Abstract
Platinum-based compounds are widely used and effective chemotherapeutic agents; however, sensory peripheral neuropathy is a dose-limiting and long term side effect for 20-30% of patients. A critical question is whether the mechanisms of cell death underlying clinical efficacy can be separated from the effects on neurons in order to develop strategies that prevent platinum-induced neuropathy. In rodent dorsal root ganglion neurons (DRG), cisplatin has been shown to bind and damage neuronal DNA, inducing apoptosis; however genetic manipulation in order to study mechanisms of this phenomenon in the rodent model system is costly and time-consuming. Drosophila melanogaster are commonly used to study neurological disorders, have DNA damage-apoptosis mechanisms homologous to mammalian systems, and have readily-available, inexpensive tools for rapid genetic manipulation. We therefore sought to develop adult Drosophila as a new model to study cisplatin-induced neurotoxicity. Adult Drosophila were exposed to 10, 25, 50, 100, 200 and 400μg/ml cisplatin for 3 days and observed for fly survival and geotactic climbing behavior, cisplatin-DNA binding and cellular apoptosis. On day 3, 50μg/ml cisplatin reduced the number of flies able to climb above 2 cm to 43% while fly survival was maintained at 92%. 100% lethality was observed at 400μg/ml cisplatin. Whole fly platinum-genomic DNA adducts were measured and found to be comparable to adduct levels previously measured in rat DRG neurons. Brain, ovaries, kidney and heart harvested from cisplatin treated flies were stained for active caspase 3. Apoptosis was found in ovaries and brain but not in heart and kidney. Brain apoptosis was confirmed by transmission electron microscopy. Expression of the anti-apoptotic baculoviral protein, p35, in neurons using the Gal4-UAS system prevented cisplatin-induced apoptosis in the brain and restored climbing behavior. In conclusion, cisplatin-induced behavioral and apoptotic changes in Drosophila resemble those seen in mammals. Furthermore, the use of lethality and climbing assays combined with powerful gene manipulation, make Drosophila a suitable model to study mechanisms of cisplatin neurotoxicity.
Keywords: chemotherapy, cisplatin, neuropathy, apoptosis, neurotoxicity, neuroprotection, model, Drosophila, behavior, genetics
Introduction
Chemotherapy induced peripheral neuropathy is a serious, life long side effect that impairs quality of life for long-term cancer survivors. Cisplatin and oxaliplatin are used to treat ovarian, testicular and metastatic colorectal cancers. Unfortunately, platinum drugs induce neuropathy in 20-30% of patients, which is the dose-limiting side effect (Thompson et al., 1984; Cavaletti, 2008; Windebank and Grisold, 2008). Mammalian models have been developed to investigate the mechanisms of platinum-induced neurotoxicity (Cavaletti et al., 1998; Carozzi et al., 2010b; Carozzi et al., 2010a). Cisplatin and oxaliplatin induce sensory behavioral changes in mice, in vivo (Ta et al., 2009). Mice treated with cisplatin exhibit thermal hyperalgesia to heat stimuli and mechanical allodynia. The chemotherapeutic mechanism of cisplatin and oxaliplatin is through DNA binding, inducing DNA damage that leads to apoptosis. Likewise, data from mammalian in vitro and in vivo models have demonstrated that sensory neurons undergo cisplatin-induced apoptosis through a DNA damage mechanism that is similar to the mechanisms observed in cancer cells (Fischer et al., 2001; McDonald and Windebank, 2002; McDonald et al., 2005; Ta et al., 2006).
Drosophila melanogaster have been used to study the genetics of human neurodegenerative diseases (Feany, 2010). In Parkinson’s disease (PD), mutations in Drosophila parkin and PINK1 have been linked to the mitochondrial pathology of PD. Expression of human α-synuclein in Drosophila using the GAL4-UAS system induces a PD phenotype and allows for the study of toxicity induced by α-synuclein aggregation (Botella et al., 2009). Alzheimer’s disease can be modeled in transgenic Drosophila expressing genes which induce the production of amyloid-ß peptide. Aggregation of amyloid-ß is observed in these flies along with age related neurodegeneration and memory loss (Iijima and Iijima-Ando, 2008).
The genotoxic and mutagenic effects of cisplatin have been studied in Drosophila (Woodruff et al., 1980; Katz, 1987). Genotoxic potency of cisplatin was measured using a wing spot assay (Katz, 1998). Cisplatin induced an increase in large spots indicating genotoxic alterations. Also, platinum-DNA adducts measured in Drosophila larva induced DNA damage in larval brain (Garcia Sar et al., 2008). Many critical biological systems are highly conserved between Drosophila and mammals with many genes involved in the mammalian apoptotic pathway having Drosophila homologues (Steller, 2008; Xu et al., 2009). It is therefore likely cisplatin induces cellular apoptosis in Drosophila similar to that seen in mammals (Ta et al., 2006; Wang and Youle, 2009).
We and others have used in vitro and in vivo rodent models to study cisplatin-induced neurotoxicity (Cavaletti et al., 1998; Meijer et al., 1999; Cavaletti et al., 2002; McDonald and Windebank, 2002; McDonald et al., 2005; Ta et al., 2006; Ta et al., 2009). Cell culture is a simple model system to study the direct effect of drugs on specific cell populations and rodent models are an effective way to study whole body drug effects. However, culture systems have limitations and molecular manipulation of death pathways in rodent models is costly and time consuming. Drosophila melanogaster are an established model in the study of disease genetics. Transgenic animals are easy to generate with a variety of tools to control the expression or inhibition of specific genes and can be done in a relatively short amount of time with little cost. Established neurobehavioral assays have also been developed to reliably quantitate phenotypes in Drosophila. Our experiments were designed to establish a model to study cisplatin neurotoxicity in Drosophila melanogaster.
