Abstract
Depression is one of the most common psychological diseases with significant potential morbidity and mortality. Although the underlying pathophysiology of depression has not been clearly defined, preclinical and clinical evidence suggest disturbances in serotonin (5-HT), norepinephrine (NE), and dopamine (DA) neurotransmission in the central nervous system. Virtually all currently available antidepressants act on one or more of the following mechanisms: inhibition of reuptake of 5-HT or NE (and DA), antagonism of inhibitory presynaptic 5-HT or NE receptors, or inhibition of monoamine oxidase. All of these mechanisms result in an enhanced neurotransmission of 5-HT and/or NE. Evidence for the involvement of NE in depression is abundant, and recent studies on neuronal pathways and symptoms highlight the specific role of NE in this disorder. NE plays a determinant role in executive functioning regulating cognition, motivation, and intellect, which are fundamental in social relationships. Social dysfunction is possibly one of the most important factors affecting the quality of life in depressed patients.
Keywords: serotonin, antidepressants, neurotransmission, symptoms
Introduction
Depression is associated with significant potential morbidity and mortality contributing to suicide, medical illness, disruption of interpersonal relationships, lost work time, and often leading to substance abuse.1 The underlying pathophysiology of depression is not clearly understood, but biological, psychological, and social factors all play a causal role in depression.2
Imaging studies have shown that patients with depression have smaller hippocampal volume compared with controls,3 and there may be a link between depression and hippocampal neurogenesis.4 Evidence also suggests that major depression may involve an overactive hypothalamic-pituitary-adrenal axis which results in an effect similar to the neuroendocrine response to stress.5 The hormone, estrogen, has also been implicated in depressive disorders6–8 and in their treatment.9 The involvement of pro-inflammatory cytokines in depression is strongly suggested by meta-analyses of clinical studies showing higher blood concentrations of interleukin (IL)-6 and tumor necrosis factor (TNF)-α in depressed patients compared with controls.10,11 Other possible disease mechanisms that have been suggested include changes in glutamatergic neurotransmission, reduced neurotransmission of gamma-butyric acid, abnormal circadian rhythms, deficient neurosteroid synthesis, impaired endogenous opioid function, acetylcholine imbalance, tyroxine abnormalities, and dysfunction of specific brain structures and circuits.12 In spite of these new hypotheses, one of the oldest, the monoamine hypothesis which postulates a deficiency of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain,13,14 is still driving clinical development of new antidepressants. Virtually all currently available antidepressants act on one or more mechanisms compatible with the monoamine hypothesis: inhibition of reuptake of 5-HT or NE; antagonism of presynaptic inhibitory 5-HT or NE receptors; or inhibition of monoamine oxidase. All of these mechanisms result in an enhanced neurotransmission of 5-HT and/or NE. The confirmation of the clinical activity of these antidepressants has done much to reinforce the monoamine hypothesis.
An association of specific features and symptoms of depression and a deficiency or dysfunction of certain neurotransmitters has been proposed.15 Thus, a 5-HT deficiency is related to anxiety, obsessions, and compulsions; reduced NE neurotransmission is associated with decreased alertness, low energy, problems of inattention, concentration, and cognitive ability; while dysfunctional dopamine (DA) activity is implicated in problems of motivation, pleasure, and reward. Interestingly, increased 5-HT activity can be associated with certain symptoms such as fatigue.16
Evidence for the involvement of 5-HT in depression has been the subject of numerous studies.17 The role of NE15,18 and DA19,20 has been less extensively studied. This review briefly summarizes the involvement of NE in depression, highlighting the importance of the relationship between NE pathways and specific symptoms.
Evidence for the involvement of NE in depression
Several lines of evidence suggest that NE is a neurotransmitter of major importance in the pathophysiology and treatment of depressive disorders.21
NE projections from the locus coeruleus innervate the limbic system, which is implicated in the regulation of emotions.
Numerous differences have been found in elements of the NE system in postmortem brains from depressed patients and healthy controls.
Genetic studies show that mice with genetically engineered functional enhancement of the NE system are protected from stress-induced depression-like behaviors.
