Abstract
We sought to protect CBA mice against tuberculosis using in vivo transfer of a T-cell line previously shown to be capable of I-A-restricted recognition of peritoneal macrophages infected in vitro with Mycobacterium tuberculosis. This line induces total bacteriostasis in vitro. In mice that received 500 rads of irradiation 48 h before infection, the T-cell line caused significant prolongation of life when given intravenously with a challenge dose of 5 x 10(6) organisms. Similar experiments with two other T-cell lines showed that these lines offered no protection. Bacterial load at the time of death was inversely related to the time of survival. Thus, death occurred at a lower bacterial load in adoptively protected mice, implying the contribution of an immunopathological component in these animals. The protective T-cell line, which was CD4+ CD8-, had no effect on the rate of growth of strain BCG in CBA nu/nu mice or M. tuberculosis in fully T-cell-deprived mice. This could indicate that CD8+ cells play a role in this system or that there is a need for the recruitment of interleukin 2-producing cells in the recipient. Experiments with monoclonal antibodies to selectively deplete T-cell subsets in normal CBA mice showed that depletion of CD4+ cells strikingly shortened survival, whereas depletion of CD8+ cells did not. However, CD8-depleted mice died with a lower bacterial load than those found in nondepleted controls, and the lesions in CD8-depleted mice were histopathologically distinct. These results suggest that the CD8+ cells either down-regulate bacteriostasis or cause immunopathology in this model and that it is the CD4+ cells that are the major protective subset in long-term protection experiments.
Full text
PDF
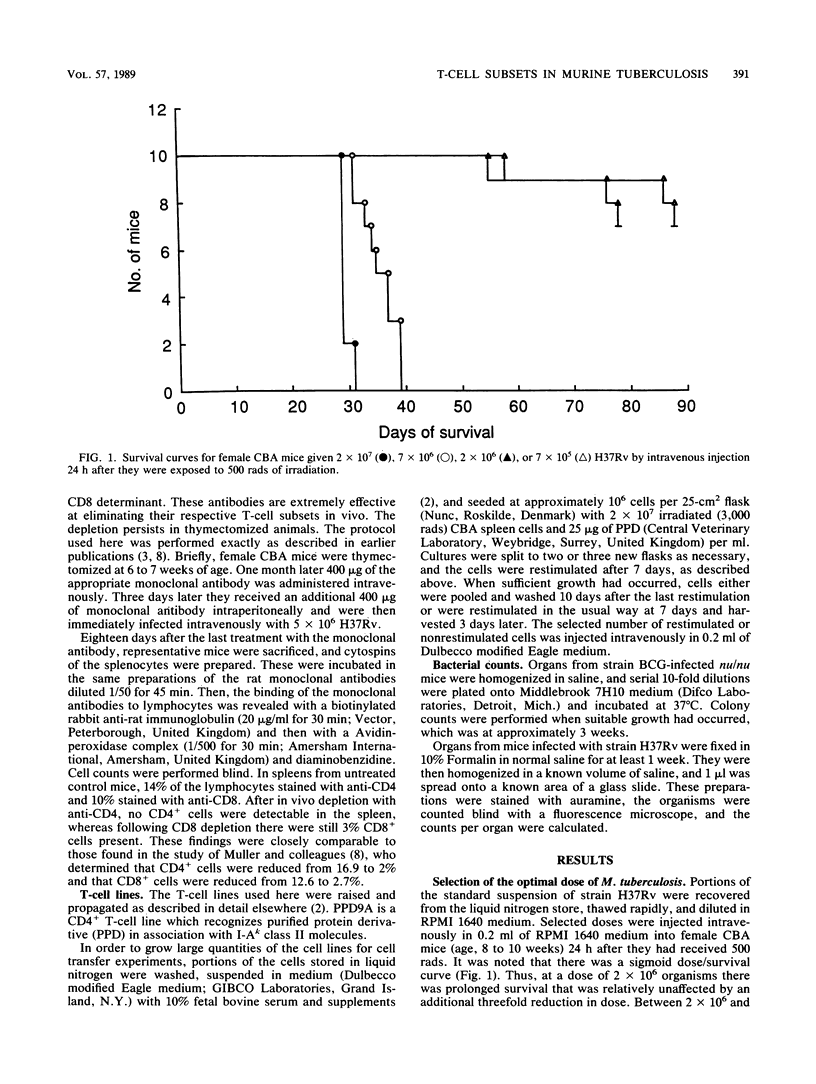
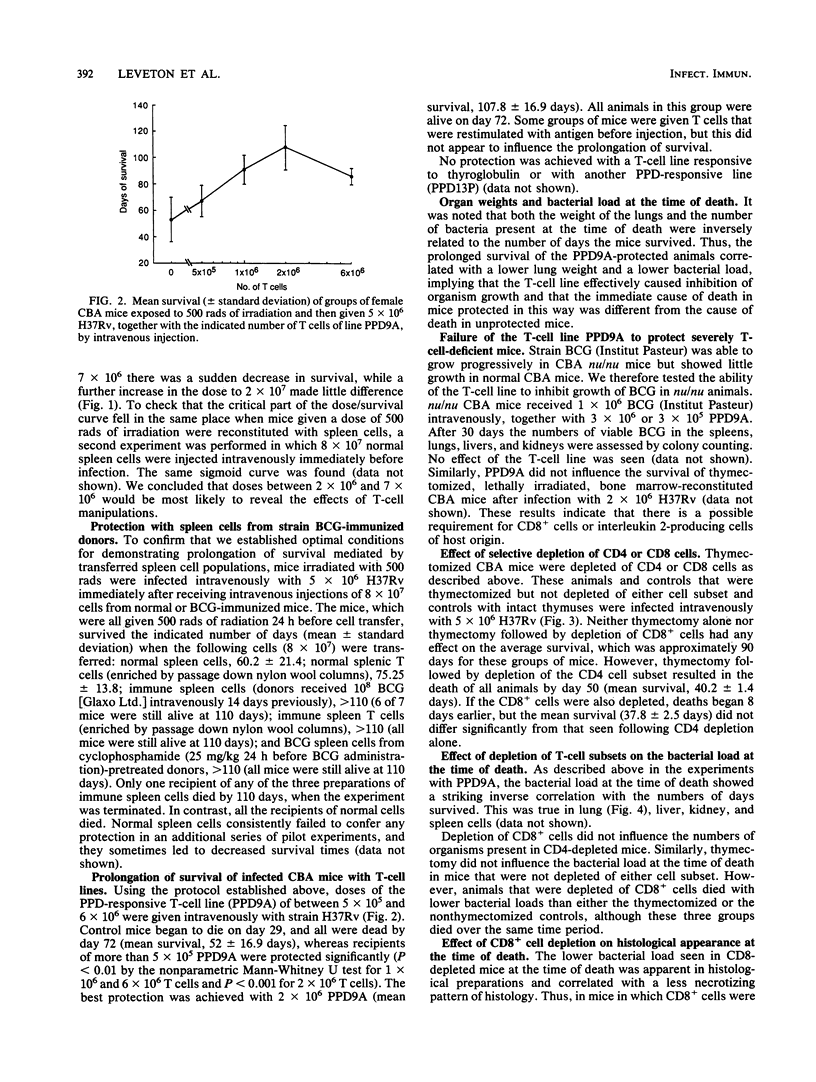
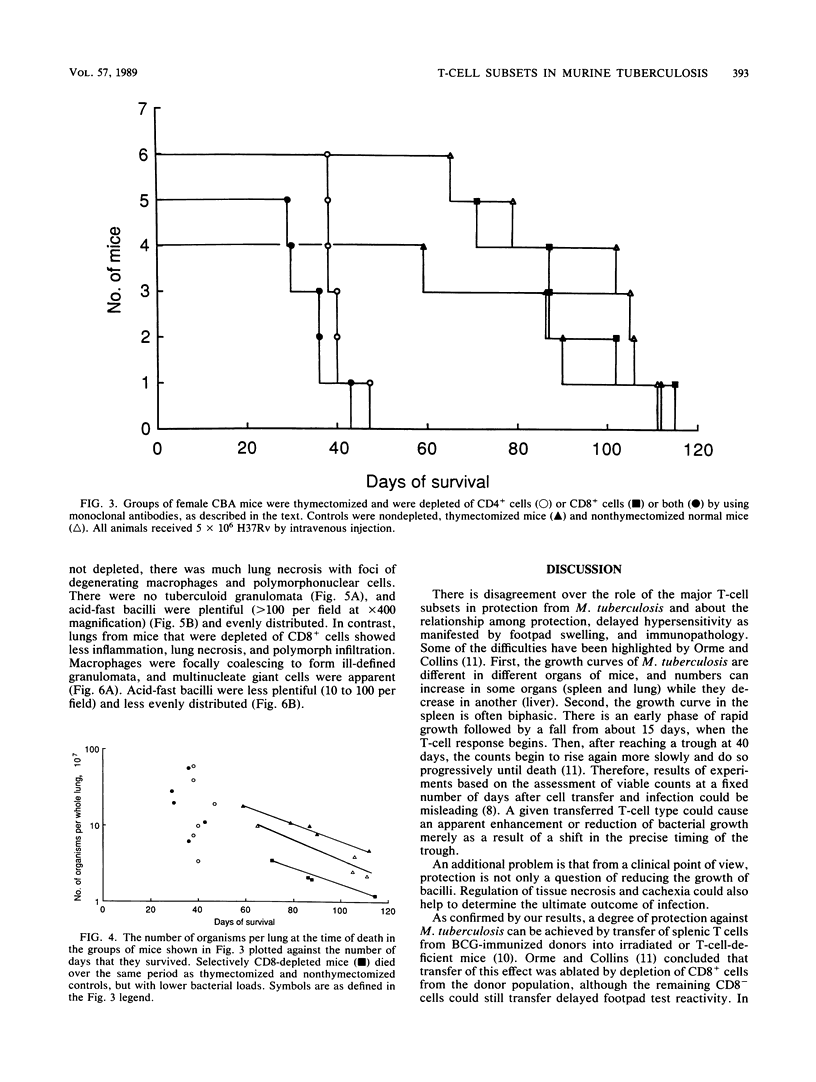
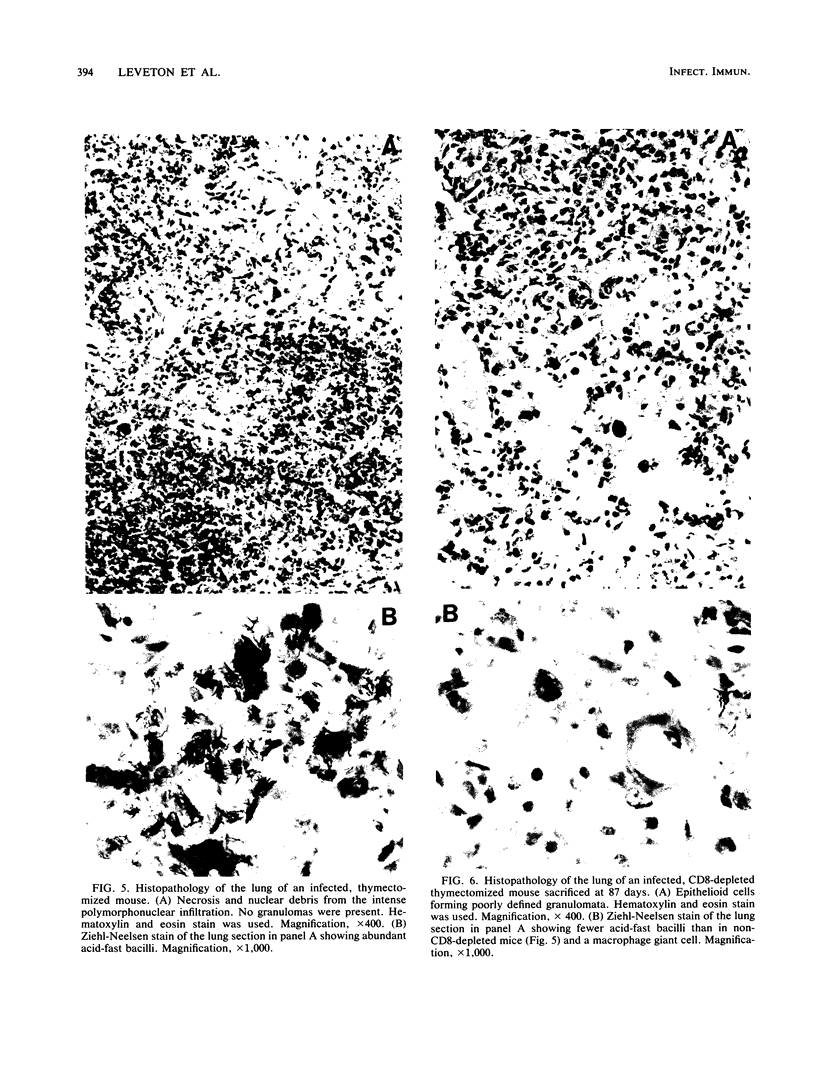

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altes C., Steele J., Stanford J. L., Rook G. A. The effect of lymphokines on the ability of macrophages to protect mycobacteria from a bactericidal antibiotic. Tubercle. 1985 Dec;66(4):261–266. doi: 10.1016/0041-3879(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Champion B. R., Varey A. M., Katz D., Cooke A., Roitt I. M. Autoreactive T-cell lines specific for mouse thyroglobulin. Immunology. 1985 Mar;54(3):513–519. [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Martin G., Waldmann H. Monoclonal antibodies for the prevention of graft-versus-host disease and marrow graft rejection. The depletion of T cell subsets in vitro and in vivo. Transplantation. 1986 Sep;42(3):239–247. doi: 10.1097/00007890-198609000-00003. [DOI] [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., De Souza B. J. Cell-mediated immunity in mice vaccinated against malaria. Clin Exp Immunol. 1978 Nov;34(2):147–158. [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Pedrazzini T., Hug K., Louis J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987 Sep 15;139(6):2032–2037. [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]