Methods
Drosophila treatment
Oregon Red Drosophila melanogaster were raised in vials containing molasses, cornmeal, yeast, agar, TegoSept and proprionic acid at 25° C. For our experiments, flies were placed into empty vials with 50 flies per vial and 2-3 vials per condition. Flies were treated with 0, 10, 25, 50, 100, 200 and 400μg/ml cisplatin (APP Pharma., Schaumburg, IL) diluted in10% sucrose-DPBS for three days. After an 8-10 hour starvation period, flies were given 200μl of the appropriate drug concentration. Additional drug was given 24 and 48 hours later. On day 3, flies were removed from the drug and placed into normal food vials to lay eggs. On day 6, the flies were removed from the vials and either harvested for immunostaining or placed into new food vials and kept until day 9.
Quantitation of Platinum levels
To measure platinum levels in Drosophila, flies were treated with or without 100μg/ml cisplatin for 3 days. Drosophila were anesthetized with CO2, placed into 95% ethanol, dehydrated and weighed. Whole flies were homogenized in Dulbecco’s phosphate buffered saline (DPBS) and hydrochloric acid was added to a concentration of 6N for whole body platinum measurements. For platinum-total DNA measurements, genomic DNA was isolated from treated flies using a Genomic-tip, DNA isolation kit (Qiagen, Valencia, CA). Total DNA was measured using a Nano-Drop spectrophotometer (Bio-Rad, Hercules, CA) and the sample was diluted to 6N hydrochloric acid in DPBS. Platinum levels were measured using Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) (McDonald et al, 2005) and expressed as ng platinum/mg fly weight or ng platinum/mg total DNA.
Lethality and Climbing Assays
On days 3, 6 and 9 of our experiments, live flies were counted and tested for geotactic climbing ability. Lethality was expressed as percent of surviving flies compared to the number of live flies at the beginning of the experiment. The climbing assay was modified from Orso and colleagues (Orso et al., 2005). From each test vial, 10 flies were placed into an empty vial with a mark placed 2cm from the bottom. The flies were gently knocked to the bottom of the vial by tapping on the counter and the number of flies that climbed above a 2cm line were counted after 20 seconds. This was repeated 5 times per vial with 2 vials per condition. Climbing ability was expressed as a percent of the number of flies above the 2cm line as compared to the total number of flies.
Ovary Apoptosis
Cisplatin treated flies were anesthetized on day 6 using CO2. Ovaries were removed in PBS and fixed with 4% paraformaldehyde. The ovaries were stained with 1:500 anti-active caspase 3 (Cell Signaling Tech. Inc., Danvers, MA) polyclonal antibody and 1:200 anti rabbit Cy 2 (Jackson Immunoresearch, West Grove, PA) followed by 1μg/ml bisbenzimide to stain nuclei. Ovaries were imaged using a Zeiss Axiovert fluorescent microscope (Carl Zeiss Microimaging Inc., Thornwood, NY). Oocytes contain multiple cells and not every cell within the oocyte is at the same stage of apoptosis making for an uneven morphology, we therefore counted the individual oocytes containing any caspase 3 activity. The caspase positive oocytes were counted and expressed as a percent of the total number of oocytes.
Heart, Kidney and Brain Apoptosis
On day 6, flies treated with 50μg/ml cisplatin were anesthetized with CO2 and prefixed with a 1.25% formaldehyde-octane fixative for 30 min. The heads and bodies were separated and the thorax cut away from the abdomen. Fly abdomens were postfixed in 0.016 % formaldehyde for 90 min. The heart and kidney were stained in the abdomen with 1:200 anti-active caspase 3 polyclonal antibody and 1:200 anti-rabbit FITC (Jackson Immunoresearch, West Grove, PA), followed by 1μg/ml bisbenzimide. The heart was additionally stained with 1:2 anti-pericardin (DSHB, U. of Iowa) monoclonal antibody and 1:100 anti-mouse Cy3 to stain heart myocytes. Images were acquired using a Zeiss LSM 500 laser scanning confocal microscope (Carl Zeiss Microimaging Inc., Thornwood, NY). Brain apoptosis was quantitated by systematically acquiring images from all different brain regions. Four images were acquired per brain with 8-10 brains per condition. Percent apoptosis was calculated by counting the number of eLav positive neurons compared to the number neurons co-labeled with active caspase 3.
Electron microscopy
Drosophila brains treated with 100μg/ml cisplatin were dissected fresh, fixed in Trump’s fixative consisting of 4% formaldehyde and 1% glutaraldehyde in PBS at pH 7.2, post fixed in 1% osmium tetroxide and stained en bloc with 2% uranyl acetate. The brains were embedded in mixture of Epon and araldite. All reagents were obtained from Electron Microscope Services (Ft. Washington, PA). Ultrathin, 100nm sections were cut from the same blocks, mounted on 200μm mesh copper grids, stained with lead citrate. Images were acquired using a Joel ExII Transmission Electron Microscope.
p35 Experiments
Drosophila were bred to express p35 via the eLav neuron-specific promoter. P35 is a baculoviral anti-apoptotic protein that inhibits caspase activity (Martin et al., 2009). Elav-Gal4 flies were obtained from Corey Goodman’s Laboratory (University of California, Berkeley) and UAS-p35 flies were obtained from Bruce Hay’s Laboratory (California Institute of Technology). Each fly strain was bred with w;Adv/SM5-TM6B balancer flies. Flies were selected from F1 with white eyes and curly wings. The elav-Gal4/balancers were bred to the UAS-p35/balancers to create elav-Gal4/UAS-p35 expressing flies. These flies were treated with 100μg/ml cisplatin for 3 days followed by survival and climbing assay. On day 6, flies were anesthetized with CO2 and tissue fixed with a formaldehyde-octane fixative as previously described. Brains were stained with either 1:400 anti-active caspase 3 for apoptosis or 1:1000 anti-p35 (IMGENEX, San Diego, CA) polyclonal antibody for p35 expression and 1:800 anti-rabbit Cy 2 followed by 1:5 rat anti-eLav antibody and 1:400 anti-rat Cy3 to stain all neurons. Ovaries were removed from the abdomen and stained with either 1:1000 anti-active caspase 3 or 1:1000 anti-p35 and 1:800 anti-rabbit Cy2 for active and 1μg/ml bisbenzimide to stain nuclei. Images were acquired using laser scanning confocal microscopy.
