Abstract
Memory T cells are distinguished from naive T cells by their rapid production of effector cytokines, although mechanisms for this recall response remain undefined. Here, we investigated transcriptional mechanisms for rapid IFN-γ production by antigen-specific memory CD4 T cells. In naive CD4 T cells, IFN-γ production only occurred following sustained antigen activation and was associated with high expression of the T-bet transcription factor required for Th1 differentiation, and T-bet binding to the IFN-γ promoter as assessed by chromatin immunoprecipitation analysis (ChIP). By contrast, immediate IFN-γ production by antigen-stimulated memory CD4 T cells occurred in the absence of significant nuclear T-bet expression or T-bet engagement on the IFN-γ promoter. We identified rapid induction of NFκB transcriptional activity and increased engagement of NFκB on the IFN-γ promoter at rapid times following TCR stimulation of memory compared to naive CD4 T cells. Moreover, pharmacologic inhibition of NFκB activity or peptide-mediated inhibition of NFκB p50 translocation abrogated early memory T cell signaling and TCR-mediated effector function. Our results reveal a molecular mechanism for memory T cell recall through enhanced NFκB p50 activation and promoter engagement, with important implications for memory T cell modulation in vaccines, autoimmunity and transplantation.
Keywords: Memory, T cells, Cell activation, transcription, signal transduction
INTRODUCTION
Memory T cells exhibit enhanced functional properties compared to naive T cells in their rapid production of effector cytokines upon antigen stimulation leading to efficacious secondary immune responses (1–2). In contrast to naive CD4 T cells which can only produce effector cytokines such as IFN-γ after sustained antigenic stimulation over days, memory CD4 T cells can produce IFN-γ within hours of TCR stimulation. The signaling pathways and mechanisms controlling these different effector responses and expedient memory T cell recall are not known. While it has been established that the acquisition of primary effector capacity such as IFN-γ production is controlled by upregulation of specific transcription factors in activated naive CD4 T cells, it is not known whether rapid IFN-γ secretion by memory T cells is controlled at the transcriptional level, and/or whether differences in transcription factor mobilization or regulation play a role.
IFN-γ transcription in CD4 T cells is predominantly regulated by the T-box transcription factor, T-bet. T-bet expression is both necessary and sufficient for IFN-γ production and differentiation of Th1 effector cells during a primary response from naive CD4 T cell precursors (3). In addition, CD4 T cells from T-bet-deficient mice are substantially impaired in IFN-γ production in both primary and secondary responses (4–5). Whether T-bet is required in wildtype memory CD4 T cells for rapid IFN-γ production and its role in secondary responses is not known.
IFN-γ production in CD4 T cells is also regulated by the ubiquitous transcription factor, NFκB. NFκB comprises a family of transcription factor subunits that control expression of multiple immune-related genes in both innate cells and lymphocytes (6). In resting cells, dimers or heterodimers of NFκB subunits p50 and/or p65(RelA) are associated with precursor and inhibitor molecules p105 and IκB, respectively (7). Antigen engagement of the T cell receptor (TCR) triggers a signaling cascade resulting in inhibitor degradation and nuclear translocation of active NFκB subunits for transcriptional regulation of multiple genes including IL-2, the high affinity IL-2 receptor (CD25), and IFN-γ (8–9). The role of NFκB transcriptional activity in memory CD4 T cell function has not been defined, and the subunits important for memory T cell recall are not known.
In this study, we hypothesized that rapid recall production of the effector cytokine IFN-γ by memory CD4 T cells was regulated by the enhanced activity of transcription factors. We focused on the role of T-bet and NFκB in memory recall, due to their known role in regulating IFN-γ production during primary responses. Here, we demonstrate that rapid IFN-γ production by antigen-specific memory CD4 T cells is regulated on the transcriptional level and occurred from a population that did not upregulate T-bet expression, but was associated with NFκB p50 nuclear translocation and increased nuclear p50 expression. Chromatin immunoprecipitation (ChIP) analyses further revealed that NFκB but not T-bet was engaged on the IFN-γ promoter in memory CD4 T cells at early times after antigen stimulation. Moreover, inhibition of NFκB activity by PDTC, a pharmacological inhibitor, or by a cell-permeable peptide inhibitor of NFκB p50 translocation abrogated early recall responses by memory CD4 T cells. Our results indicate differential control of IFN-γ transcription in naive and memory CD4 T cells, and a molecular mechanism for rapid memory recall responses through NFκB p50 activity and promoter engagement. The identification of a transcriptional regulator for memory immune responses is important for memory modulation in vaccines, autoimmunity and transplantation.
