liver function by PET. II. A first clinical PET study of the liver in normal volunteers. Kaku Igaku. 1987;24:871–4. [PubMed] [Google Scholar]
- 7.Sørensen M, Munk OL, Mortensen FV, Olsen AK, Bender D, Bass L, et al. Hepatic uptake and metabolism of galactose can be quantified in vivo by 2-[18F]fluoro-2-deoxygalactose positron emission tomography. Am J Physiol Gastrointest Liver Physiol. 2008;295:G27–36. doi: 10.1152/ajpgi.00004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishiwata K, Yamaguchi K, Kameyama M, Fukuda H, Tada M, Matsuzawa T, et al. 2-Deoxy-2-[18F]fluoro-D-galactose as an in vivo tracer for imaging galactose metabolism in tumors with positron emission tomography. Int J Rad Appl Instrum B. 1989;16:247–54. doi: 10.1016/0883-2897(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 9.Paul R, Aho K, Bergman J, Haaparanta M, Kulmala J, Reissell A, et al. Imaging of rats with mammary cancer with two 2-deoxy-2-[18F]fluoro-D-hexoses. Int J Radiat Appl Instrum B. 1989;16:449–53. doi: 10.1016/0883-2897(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 10.Ishiwata K, Takahashi T, Iwata R, Tomura M, Tada M, Itoh J, et al. Tumor diagnosis by PET: Potential of seven tracers examined in five experimental tumors including an artificial metastasis model. Int J Radiat Appl Instrum B. 1992;19:611–8. doi: 10.1016/0883-2897(92)90095-g. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda H, Takashi J, Fujiwara T, Yamaguchi K, Abe Y, Kubota K, et al. High accumulation of 2-deoxy-fluorine-18-fluoro-D-galactose by well-differentiated hepatomas of mice and rats. J Nucl Med. 1993;34:780–6. [PubMed] [Google Scholar]
- 12.Sørensen M. Determination of hepatic galactose elimination capacity using 2-[18F]fluoro-2-deoxy-D-galactose PET/CT. Reproducibility of the method and metabolic heterogeniety in a normal pig liver model. Scand J Gastroenterol. 2010 Aug 9th;:1–6. doi: 10.3109/00365521.2010.510574. [early online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redaelli CA, Dufour JF, Wagner M, Schilling M, Hüsler J, Krähenbühl L, et al. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77–85. doi: 10.1097/00000658-200201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sørensen M. Molecular imaging of liver tumours (abstract) Scand J Gastroenterol Suppl. 2010;45(suppl 247):42. [Google Scholar]
- 15.Tada M, Matsuzawa T, Ohrui H, Fukada H, Ido T, Takahashi T, et al. Synthesis of some 2-deoxy-2-fluoro[18F]hexopyranoses, potential diagnostic imaging agents. Heterocycles. 1984;22(3):565–8. [Google Scholar]
- 16.Tada M, Matsuzawa T, Yamaguchi K, Abi Y, Fukuda H, Iroh M. Synthesis of 18F-labelled 2-deoxy-2-fluoro-D-galactopyranose using the acetyl hypofluorite procedure. Carbohydr Res. 1987;161:314–7. [Google Scholar]
- 17.Oberdorfer F, Traving BC, Maier-Borst W, Hull WE. Preparation and characterization of 2-deoxy-2-[18F] fluoro-D-galactose. J Labelled Compd Radiopharm. 1988;25:465–81. [Google Scholar]
- 18.Langstrom B, Johan U. Production of [18F]-F2 from [18F]-fluoride using a plasma induced scrambling procedure. WO/US2009/0104087A1. International application no.: PCT/IB2007/001076 2009 Publication no.
- 19.Haradahira T, Maeda M, Kai Y, Kojima M. A new synthesis of 2-deoxy-2-[18F]fluoro-D-galactose using [18F]fluoride ion. J Labelled Compd Radiopharm. 1988;25:721–9. [Google Scholar]
- 20.Eur Pharm. 2002:1325. [Google Scholar]
- 21.Hamacher K, Coenen HH, Stöcklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27:235–8. [PubMed] [Google Scholar]
- 22.Füchtner F, Steinbach J, Mäding P, Johannsen B. Basic hydrolysis of 2-[18F]fluoro-1,3,4,6-tetra-O-acetyl-D-glucose in the preparation of 2-[18F]fluoro-2-deoxy-D-glucose. Appl Radiat Isot. 1996;47:61–6. [Google Scholar]
- 23.International Commission of Radiological Protection. Report no. 80. [PubMed] [Google Scholar]
- 24.Fukuda H, Yamaguchi K, Matsuzawa T, Abe Y, Yamada K, Yoshioka S, Sato T, Tada M, Ogata Y, Takahashi T, et al. 2-Deoxy-2-[18F]fluoro-D-galactose: a new tracer for the evaluation of liver function by PET. Evaluation of toxicity and radiation dose. Kaku Igaku. 1987;24:165–9. [PubMed] [Google Scholar]