Cyclophosphamide Experiments
Drosophila were treated with 0, 200, 400, 800, 1400, 2000, 3000 and 4000μg/ml cyclophosphamide using the same experimental parameters as cisplatin treatment. Cychophosphamide, like cisplatin, is a chemotherapeutic drug that binds the N7 position of guanine inducing inter- and intrastrand DNA crosslinks and DNA damage (Emadi et al., 2009), however, does not induce neurotoxicity. Survival and climbing behavior assays were performed on surviving flies followed by active caspase 3 immunostaining of brain, kidney and ovaries. On day 6, flies were anesthetized with CO2 and tissue fixed with a formaldehyde-octane fixative as previously described. Brains were stained with 1:400 anti-active caspase 3 and 1:800 anti-rabbit Cy 2 to stain for apoptosis and with 1:5 rat anti-eLav and 1:400 anti-rat Cy3 to stain all neurons. Ovaries and kidneys were removed from the abdomen and stained with either 1:1000 anti-active caspase 3 and 1:800 anti-rabbit Cy2 and 1μg/ml bisbenzimide to stain nuclei. Images were acquired using laser scanning confocal microscopy.
Statistical Methods
Data was analyzed for means and SEM using one-way analysis of variance (ANOVA) of data with parametric distribution (Gaussian). Statistical significance was analyzed using Tukey-Kramer multiple comparison post-test. Brain apoptosis was analyzed by two-tailed paired t test.
Results
Fly Platinum Levels
Platinum levels were measured in whole fly and total DNA isolated from whole fly (Table 1). Whole body platinum levels were 18.9ng elemental platinum/mg fly body weight in flies treated with 100μg/ml cisplatin for 3 days. Platinum binding to genomic DNA was 118ng platinum per mg total DNA which is equivalent to 1 platinum molecule per 2500 base pairs of DNA. Background controls measured 0.003ng (whole body) and <0.002ng (total DNA) elemental platinum/mg fly body weight.
Table 1.
Platinum levels in whole fly and genomic DNA isolated from whole fly were measure by Inductively Coupled Plasma Mass Spectroscopy.
| Cisplatin (μg/ml) | 0 | 100 |
|---|---|---|
| ng Pt/mg fly | 0.003 | 18.9 |
| ng Pt/mg tDNA | <0.002 | 118 (1Pt:2500bp DNA) |
Survival and behavior changes
Cisplatin killed Drosophila in a dose dependent manner (Fig. 1A). Flies were exposed to 0, 10, 25, 50,100, 200 and 400μg/ml cisplatin and percent survival determined at 3 days: 92%, 92%, 87%, 92%, 74%, 20%, 1%, 6 days: 90%, 89%, 82%, 89%, 60%, 16%, 0% and 9 days: 86%, 88%, 82%, 79%, 54%, 12%, 0%. There was no significant fly death induced by 10, 25 or 50μg/ml cisplatin at 3, 6 and 9 days (P>0.05). Survival decreased with 100 and 200μg/ml cisplatin (P<0.01 to P<0.001) and 100% lethality was observed with 400μg/ml cisplatin.
Figure 1.
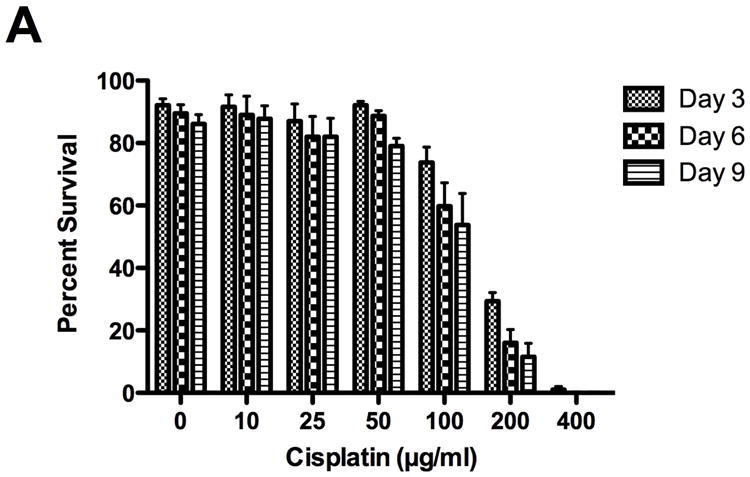
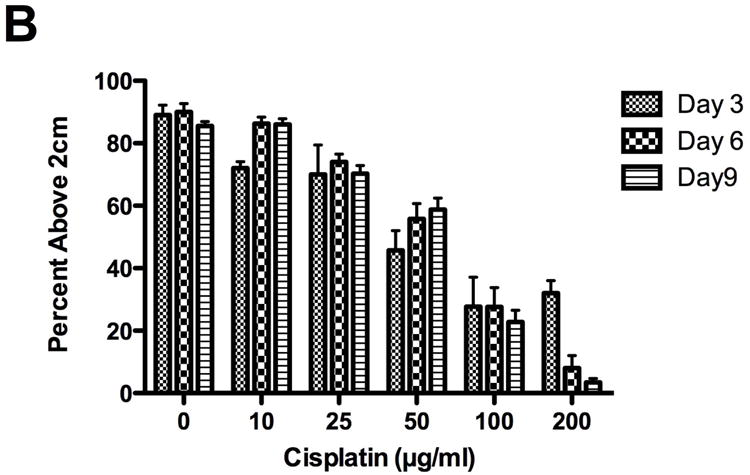
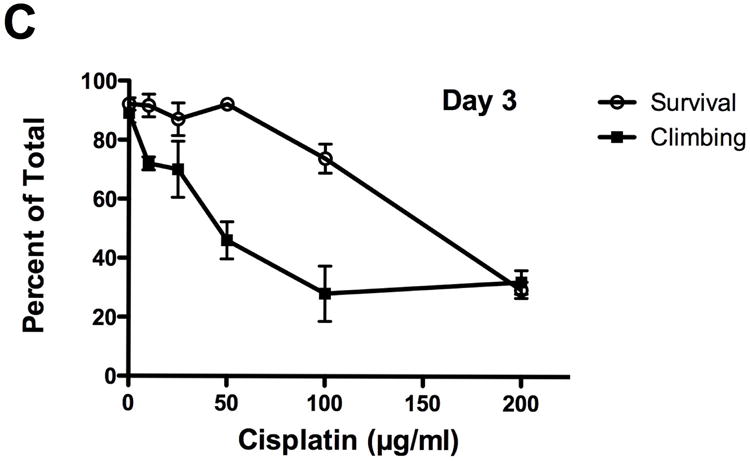
Cisplatin decreased fly survival and inhibited geotaxis climbing behavior. Lethality and climbing behavior were measured in cisplatin treated flies on days 3, 6 and 9. Cisplatin induced a dose dependent decrease in fly (A) survival (p<0.001) and (B) geotaxis climbing ability (p<0.0001). (C) On day 3, climbing ability was impaired at non-lethal cisplatin concentrations. Survival in Drosophila treated with 50μg/ml cisplatin was 92%, with only 46% of the flies able to climb above 2cm.