MATERIALS AND METHODS
Mice
BALB/c mice (8–16 weeks of age) were obtained from the National Cancer Institute Biological Testing Branch. DO11.10, DO11.10XRAG2−/− and RAG2−/− mice (Taconic Farms, Germantown, NY) were maintained in the Animal Facility at the University of Maryland (Baltimore, MD) and Columbia University under specific pathogen-free conditions. All animal procedures were approved by the Institutional Animal Care and Use committee for both Institutions.
Antibodies and Reagents
Fluorescently-conjugated anti-IFN-γ, -IL-2, -CD25, -CD44,- CD62L, and -CD4 antibodies were purchased from BD Pharmingen (San Diego, CA); anti-T-bet was from eBioscience (San Diego, CA), and KJ1-26 specific for DO11.10 TCR was from CALTAG Laboratories (Burlingame, CA). ChIP Grade antibodies specific for NFκB(p50) and T-bet were from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-Histone H3 and anti-p50/105 antibodies were from Abcam, Inc.(Cambridge, MA). For western blotting, rabbit anti- p105/50 was purchased from Abcam (Cambridge, MA, USA), anti-Actin (sc47778) from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and mouse anti-T-bet (4B10) was purchased from BD Pharmingen. HPLC-purified OVA peptide (323–339, IASQAVHAAHAEINEAGA) was synthesized by the Biopolymer Laboratory at the University of Maryland. PDTC (Ammonium Pyrrolidine Dithiocarbamate) was obtained from Sigma (St. Louis, MO), and cell-permeable p50 inhibitor and inactive control peptides were purchased from Cal Biochem/EMD Biosciences (San Diego, CA).
Generation and isolation of naive and memory CD4 T Cells
OVA-specific naïve CD4 T cells were isolated from the spleens of DO11.10 or DO11.10XRAG2−/− mice as described (10). OVA- specific memory CD4 T cells were generated as described (10–11) by in vitro priming of DO11.10 CD4 T cells with 1.0μg/ml OVA peptide and APC and adoptive transfer of the resultant primed/effector cells into RAG2−/− adoptive hosts, with persisting memory CD4 T cells recovered 2–5 months post-transfer. Polyclonal naive and memory CD4 T cells were isolated from whole CD4 T cells based on CD44 expression using anti-CD44-conjugated magnetic MACS microbeads and separated on a MACS magnet into CD44lo (naive) and CD44hi (memory) CD4 T cell subsets as previously described (12–13), and were also isolated based on CD62L expression into CD62Lhi (naive) and CD62Llo (memory) CD4 T cells by incubating with APC conjugated anti-CD62L antibody (eBioscience), followed by anti-APC conjugated magnetic microbeads (Miltenyi Biotec) and separation on a MACS magnet. Purification of polyclonal naïve and memory CD4 T cells yielded >90% pure cells by either approach.
Intracellular cytokine staining analysis
CD4 T cells were cultured with APC and 1μg/ml OVA peptide or with anti-CD3(5μg/ml)/anti-CD28 (5μg/ml) antibodies in the presence of monensin (Golgistop, BD Pharmingen) added 6 hours prior to cell harvest. Cytokine production was assessed by intracellular cytokine staining (ICS) as described (14) and analyzed using LSR II and FACSDiva software (BD-Biosciences).
Real-time PCR analysis
OVA-specific naive and memory CD4 T cells were isolated from DO11.10XRAG2−/− mice and from RAG2−/− adoptive hosts of primed DO11.10 CD4 T cells, activated in vitro with anti-CD3/anti-CD28 antibodies as above, and isolated at 0–72 hrs. RNA was isolated from 3–5×106 cells using the RNAeasy mini kit (Qiagen, Inc., Valencia, CA), and 5μg total RNA was used to generate cDNA using Superscript III first strand synthesis system (Invitrogen, Carlsbad, CA). IFN-γ sequences were amplified from cDNA using primers 5′-TCTGAGCAATGAACGCTACAC-3′ (sense) and 5′TCTTCCACATCTATGCCACTT-3′(anti-sence) along with control HGPRT and GAPDH sequences in SYBR green PCR master mix (Applied Biosystems, Carlsbad, CA), using the 7900 HT fast real-time PCR systems (Applied Biosystems).