Experimental depletion of NE in the brain results in a return of depressive symptoms after successful treatment with NE antidepressant drugs.
Therapeutic agents which specifically increase NE activity are effective antidepressants.
NE neuroanatomy
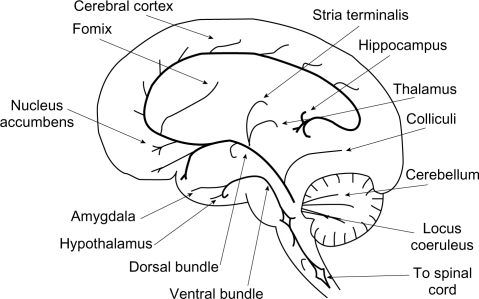
Noradrenergic pathways in the brain arise from the cell bodies in the locus coeruleus and project to different cerebral regions and to the spinal cord (Figure 1). In addition to major projections to the frontal cortex, NE neurons project to the limbic system, whose various components such as the amygdala, hippocampus, and hypothalamus are implicated in emotion and cognition as well as a number of functions modified in depressed patients such as appetite, response to pain, levels of pleasure, sexual satisfaction, and aggressive behavior.22
Figure 1.
Sagittal section of the human brain, showing the principal noradrenergic pathways.
Adapted with permission from Moret C. Understanding neurotransmission in the brain.
Available from: http://www.psy-world.com/unt_noradr.htm.
Imaging studies indicate that major depression is associated with abnormal metabolism in limbic and paralimbic structures of the prefrontal cortex. This abnormal metabolism is normalized in the amygdala and prefrontal cortex in patients showing a persistent antidepressant response.22
Stahl23 has suggested that it can be instructive to consider brain neuroanatomy in terms of specific functional centers (Table 1).23–26 The “emotional” and “somatic” centers in the brain receive projections from both NE and 5-HT as well as DA pathways. The “cognitive” centers, on the other hand, receive input only from NE as well as DA and histaminergic projections, but not 5-HT projections.23–26
Table 1.
Neuronal projections to different brain “centers”
| Emotional centers |
| NE projections from the locus coeruleus to the hypothalamus |
| NE projections from the locus coeruleus to the amygdala and prefrontal cortex |
| 5-HT projections from the midbrain raphe to the hypothalamus |
| 5-HT projections from the midbrain raphe to the amygdala and prefrontal cortex |
| DA projections from the ventral tegmentum to the nucleus accumbens |
| Somatic centers |
| NE projections from the locus coeruleus to the hypothalamus |
| NE projections from the locus coeruleus to the cerebellum |
| NE projections from the locus coeruleus to the spinal cord |
| 5-HT projections from the midbrain raphe to the hypothalamus |
| 5-HT projections from the midbrain raphe to the striatum |
| 5-HT projections from the midbrain raphe to the spinal cord |
| DA projections from the substantia nigra to the striatum |
| Cognitive centers |
| NE projections from the locus coeruleus to the dorsolateral prefrontal cortex |
| DA projections from the ventral tegmentum to the dorsolateral prefrontal cortex |
| Histamine projections from the hypothalamus to the dorsolateral prefrontal cortex |
Executive function is a complex organization of higher mental functions that process mental and environmental input to enable efficient problem-solving capacity in a way that is acceptable to both the individual and society. It includes inhibition of irrelevant or unacceptable behavior, the suppression of nonpertinent information, the regulation of verbal and nonverbal working memory, self-regulation of affect, motivation and arousal, planning, decision-making, self-monitoring of the problem-solving process, and self-evaluation of the results of the action taken. Anatomically, this occurs in the prefrontal lobe of the cortex and its afferent and efferent structures involving the neurotransmitters NE and DA and to a lesser degree acetylcholine and 5-HT.27
Executive function is also fundamental to social relationships. Social dysfunction in depression is possibly one of the most important factors affecting the quality of life of patients. Considerable clinical data suggest the importance of NE in the improvement of clinical dysfunction in depression.28
Biochemical differences between depressed patients and healthy controls
An early study29 found increased β-adrenergic receptor binding in the frontal cortex of suicide victims. More recently, post-mortem and functional imaging studies in the prefrontal cortex of depressed suicide victims have shown altered density and sensitivity of α2A-adrenoceptors which modulate NE release.30–32 In addition, decreased NE transporter binding has been reported in locus coeruleus of postmortem samples from subjects diagnosed with major depression.33 Alterations in putative peripheral markers of central NE function, such as α2-adrenoceptor density in platelets, have also been found in depressed patients.34,35 These modifications may all be part of the primary causal physiopathology of depression. Alternatively, at least some of them could be the result of compensatory modifications resulting from changes of NE neurotransmission in depressed patients. Whatever the interpretation, these data imply an important role for NE in depression.