Inhibition of geotactic climbing behavior by cisplatin was dose dependent (Fig. 1B). Flies above the 2cm line at the end of the 20 second time period were counted. Percent of flies above the 2cm line treated with 0, 10, 25, 50, 100 and 200μg/ml cisplatin was 89%, 72%, 70%, 46%, 28%, 32% at 3 days, 90%, 86%, 74%, 56%, 28%, 8% at 6 days and 86%, 86%, 70%, 59%, 23%, 3% at 9 days. There were not enough flies to do the assay with 400μg/ml cisplatin. No significant decrease in climbing was observed with 10 or 25μg/ml cisplatin at 3 and 6 days (p>0.05). Climbing was significantly impaired with 50, 100 and 200μg/ml cisplatin at 3, 6 and 9 days (P<0.01 to P<0.001). Inhibition of climbing behavior was observed at non-lethal doses of cisplatin (Fig. 1C). On day 3, 50μg/ml cisplatin inhibited climbing behavior 46% while 92% of the flies survived.
Caspase activity
Caspase 3 activation was observed in ovaries and brain isolated from cisplatin treated flies. Oocytes are a rapidly dividing cell and were used as a positive control for cisplatin-induced apoptosis. Ovaries (Fig. 2A) were isolated from Drosophila treated with or without 50μg/ml cisplatin and immunostained for active caspase 3 and bisbenzimide. Control ovaries (Fig. 2B) had normal nuclei as visualized with bisbenzimide and no active caspase 3 staining. Immunostaining of cisplatin treated ovaries (Fig. 2C) showed apoptosis with fragmented nuclei and positive caspase 3 staining in oocytes. Apoptosis was quantitated in ovaries isolated from flies treated with 0, 10, 25, 50, 100 and 200μg/ml cisplatin (Fig. 2D) by counting the number of caspase 3 positive oocytes. Apoptosis was: 3%, 5%, 11%, 19%, 32% and 33%. When the concentration of cisplatin was increased to 100μg/ml cisplatin reproduction was interrupted and the first generation of flies was lost (data not shown).
Figure 2.
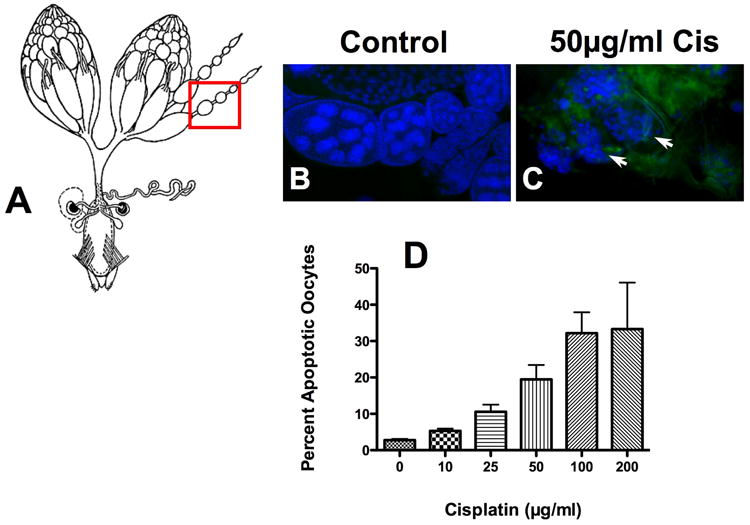
Cisplatin-induced apoptosis in Drosophila oocytes. To determine if drug delivery was effective, ovaries harvested from flies treated with and without 50μg/ml cisplatin were immunostained and oocytes were quantitated for apoptosis. (A) Ovaries (www.flybase.org/reports/FBim0000078 permissions requested) were stained for active caspase 3 and nuclei were visualized using bisbenzimide (blue). Normal oocytes with no caspase 3 activation and intact nuclei were observed in (B) controls. Fragmented nuclei (arrows) with positive caspase 3 staining (green) along with a disorganized morphology were observed in (C) cisplatin treated oocytes. (D) Caspase 3 positive oocytes were counted and expressed as a percent of the total number of oocytes (p=0.098). Despite the disorganized morphology, we were still able to count individual oocytes.