Promoter-reporter assays
The following promoter-luciferase constructs for transcriptional reporter assays were obtained from Agilent Technologies Inc. (Santa Clara, CA): NF-κB (NFκB-Luc), NFAT (NFAT-Luc) promoter-luciferase, and the pCIS-CK negative control plasmid. Positive control pGL3 plasmid with firefly luciferase driven by the CMV promoter, and Renilla Luciferase Reporter Vector (pRL-CMV) were obtained from Promega Corporation (Madison, WI). Whole unfractionated CD4 T cells or CD44hi cells isolated from BALB/c mice were transfected directly or activated with anti-CD3/anti-CD28 antibodies for 24hrs prior to transfection with the indicated reporter constructs using nucleofection with the mouse T cell transfection kit V (Lonza, Inc., Cologne, Germany) as previously described (15). Following transfection, cells were incubated overnight at 37°C/5% CO2 in complete Clicks medium, and subsequently lysed in Passive Lysis Buffer (Promega Corp.) provided as part of the Dual-Luciferase Reporter Assay System (Promega). An aliquot of each lysate was mixed with luciferin substrate and within 30s, luciferase activity was measured based on light emission at 562 nm using the Turner Designs Model TD-20/20 Luminometer (Promega) which automatically measures both Firefly and Renilla luciferase activities. Readings for each sample were normalized by dividing the firefly/renilla units and activities within resting and TCR-stimulated T cells were expressed as the percent of the positive control (pCMV-GL3).
Chromatin Immunoprecipitation (ChIP) and PCR
ChIP analysis of T-bet and NFκB binding to the IFN-γ promoter was performed using the QuikChIP Assay Kit according to the manufacturer’s instructions (IMGENEX Corporation, San Diego, CA). OVA-specific naïve and memory CD4 T cells were activated with anti-CD3(5μg/ml)+ anti-CD28(2.5μg/ml) antibodies in complete media at 37°C for 6–72hrs, fixed and lysed in SDS Lysis Buffer (IMGENEX). After sonication to shear the DNA, samples were immunoprecipitated with anti-histone H3, anti-p50, anti-T-bet or no antibodies followed by protein A agarose at 4°C. ChIP sample DNA was amplified by PCR (40 cycles) using primers corresponding to sequences in the mouse IFN-γ promoter containing the NFκB (16) or T-bet (17) binding sites. For quantitative PCR, 20ng of total cDNA was amplified using SYBR Green as above on an Applied Biosystems 7900HT. Ct values were compared using the delta-delta-Ct method (Applied Biosystems) using mouse IgG samples for negative controls.
Western Blotting
Polyclonal naive and memory CD4 T cells were isolated to >90% purity by MACS separation as above, and were either lysed directly or activated for 6–48hrs with anti-CD3/anti-CD28 antibodies in complete media at 37°C prior to lysing. For isolation of cytoplasmic and nuclear fractions, cells were initially lysed in buffer A (10mM HEPES/10mM KCL/10mM EDTA/1mM DTT/0.4% IGEPAL, 1μg/ml protease inhibitor cocktail) at 4°C, followed by centrifugation to yield the cytoplasmic fraction in the supernatant and nuclear fraction in the pellet. Nuclear pellets were solubilized in lysis buffer B (20mM HEPES/400mM NaCl/1mM EDTA/10% Glycerol/1mM DTT, 1μg/ml protease inhibitor cocktail) at 4°C, followed by isolation of the nuclear lysate by centrifugation. Nuclear and cytoplasmic lysates were resolved on 4–20%SDS gels, and immunoblotted with anti-actin, anti-T-bet and anti-p50/105 antibodies as described (18).