Genetic studies of NE function
Genetic studies of NE function have indicated the multiple roles that NE plays in normal and pathological states. Functional deletion (knockout) of the NE transporter in mice results in increased extracellular levels of NE.36 This model functionally mimics the therapeutic effects of selective NE antidepressants. Recently, this model has shown that NE transporter (NET) knockout mice are resistant to the stress-induced depressive-like changes in behavior and brain neurotrophin expression that are seen in wild-type mice.37 Human genetic studies have shown that variations in the gene coding for NET which alter neurotransmitter release are related to individual differences in behavior and susceptibility to depression.38 The polymorphism, NET-T182C, for example, is associated with an increased susceptibility to depression.39
Catechol-O-methyltransferase (COMT) 158Val/Met is a polymorphism of a major enzyme in catecholamine inactivation. The alleles encoding Val and Met are associated with relatively high and relatively low COMT activity, respectively. The Val/Val genotype, a high-activity COMT genotype, was significantly less frequent in male suicide completers than in male controls.40 An association of 158Val/Met polymorphism with major depression is still unclear, since some studies have found the Met allele (low COMT activity) to be associated with major depression41 while others have not.42,43
NE depletion studies
Studies in depressed patients in remission and no longer taking medication have shown that a drastic reduction of NE levels (by inhibition of the key synthetic enzyme, tyrosine hydroxylase, with α-methyl-p-tyrosine) results in a rapid reappearance of depressive symptoms. Interestingly, however, catecholamine depletion in healthy control volunteers does not result in depressed mood.18,44,45
Clinical activity of noradrenergic antidepressants
A considerable proportion of patients fail to respond adequately to selective serotonin reuptake inhibitors (SSRIs). Analysis of the unresolved symptoms suggests that a specific set of symptoms related to decreased positive affect respond poorly to serotonergic antidepressants, namely loss of pleasure, loss of interest, fatigue, and loss of energy.46 There is evidence to suggest that antidepressants that enhance NE and DA activity offer a therapeutic advantage over 5-HT antidepressants in the treatment of symptoms associated with reduced positive affect.
The undisputed antidepressant action of NE-selective tricyclic antidepressants such as desipramine and nortriptyline suggests a major involvement of NE neurotransmission in depression, although these compounds or their metabolites also have some action on the 5-HT system. The selective NE reuptake inhibitor, reboxetine, has demonstrated equivalent efficacy to the TCA (tricyclic antidepressants) in some studies47,48 and is approved as an antidepressant in Europe but not in the USA. A recent publication49 suggests, however, that there may be considerable publication bias and that if unpublished studies are also considered, the antidepressant activity is unclear.
The SNRIs (serotonin and norepinephrine reuptake inhibitors) venlafaxine, milnacipran, and duloxetine show at least equivalent antidepressant efficacy to the SSRIs, and there is evidence that they may be more effective than the SSRIs in achieving remission.50
Conclusion
Although 5-HT has been the most studied neurotransmitter in depression, converging lines of evidence suggest that NE is of major importance in the pathophysiology and treatment of depressive disorder. NE projections from the locus ceoruleus innervate the limbic system, which is implicated in the regulation of emotions and cognition. Substantial functional biochemical differences exist in the NE system in postmortem brains from depressed patients and healthy controls. Genetic manipulation of the NE system that increases NE neurotransmission protects animals from stress-induced depressive behavior, while chemical manipulation that depletes the brain of NE increases the susceptibility of recovered depressed patients to a depressive relapse. Therapeutic agents which specifically increase NE activity are effective antidepressants, and there is evidence that those acting simultaneously on 5-HT and NE neurotransmission may have an antidepressant action superior to SSRIs.50
Footnotes
Disclosure
Dr Chantal Moret has no potential conflict of interest. Dr Mike Briley is a consultant for Pierre Fabre Médicament, Asahi Kasei Pharma, Germania Pharmaceutica, Janssen Pharmaceutica, and Cypress BioScience.