Drosophila brain (Fig. 3A) was harvested from cisplatin treated flies and stained for active caspase 3 and a neuron specific nuclear transcription protein, eLav. Control brain stained for eLav (Fig. 3B) and active caspase 3 (Fig. 3D) showed no caspase staining in neurons (Fig. 3F). Brain from cisplatin treated flies stained for eLav (Fig. 3C) and active caspase 3 (Fig. 3E), showed positive caspase 3 staining co-localized with eLav neuronal staining (Fig. 3G). Apoptosis was quantitated in brains treated with 50μg/ml cisplatin (Fig. 3H). Control brain (n=10) had 1.11% apoptosis as compared to 16.18% apoptosis in cisplatin treated brain (n=8) (P=0.0007). Brain apoptosis in was confirmed by electron microscopy. Images of control (Fig. 3I) brain show normal neurons with intact plasma and nuclear membranes while nuclear fragmentation in cells with intact plasma membranes were observed in brains treated with 100μg/ml cisplatin (Fig. 3J).
Figure 3.
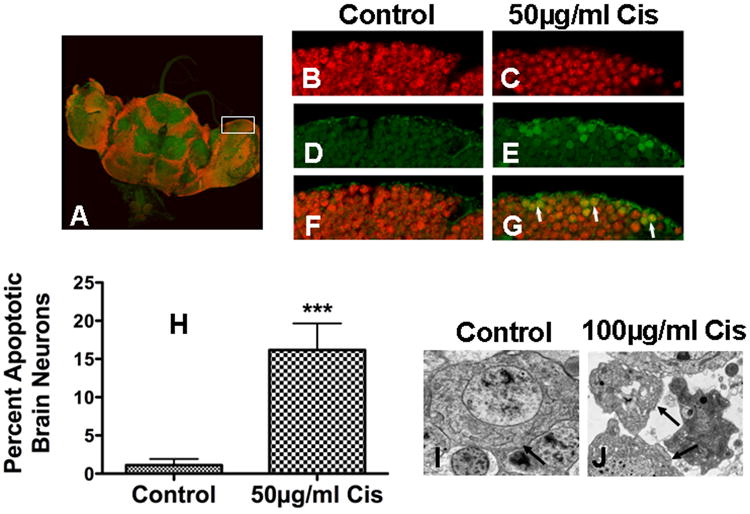
Cisplatin-induced apoptosis in Drosophila brain neurons. (A) Brain harvested from cisplatin treated (50μg/ml) flies were stained for active caspase 3 and eLav, a pan neuronal marker. Control and cisplatin treated brain neurons stained for (B, C) eLav (red) and (D, E) active caspase 3 (green), showed (F) no caspase 3 staining in control brain and (G) positive caspase 3 staining with co-localized eLav staining in cisplaitn treated brain (arrows). (H) Caspase 3 staining was found in 1.11% of control brain neurons (n=10) versus 16.18% in cisplatin treated brain neurons (n=8) (p=0.0007). Electron micrographs of (I) control brain and (J) brain treated with 100μg/ml cisplatin show apoptosis with intact plasma membrane (arrows) and fragmented nuclei.
There was no caspase 3 activation in heart or kidney harvested from cisplatin treated flies. Drosophila heart (Fig. 4A) was stained with antibodies for active caspase 3, pericardin and bisbenzimide. There was no nuclear fragmentation or active caspase 3 staining in control heart (Fig. 4B) or hearts treated with 50μg/ml cisplatin (Fig. 4C). The same results were found in Drosophila kidney (Fig. 4D) with no active caspase 3 activity or nuclear fragmentation in control (Fig. 4E) or cisplatin treated kidney (Fig. 4F).
Figure 4.
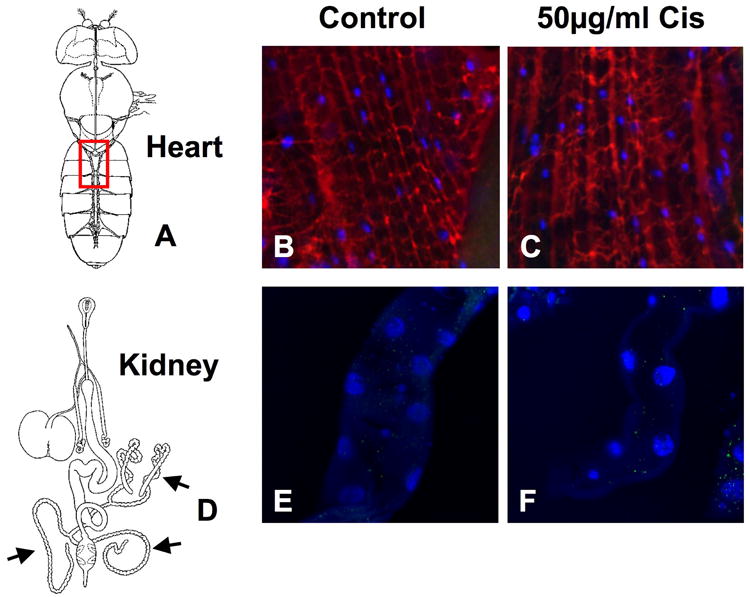
No apoptosis was observed in cisplatin treated heart or kidney. Heart and kidney from Drosophila treated with and without 50μg/ml cisplatin was observed for apoptosis. (A) Heart (www.flybase.org/reports/FBim0000047 permissions requested) was stained for active caspase 3 (green), pericardin (red) and nuclei visualized using bisbenzimide (blue). No caspase activity or nuclear fragmentation was observed in (B) control or (C) cisplatin treated heart. (D) Kidney (www.flybase.org/reports/FBim0000045 permissions requested) stained for active caspase 3 (green) and nuclei (blue) showed no positive caspase 3 staining or nuclear fragmentation in (E) controls or (F) cisplatin treated kidney.