RESULTS
Rapid IFN-γ production by memory T cells is transcriptionally controlled
We compared the kinetics of effector cytokine production in antigen-specific naive and memory CD4 T cells following antigen stimulation. Naive, OVA-specific CD4 T cells were obtained from DO11.10 TCR transgenic mice, either on BALB/c or RAG2−/− backgrounds as previously described (10). OVA-specific memory CD4 T cells were generated by adoptive transfer of primed DO11.10 CD4 T cells into adoptive hosts, and recovery of persisting memory CD4 T cells from host mice 2–4 months post-transfer as described (10–11, 19). Consistent with our earlier findings (10), antigenic stimulation of OVA-specific naive and memory CD4 T cells resulted in a Th1 cytokine profile with IFN-γ as the predominant effector cytokine (data not shown), and distinct kinetic profiles. Naive CD4 T cells exhibited peak IFN-γ production after 48–72hrs of stimulation, with negligible IFN-γ production at 6–24hrs post-stimulation (Fig. 1A). By contrast, a high frequency of memory CD4 T cells (25–40%) produced IFN-γ within 6hrs of antigen activation, with peak IFN-γ production at 24hrs, demonstrating the enhanced kinetics and magnitude of effector cytokine production that is a distinguishing feature of memory CD4 T cells.
FIGURE 1. Kinetics of IFN-γ secretion and transcript expression in naive and memory CD4 T cells.

(A) Rapid and enhanced production of IFN-γ in antigen-stimulated memory versus naive CD4 T cells. OVA-specific naive and memory CD4 T cells (see methods) were stimulated with OVA peptide and APC for 0–72hrs, and IFN-γ production was determined by intracellular cytokine staining. Results are expressed as percent of antigen-specific IFN-γ+ cells gated on CD4+KJ1-26+ cells at each timepoint, from one experiment representative of five independent experiments. (B) IFN-γ transcript expression in resting and activated naive and memory CD4 T cells. OVA-specific naive and memory CD4 T cells isolated as in (A) were activated with anti-CD3/anti-CD28 antibodies for 6–72hrs, and RNA was isolated from cell lysates of resting dn differentially activated, and IFN-γ-specific mRNA was amplified by quantitative realtime PCR (see methods). Results are expressed as expression of IFN-γ transcripts relative to control HPRT transcripts. Inset shows level of IFN-γ mRNA in naïve CD4 T cells with reduced scale, for direct comparison of peak mRNA production>
We investigated whether the rapid production of IFN-γ from memory CD4 T cells was due to production of new transcripts or derived from pre-existing IFN-γ transcripts. To distinguish these possibilities, we quantitated the level of IFN-γ mRNA in resting and activated OVA-specific naive and memory subsets by real-time PCR over a similar kinetic window of activation (Fig. 1B). In resting naive and memory CD4 T cells, the level of IFN-γ mRNA was below detection, indicating that neither subset harbors significant levels of pre-formed IFN-γ transcripts. However, the kinetics and magnitude of IFN-γ-specific mRNA differed vastly between the two subsets. Notably, memory CD4 T cells exhibited a dramatic increase and peak levels of IFN-γ transcripts after only 6hrs of TCR stimulation, whereas stimulation of naïve CD4 T cells resulted in peak IFN-γ mRNA content after 24–48hrs (Fig. 1B, also see inset). In addition, memory CD4 T cells produced >10-fold more IFN-γ mRNA at peak timepoints compared to activated naive CD4 T cells (Fig. 1B and inset). These results indicate that the enhanced kinetics and magnitude of IFN-γ production by memory CD4 T cells is controlled at the level of mRNA, and that preformed mRNA for IFN-γ is not measurably present in memory CD4 T cells.
Lack of constitutive or rapidly upregulated T-bet expression in memory CD4 T cells
We hypothesized that rapid production of IFN-γ mRNA in memory CD4 T cells could be due to elevated expression of the T-bet transcription factor that is required for IFN-γ transcription by Th1 effector cells (3). However, we found intracellular T-bet expression to be comparably low/negative in resting antigen-specific naive and memory CD4 T cells (Fig. 2A), as well as in resting polyclonal naive (CD44lo) and memory (CD44hi) CD4 T cells (Fig. 2B). After activation with antigen, there was minimal upregulation of T-bet in OVA-specific naive and memory CD4 T cells at 6hrs, with maximal T-bet upregulation observed after 48hrs of antigenic stimulation for memory CD4 T cells and 72hrs for naive CD4 T cells (Fig. 2A). TCR/CD3 stimulation of polyclonal CD4 T cells with anti-CD3/anti-CD28 antibodies resulted in maximal T-bet upregulation in both naive and memory CD4 T cells after 48hrs, although T-bet upregulation was greater in polyclonal memory compared to naive CD4 T cells after 24hrs of activation (Fig. 2B). These results show enhanced upregulation of T-bet expression following TCR stimulation of memory compared to naive subsets only after sustained stimulation (24–48hrs), and beyond the timepoint for rapid recall.