References
- 1.Halverson JL. Depression. http://emedicine.medscape.com/article/286759-overview 2010.
- 2.United States Surgeon General Mental Health: A Report of the Surgeon General – Chapter 2 The fundamentals of mental health and mental illness 1999. Available from: http://www.surgeongeneral.gov/library/mentalhealth/pdfs/c2.pdf. Accessed April 11, 2011.
- 3.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 4.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteleone P. Endocrine disturbances and psychiatric disorders. Curr Opin Psychiatry. 2001;14:605–610. [Google Scholar]
- 6.Cutter WJ, Norbury R, Murphy DG. Oestrogen, brain function, and neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 2003;74:837–840. doi: 10.1136/jnnp.74.7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders reproductive life cycle factors. Adv Nurs Sci. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lasiuk GC, Hegadoren KM. The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biol Res Nurs. 2007;9:147–160. doi: 10.1177/1099800407305600. [DOI] [PubMed] [Google Scholar]
- 9.Estrada-Camarena E, López-Rubalcava C, Vega-Rivera N, Récamier-Carballo S, Fernández-Guasti A. Antidepressant effects of estrogens: a basic approximation. Behav Pharmacol. 2010;21:451–464. doi: 10.1097/FBP.0b013e32833db7e9. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Perović B, Jovanović M, Miljković B, Vezmar S. Getting the balance right: established and emerging therapies for major depressive disorders. Neuropsychiatr Dis Treat. 2010;6:343–364. doi: 10.2147/ndt.s10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69(Suppl E1):4–7. [PubMed] [Google Scholar]
- 16.Marin H, Menza MA. The management of fatigue in depressed patients. Essent Psychopharmacol. 2005;6:185–192. [PubMed] [Google Scholar]
- 17.Cowen PJ. Serotonin and depression: pathophysiological mechanism or marketing myth. Trends Pharmacol Sci. 2008;29:433–436. doi: 10.1016/j.tips.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61(Suppl 1):5–12. [PubMed] [Google Scholar]
- 19.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery SA. The under-recognized role of dopamine in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23:63–69. doi: 10.1097/YIC.0b013e3282f2b3cb. [DOI] [PubMed] [Google Scholar]
- 21.Stahl SM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 3rd ed. New York, NY: Cambridge University Press; 2008. [Google Scholar]
- 22.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 23.Stahl SM. Symptoms and circuits, part 1: major depressive disorder. J Clin Psychiatry. 2003;64:1282–1283. doi: 10.4088/jcp.v64n1101. [DOI] [PubMed] [Google Scholar]
- 24.Stahl SM, Zhang L, Damatarca C, Grady M. Brain circuits determine destiny in depression: a novel approach to psychopharmacology of wakefulness, fatigue and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(Suppl 14):6–17. [PubMed] [Google Scholar]
- 25.Stahl SM. Symptoms and circuits, part 3: schizophrenia. J Clin Psychiatry. 2004;65:8–9. doi: 10.4088/jcp.v65n0102. [DOI] [PubMed] [Google Scholar]
- 26.Stahl SM. Brainstorms: symptoms and circuits, part 2: anxiety disorders. J Clin Psychiatry. 2003;64:1408–1409. doi: 10.4088/jcp.v64n1201. [DOI] [PubMed] [Google Scholar]
- 27.Papazian O, Alfonso I, Luzondo RJ. Executive function disorders. Rev Neurol. 2006;42(Suppl 3):S45–S50. [PubMed] [Google Scholar]