p35 inhibition of cisplatin neurotoxicity
ELav-Gal4 and UAS-p35 Drosophila were bred to express non-apoptotic p35 behind the eLav promotor in neurons (Fig. 5A). Brains harvested from wild type and eLav-p35 Drosophila were immunostained for p35 expression and caspase 3 activation. There was no expression of p35 in wild type brain (Fig. 5B). Expression of p35 was observed in eLav-p35 brain neurons (Fig. 5C) and this expression prevented caspase activation in response to 100μg/ml cisplatin treatment (Fig. 5E). To ensure p35 protection was neuron specific and drug treatment was sufficient to induce apoptosis, we stained wild type and eLav-p35 ovaries for p35 and active caspase 3. No p35 expression was observed in wild type (Fig. 5F) and eLav-p35 (Fig. 5G) ovaries and cisplatin induced caspase activation in eLav-p35 ovaries (Fig. 5I). No caspase activation was observed in control eLav-p35 brain (Fig. 5D) or non-treated eLav-p35 ovaries (Fig. 5H). ELav-p35 Drosophila treated with or without 100μg/ml cisplatin were observed on day 6 for survival (Fig. 5J) and climbing behavior (Fig. 5K). Survival of control flies was 96% in wild type and 100% in eLav-p35 flies. Cisplatin treatment reduced fly survival to 66% and expression of p35 in brain neurons prevented fly death with 98% survival (p=0.0347). The percent of flies able to climb above 2cm was 79% in wild type controls and 93% in eLav-p35 controls. Climbing was reduced to 7% in wild type cisplatin treated flies and was significantly increased to 73% by p35 expression in neurons (P<0.0001).
Figure 5.
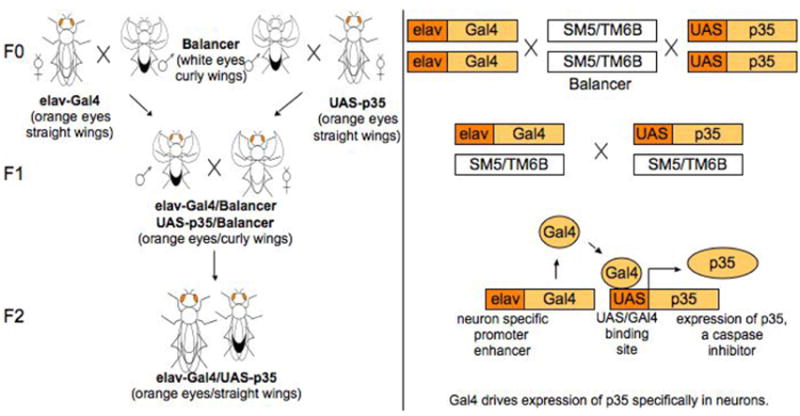
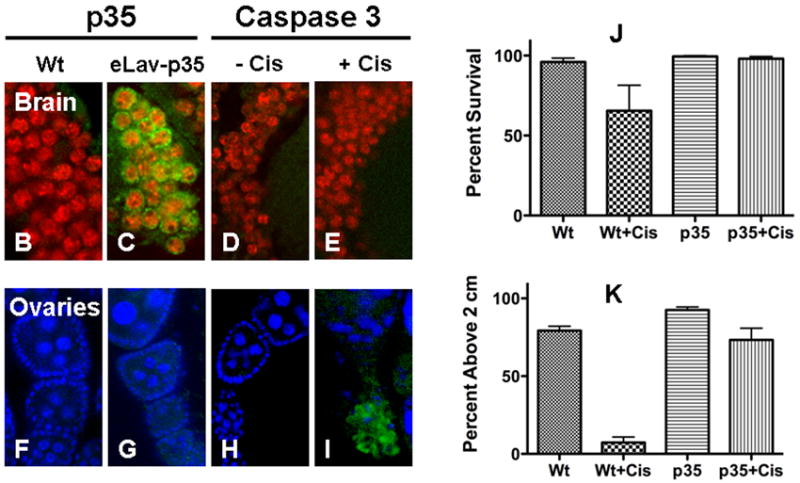
Using the Gal4-UAS system, (A) eLav-Gal4 flies and UAS-p35 Drosophila were bred with balancer flies to generate eLav-p35 flies. ELav-p35 Drosophila targeted expression of p35, a baculovirus anti-apoptotic protein, specifically in neurons. Expression of the caspase-inhibitor p35 in Drosophila brain prevented cisplatin induced apoptosis, lethality and climbing inhibition. ELav-p35 Drosophila were treated with or without 100μg/ml cisplatin and immunotstained for p35 expression and caspase 3 activation. p35 was not expressed in (B) wild type brain (Wt) but was expressed in (C) eLav-p35 brain neurons as indicated by co-localization of p35 (green) with eLav staining (red). Expression of p35 prevented caspase 3 activation in eLav-p35 brains treated (D) without or (E) with cisplatin. In Ovaries, no expression of p35 was observed in either (F) Wt or (G) eLav-p35 oocytes and no caspase 3 staining was observed in (H) control eLav-p35 oocytes. Cisplatin treatment induced positive active caspase 3 staining in (I) eLav-p35 oocytes. p35 expression in neurons improved (J) fly survival and prevented (K) climbing inhibition induced by cisplatin treatment. Wild type (Wt) and eLav-p35 (p35) flies were treated with 100μg/ml cisplatin. Cisplatin reduced fly survival and inhibited climbing ability in wild type flies (Wt+Cis). Survival was slightly improved (p=0.0347) and climbing inhibition significant improved (p<0.0001) by the expression of neuronal p35 in cisplatin treated eLav-p35 flies (p35+Cis).
Cyclophosphamide
Survival in cyclophosphamide treated flies decrease in a dose dependent manner while climbing behavior of surviving flies was unaffected (Fig. 6A). Flies were treated with 0, 200, 400, 800, 1400, 2000, 3000, and 4000μg/ml cyclophosphamide. On day 3 survival was 89%, 86%, 82%, 83%, 76%, 51%, 40% and 17% (p=0.0001) while percent flies able to climb above 2cm was 70%, 53% 78%, 76%, 84%, 65% and 88% (p=.0.0104) there were not enough flies to do the climbing assay with 4000μg/ml cyclophosphamide. In Drosophila treated with 3000μg/ml cyclophosphamide, no active caspase 3 was observed in brain neurons (Fig. 6B), however, active caspase 3 with fragmented nuclei was observed in both kidney (Fig. 6C) and ovaries (Fig. 6D).