FIGURE 2. Early IFN-γ production by memory CD4 T cells is not associated with upregulation of T-bet expression or promoter engagement.
(A) T-bet expression in resting and antigen-stimulated naive and memory CD4 T cells. OVA-specific naive and memory CD4 T cells were stimulated with OVA/APC as in (A) and T-bet expression assessed by intracellular staining with T-bet specific antibodies. Histograms are gated on CD4+KJ1-26+ cells. (B) T-bet expression in resting and TCR-stimulated polyclonal naive and memory CD4 T cells. Polyclonal naive (CD44lo) and memory (CD44hi) CD4 T cells were sorted from BALB/c splenic CD4 T cells using anti-CD44PE- and PE-coupled magnetic microbeads and MACS™ columns (Miltenyi Biotech, Auburn, CA). Naive (CD44lo) and memory (CD44hi) CD4 T cells were activated with anti-CD3/anti-CD28 antibodies and T-bet expression was assessed at the indicated times. Histograms shown are gated on live CD4+ T cells. (C) T-bet expression as a function of IFN-γ production in resting and antigen-activated OVA-specific naive and memory CD4 T cells. T-bet and IFN-γ expression are shown gated on CD4+KJ1-26+ cells. Results are representative of five independent experiments.
We further evaluated whether IFN-γ production from naive or memory CD4 T cells was occurring from cells that had upregulated T-bet (Fig. 2C). In OVA-specific naive CD4 T cells, IFN-γ production was detected 24–48hrs after antigenic stimulation exclusively from the T-bethi population (Fig. 2C, upper), consistent with the requirement for T-bet expression for Th1 generation. By contrast, in memory CD4 T cells, IFN-γ production at early times of antigenic stimulation (6–24hrs) occurred from T-betlo cells, and was only associated with T-bethi cells after sustained (48–72hrs) antigens stimulation (Fig. 2C, lower). This coordinated analysis of T-bet and IFN-γ production revealed that early IFN-γ production by memory T cells occurred from distinct populations relative to T-bet expression, with rapid IFN-γ production occurring in the absence of substantial T-bet expression or upregulation.
NFκB transcriptional activity is detected at early times following TCR stimulation in CD4 T cells
We investigated whether the transcription factors NFκB or NFAT, previously shown to be involved in IFN-γ transcription in cell lines or primary T cells (8–9) were activated at early times following TCR stimulation in CD4 T cells using luciferase reporter assays. We initially performed this analysis in whole, unfractionated CD4 T cells from BALB/c mice as we previously showed that the population producing IFN-γ at early times following activation corresponds to memory phenotype CD44hi cells (10). We transfected resting and anti-CD3/CD28-stimulated CD4 T cells with promoter-reporter constructs in which luciferase was driven by NFκB-binding (NFκB-Luc) or NFAT-binding promoter elements (NFAT(IL-2)-luc), or a constitutively active CMV promoter (pGL3) as a positive control and a promoterless luciferase plasmid (pCIS-CK) as a negative control. All luciferase constructs were co-transfected with a plasmid expressing renilla luciferase (pRL-CMV) for normalization of luciferase activity between samples using the dual luciferase reporter assay (see methods). In resting CD4 T cells, there was negligible NFAT activity and a low level of basal NFκB activity. Following TCR stimulation for 24hrs, NFκB activity increased substantially (3–4fold), whereas NFAT activity was not significantly higher than the negative control (Fig. 3A), consistent with other studies which also found negligible NFAT activity in Th1-like effector cells or following 24hrs activation of primary mouse T cells (20). We also fractionated the memory (CD44hi) subset from total CD4 T cells and found a similar low, yet detectable level of NFκB activity in resting memory-phenotype CD44hi CD4 T cells, upregulation of NFκB transcriptional activity within 24hrs of TCR stimulation of this subset (Fig. 3B), and no detectable NFAT activity at this timepoint (data not shown). Together, these results indicate that NFκB, but not NFAT activity is readily triggered early after TCR-engagement of memory CD4 T cells.
FIGURE 3. NFκB transcriptional activity is upregulated in memory CD4 T cells.