- 28.Briley M, Moret C. Improvement of social adaptation in depression with serotonin and norepinephrine reuptake inhibitors. Neuropsychiatr Dis Treat. 2010;6:647–655. doi: 10.2147/NDT.S13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- 30.Ordway GA, Schenk J, Stockmeier CA, May W, Klimek V. Elevated agonist binding to alpha 2-adrenoceptors in the locus coeruleus in major depression. Biol Psychiatry. 2003;53:315–323. doi: 10.1016/s0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- 31.Escribá PV, Ozaita A, García-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and muopioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 32.Valdizán EM, Díez-Alarcia R, González-Maeso J, et al. α(2)-Adrenoceptor functionality in postmortem frontal cortex of depressed suicide victims. Biol Psychiatry. 2010;68:869–872. doi: 10.1016/j.biopsych.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimek V, Stockmeier C, Overholser J, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurguis GN, Vo SP, Griffith JM, Rush AJ. Platelet alpha2A-adrenoceptor function in major depression: Gi coupling, effects of imipramine and relationship to treatment outcome. Psychiatry Res. 1999;89:73–95. doi: 10.1016/s0165-1781(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 35.Maes M, Van Gastel A, Delmeire L, Meltzer HY. Decreased platelet alpha-2 adrenoceptor density in major depression: effects of tricyclic antidepressants and fluoxetine. Biol Psychiatry. 1999;45:278–284. doi: 10.1016/s0006-3223(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 36.Wang YM, Xu F, Gainetdinov RR, Caron MG. Genetic approaches to studying norepinephrine function: knockout of the mouse norepinephrine transporter gene. Biol Psychiatry. 1999;46:1124–1130. doi: 10.1016/s0006-3223(99)00245-0. [DOI] [PubMed] [Google Scholar]
- 37.Haenisch B, Bilkei-Gorzo A, Caron MG, Bönisch H. Knockout of the norepinephrine transporter and pharmacologically diverse anti-depressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem. 2009;111:403–416. doi: 10.1111/j.1471-4159.2009.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z, Madras BK. Human genetics and pharmacology of neurotransmitter transporters. Handb Exp Pharmacol. 2006;175:327–371. doi: 10.1007/3-540-29784-7_16. [DOI] [PubMed] [Google Scholar]
- 40.Ono H, Shirakawa O, Nushida H, Ueno Y, Maeda K. Association between catechol-O-methyltransferase functional polymorphism and male suicide completers. Neuropsychopharmacology. 2004;29:1374–1377. doi: 10.1038/sj.npp.1300470. [DOI] [PubMed] [Google Scholar]
- 41.Ohara K, Nagai M, Suzuki Y, Ohara K. Low activity allele of catechol-o-methyltransferase gene and Japanese unipolar depression. Neuroreport. 1998;9:1305–1308. doi: 10.1097/00001756-199805110-00009. [DOI] [PubMed] [Google Scholar]
- 42.Frisch A, Postilnick D, Rockah R, et al. Association of unipolar major depressive disorder with genes of the serotonergic and dopaminergic pathways. Mol Psychiatry. 1999;4:389–392. doi: 10.1038/sj.mp.4000536. [DOI] [PubMed] [Google Scholar]
- 43.Kunugi H, Vallada HP, Hoda F, et al. No evidence for an association of affective disorders with high- or low-activity allele of catechol-o-methyltransferase gene. Biol Psychiatry. 1997;42:282–285. doi: 10.1016/S0006-3223(96)00366-6. [DOI] [PubMed] [Google Scholar]
- 44.Berman RM, Narasimhan M, Miller HL, et al. Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker. Arch Gen Psychiatry. 1999;56:395–403. doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- 45.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 46.Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21:461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- 47.Massana J. Reboxetine versus fluoxetine: an overview of efficacy and tolerability. J Clin Psychiatry. 1998;59(Suppl 14):8–10. [PubMed] [Google Scholar]
- 48.Montgomery SA. Reboxetine: additional benefits to the depressed patient. J Psychopharmacol. 1997;11(4 Suppl):S9–S15. [PubMed] [Google Scholar]
- 49.Eyding D, Lelgemann M, Grouven U, et al. Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ. 2010;341:c4737. doi: 10.1136/bmj.c4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62:1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Moret C. Understanding neurotransmission in the brain. Available from: http://www.psy-world.com/unt_noradr.htm. Accessed December 14, 2010.