Figure 6.
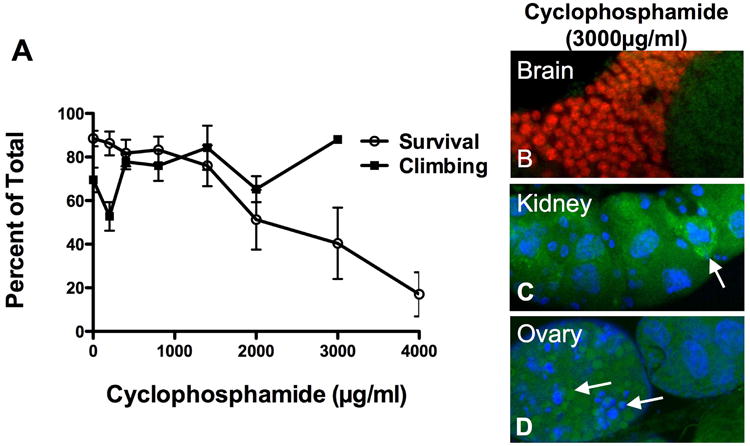
Cyclophosphamide did not inhibit geotaxis, climbing behavior or induce apoptosis in brain neurons. (A) Climbing was not impaired in cyclophosphamide treated flies (p=0.0104). At lethal concentrations, the surviving flies exhibited normal climbing ability. (B) No positive caspase 3 staining was observed in brain neurons, however, apoptosis was found in both (C) kidney and (D) ovaries.
Discussion
The goal of this study was to determine whether Drosophila has the potential to serve as a model system for studying cisplatin neurotoxicity. We determined that including cisplatin in the fly food resulted in tissue levels comparable to rodents and humans treated with the drug, that platinum DNA adducts were formed and that tissue damage was qualitatively similar to that seen in vertebrates. Cisplatin-induced neuronal apoptosis was associated with altered climbing behavior, which occurred at lower doses than what caused fly lethality. Importantly, the neuronal damage and behavioral changes were abrogated by targeting expression of an anti-apoptotic factor (p35) specifically in neurons.
Cisplatin binding to DNA in whole fly was similar to platinum levels found in cultured rat DRG. DNA isolated from embryonic rat DRG treated with 2μg/ml cisplatin had an accumulation of 1 platinum adduct per 3700 base pairs genomic DNA (McDonald et al., 2005). Drosophila fed 100μg/ml cisplatin had a whole fly DNA binding of 1 platinum molecule per 2500 base pairs genomic DNA. Although the delivery of the drug was administered orally to the Drosophila in our experiments, platinum levels bound to DNA are comparable to our rat DRG studies, in vitro. We had previously demonstrated (McDonald et al., 2005; Ta et al., 2006) that these tissue levels in rodents were comparable to those found in cisplatin treated patients and that the concentration of DNA platinum adducts in neurons was well in excess of levels associated with cell death in cancer cells. The data in our present study also establishes that while Drosophila has a rudimentary glial blood-brain barrier (Freeman and Doherty, 2006; Edwards and Meinertzhagen, 2010), there is entry of cisplatin into the Drosophila brain, which is associated with the observed effects on climbing behavior.
Cisplatin induced cellular changes were observed in various organs and cell types within the same animal. Apoptosis occurred in both rapidly dividing oocytes and differentiated, post-mitotic brain neurons. Cardiomyocytes are also non-dividing cells under normal conditions in rodents and humans, and are unaffected by cisplatin. Similarly we have shown that cisplatin does not affect Drosophila cardiomyocytes. We also noted (data not shown) a dramatic increase in apoptosis in Drosophila gut cells which may mimic gastrointestinal changes in humans (Bearcroft et al., 1999). The cell damage in the gut may also reflect oral ingestion of the drug by flies. The model was less faithful in the kidney which is affected in humans and rats (Fischer et al., 2008) but was not affected in flies. It is possible that kidney tubule cells in Drosophila do not concentrate or handle the drug in the same way as vertebrates.
Cisplatin-induced lethality and behavioral changes were associated with brain neuronal apoptosis. Neuron specific expression of anti-apoptotic p35 in cisplatin treated flies improved fly survival, prevented climbing deficiencies and inhibited brain neuron caspase 3 activation while still inducing apoptosis in ovaries. To better understand the relationship of neuron apoptosis and climbing inhibition, Drosophila were treated with cyclophosphamide, a non-neurotoxic, chemotherapeutic drug with similar therapeutic DNA binding mechanisms to cisplatin. Cyclophosphamide is an alkylating agent that binds to N7 of Guanine inducing inter- and intrastand crosslinks and apoptosis. Cyclophosphamide did kill the flies at higher concentrations, however, there were no climbing deficiencies or brain neuron apoptosis in the surviving flies. Cyclophosphamide did induce apoptosis in kidney and ovaries and may play a role in lethality. Thus, there appears to be an association between altered climbing behavior and brain neuron apoptosis.
Multiple mechanisms are involved in cisplatin-induced neurotoxicity and have been difficult to separate. Furthermore, it is still not completely understood why neurons are the only post-mitotic cells that are preferentially affected by cisplatin. Rodent models have proven a difficult model in which to answer this question. Drosophila melanogaster has the potential to be an excellent model to study the mechanisms of cisplatin and other neurotoxic drugs. Using lethality and climbing behavior, we can determine drug neurotoxicity or neuroprotection and potentially develop an assay to rapidly screen several drugs at a time. Immunostaining can be used to characterize the effect of drugs on neurons as well as dividing cells within the same animal. The ability to target gene expression or gene knockdown in specific cell populations will be a powerful tool in dissecting out different mechanisms of cisplatin toxicity in different cells types. Also, use of genetically modified Drosophila can lead to a better understanding of the mechanisms involved in drug neurotoxicity.
Research Highlights.