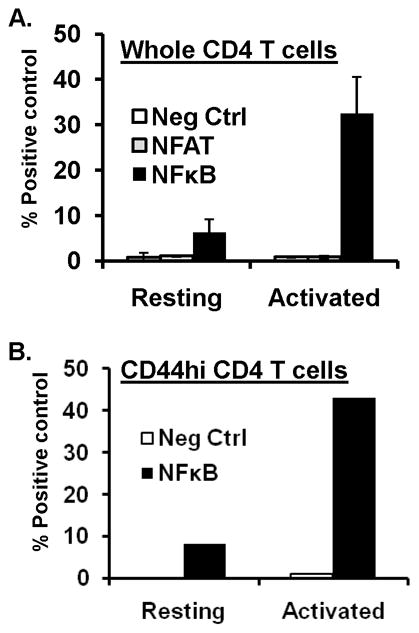
(A) NFκB activation in resting and short-term activated CD4 T cells. Total CD4 T cells purified from BALB/c mouse spleen were used directly as resting cells or activated for 24hrs with anti-CD3/anti-CD28 antibodies before cotransfection with pRL3 encoding renilla luciferase along with plasmids pNK-Luc, pNFAT-luc, pCIS-K(neg. control) or pGL3-luc (positive control) by nucleofection (see methods), and cultured overnight. Dual luciferase activities were subsequently quantitated in cell lysates. Graph show normalized luciferase activity compared to the positive (pGL3-luc) control for resting and activated CD4 T cells, respectively. Results are compiled from three independent experiments. (B) NFκB activity in resting and activated memory CD4 T cells. Memory (CD44hi) CD4 T cells were fractionated from total CD4 T cells and transfected directly or after 24hrs activation with anti-CD3/anti-CD28 antibodies with pRL3 in combination with pGL3, pNFκB-luc or pCIS-K and dual luciferase activity quantitated and graphed as in (A). Results are representative of three independent experiments.
Nuclear translocation of T-bet and NF-κB after TCR stimulation in naïve and memory CD4 T cells
We investigated whether T-bet or NFκB transcription factors were differentially expressed and/or mobilized to the nucleus in naive or memory CD4 T cells. We analyzed T-bet and NFκB p50/p105 expression in the cytoplasm and nuclear fractions of polyclonal naive and memory CD4 T cells in the resting state and following different timepoints of TCR stimulation (Fig. 4). Consistent with the flow cytometry results, we did not detect T-bet expression in resting naive or memory CD4 T cells in the cytoplasm or nucleus. T-bet expression appeared as a faint band in the cytoplasmic fraction in 6hr-stimulated naive and memory CD4 T cells with maximal cytoplasmic T-bet expression after 48hrs for both subsets (Fig. 4, upper gel). In the nucleus, T-bet was not present in resting or 6hr-activated naive or memory CD4 T cells, with nuclear T-bet detected only after 24–48hrs of activation for both subsets (Fig. 4, lower gel). We analyzed expression of NFκB p50 which is expressed as a complex with its precursor p105 inhibitor molecule in the cytoplasm (p50/p105) and as an active p50 form in the nucleus (6). Both resting naive and memory CD4 T cells had a low level of cytoplasmic p50/p105 which increased following 6–48hrs of activation (Fig. 4, upper gel). In the nucleus, a faint NFκB p50 band was detected in nuclear lysates of resting naive and memory CD4 T cells (no p105 was present, data not shown); however, after 6hrs of stimulation memory CD4 T cells exhibited a 1.8-fold increase in nuclear p50 by densitometry, while naive CD4 T cells maintained the same level of nuclear p50 as in the resting state (Fig. 4, lower). We also found a greater increase in p50 after 0–6hrs stimulation of OVA-specific memory compared to naive CD4 T cells (supplemental Fig. 1). These results indicate that at the timepoint for rapid recall (6hrs after stimulation), NFκB, but not T-bet, is present in the nucleus of naive and memory CD4 T cells and exhibits a greater fold- upregulation in memory CD4 T cells.
FIGURE 4. T-bet and NFκB expression and nuclear mobilization in resting and activated polyclonal naive and memory CD4 T cells.