We propose adult Drosophila melanogaster as a model system to study the genetics of chemotherapy induced neurotoxicity.
We found dose dependent changes in lethality and climbing behavior in cisplatin treated flies.
Apoptosis was observed in oocytes, our positive control, and brain neurons but not in kidney or heart.
Changes in climbing behavior were associated with neuronal apoptosis
Cyclophosphamide, a non-neurotoxic drug, which binds DNA similar to cisplatin, does not produce the same climbing deficiencies or neuronal apoptosis.
Acknowledgments
This work is supported by NIH-NS40471. We would like to thank Jane Meyer for her administrative role in manuscript preparation as well as Maria Caruso and Scott Gamb for their excellent technical assistance with transmission electron microscopy of the Drosophila brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bearcroft CP, Domizio P, Mourad FH, Andre EA, Farthing MJ. Cisplatin impairs fluid and electrolyte absorption in rat small intestine: a role for 5-hydroxytryptamine. Gut. 1999;44:174–179. doi: 10.1136/gut.44.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella JA, Bayersdorfer F, Gmeiner F, Schneuwly S. Modelling Parkinson’s disease in Drosophila. Neuromolecular Med. 2009;11:268–280. doi: 10.1007/s12017-009-8098-6. [DOI] [PubMed] [Google Scholar]
- Carozzi VA, Chiorazzi A, Canta A, Lapidus RG, Slusher BS, Wozniak KM, Cavaletti G. Glutamate carboxypeptidase inhibition reduces the severity of chemotherapy-induced peripheral neurotoxicity in rat. Neurotox Res. 2010a;17:380–391. doi: 10.1007/s12640-009-9114-1. [DOI] [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Oggioni N, Sala B, Chiorazzi A, Meregalli C, Bossi M, Marmiroli P, Cavaletti G. Neurophysiological and neuropathological characterization of new murine models of chemotherapy-induced chronic peripheral neuropathies. Exp Neurol. 2010b;226:301–309. doi: 10.1016/j.expneurol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer. 2008;8 doi: 10.1038/nrc2167-c1. 1p following 71. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Fabbrica D, Minoia C, Frattola L, Tredici G. Carboplatin toxic effects on the peripheral nervous system of the rat. Ann Oncol. 1998;9:443–447. doi: 10.1023/a:1008231925889. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Petruccioli MG, Marmiroli P, Rigolio R, Galbiati S, Zoia C, Ferrarese C, Tagliabue E, Dolci C, Bayssas M, Griffon Etienne G, Tredici G. Circulating nerve growth factor level changes during oxaliplatin treatment-induced neurotoxicity in the rat. Anticancer Res. 2002;22:4199–4204. [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- Feany MB. New approaches to the pathology and genetics of neurodegeneration. Am J Pathol. 2010;176:2058–2066. doi: 10.2353/ajpath.2010.091077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SJ, McDonald ES, Gross L, Windebank AJ. Alterations in cell cycle regulation underlie cisplatin induced apoptosis of dorsal root ganglion neurons in vivo. Neurobiol Dis. 2001;8:1027–1035. doi: 10.1006/nbdi.2001.0426. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Garcia Sar D, Montes-Bayon M, Aguado Ortiz L, Blanco-Gonzalez E, Sierra LM, Sanz-Medel A. In vivo detection of DNA adducts induced by cisplatin using capillary HPLC-ICP-MS and their correlation with genotoxic damage in Drosophila melanogaster. Anal Bioanal Chem. 2008;390:37–44. doi: 10.1007/s00216-007-1634-z. [DOI] [PubMed] [Google Scholar]
- Iijima K, Iijima-Ando K. Drosophila models of Alzheimer’s amyloidosis: the challenge of dissecting the complex mechanisms of toxicity of amyloid-beta 42. J Alzheimers Dis. 2008;15:523–540. doi: 10.3233/jad-2008-15402. [DOI] [PubMed] [Google Scholar]
- Katz AJ. Genotoxic effects of cisplatin in somatic tissue of Drosophila melanogaster. Environ Mol Mutagen. 1987;10:197–203. doi: 10.1002/em.2850100210. [DOI] [PubMed] [Google Scholar]
- Katz AJ. Modulation by temperature of the genotoxic potency of cisplatin in Drosophila wing spot assay. Teratog Carcinog Mutagen. 1998;18:93–100. [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Morata G. Apoptosis in Drosophila: compensatory proliferation and undead cells. Int J Dev Biol. 2009;53:1341–1347. doi: 10.1387/ijdb.072447fm. [DOI] [PubMed] [Google Scholar]
- McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves Bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9:220–233. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Meijer C, de Vries EGE, Marmiroli P, Tredici G, Frattola L, Cavaletti G. Cisplatin-induced DNA-platination in experimental dorsal root ganglia neuronopathy. Neurotoxicology. 1999;20:883–887. [PubMed] [Google Scholar]
- Orso G, Martinuzzi A, Rossetto MG, Sartori E, Feany M, Daga A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J Clin Invest. 2005;115:3026–3034. doi: 10.1172/JCI24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H. Regulation of apoptosis in Drosophila. Cell Death Differ. 2008;15:1132–1138. doi: 10.1038/cdd.2008.50. [DOI] [PubMed] [Google Scholar]
- Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009;5:9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Davis LE, Kornfeld M, Hilgers RD, Standefer JC. Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer. 1984;54:1269–1275. doi: 10.1002/1097-0142(19841001)54:7<1269::aid-cncr2820540707>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- Woodruff RC, Valencia R, Lyman RF, Earle BA, Boyce JT. The mutagenic effect of platinum compounds in Drosophila melanogaster. Environ Mutagen. 1980;2:133–138. doi: 10.1002/em.2860020205. [DOI] [PubMed] [Google Scholar]
- Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A. Genetic control of programmed cell death (apoptosis) in Drosophila. Fly (Austin) 2009;3:78–90. doi: 10.4161/fly.3.1.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]