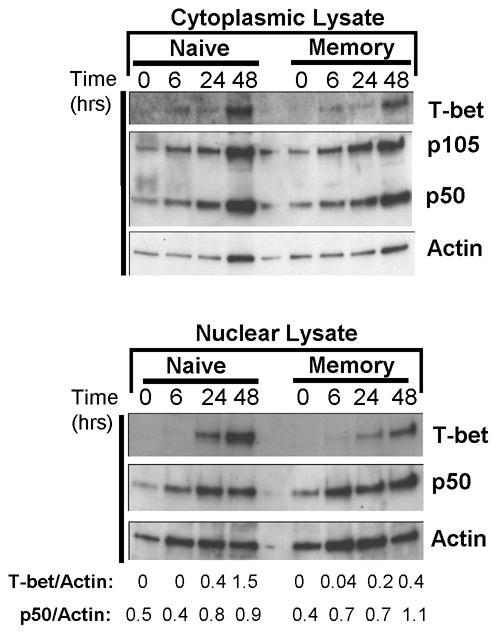
(A) Kinetics of T-bet expression in polyclonal naive and memory CD4 T cells. (B) Mobilization of T-bet and NFκB in resting and activated naïve and memory CD4 T cells. Polyclonal naive and memory CD4 T cells were lysed directly or activated with anti-CD3/CD28 antibodies for 6–48hrs, and cytoplasmic and nuclear lysates were prepared. Expression of T-bet and NFκB p50 in the cytoplasm and nucleus of resting and activated naive and memory CD4 T cells, with densitometry calculations for T-bet and p50 expression indicated below the gel. Nuclear extracts did not contain NFκB p105. Results are representative of three experiments.
Rapid engagement of NFκB but not T-bet on the IFN-γ promoter in memory CD4 T cells
To further evaluate transcriptional mechanisms controlling early IFN-γ production by memory CD4 T cells, we used chromatin immunoprecipitation (ChIP) analysis to compare T-bet versus NFκB p50 engagement on the IFN-γ promoter in resting and activated naive and memory CD4 T cells. We evaluated whether a 360bp fragment corresponding to the T-bet binding site on the IFN-γ promoter (17) was present in anti-T-bet immunoprecipitates (IPs) from OVA-specific naive and memory CD4 T cells before and after stimulation with anti-CD3/anti-CD28 antibodies (used to avoid contaminating APC) (Fig. 5A). For the ChIP assay; no Ab IPs served as negative controls (−), anti-histone H3 IPs were positive controls (+) and the input DNA (“N”) prior to immunoprecipitation was the positive PCR control. In resting memory but not naive CD4 T cells, we detected a weakly staining 360bp band associated with T-bet; however, after TCR/CD3 stimulation of both naive and memory CD4 T cells, we detected a 360kb band in anti-T-bet IPs only after 48hrs of stimulation, and not in lysates derived from 6hr or 24hr-stimulated cells (Fig. 5A). These results indicate similar delayed kinetics of T-bet binding to the IFN-γ promoter following TCR stimulation of both naive and memory subsets, and no substantial T-bet engagement on the IFN-γ promoter at early times following TCR stimulation, consistent with the the lack of nuclear T-bet expression at early timepoints observed in western blots (Fig. 4). Thus, by flow cytometry, western blotting and ChIP analysis, we do not find an association of T-bet with early IFN-γ production by memory CD4 T cells.
FIGURE 5. Differential engagement of T-bet and NFκB transcription factors on the IFN-γ promoter in naive and memory CD4 T cells.

(A) Chromatin immunoprecipitation (ChIP) analysis of T-bet bound to the IFN-γ promoter. OVA-specific naïve (“Nai”) and memory (“Mem”) CD4 T cells were crosslinked and lysed directly ex vivo or following activation for 6–48hrs with anti-CD3/anti-CD28 antibodies. DNA was sheared and immunoprecipitated with protein A-sepharose alone (negative control, “−“), anti-histone H3 (positive control, “+”) and anti-T-bet (“T”). Sequences on the IFN-γ promoter corresponding to a 360bp region of the T-bet binding sites were PCR-amplified from immunoprecipitates or input DNA (“N”) as an additional positive control. (B) ChIP analysis of NFκB binding to the IFN-γ promoter in memory CD4 T cells. OVA-specific memory CD4 T cells were isolated and activated as in (A), and prepared for ChIP analysis. Gel shows a 388bp product corresponding to the NFκB binding site on the mouse IFN-γ promoter that was PCR-amplified from immunoprecipitates using protein A agarose alone (lanes marked “−“), anti-histone H3 (lanes marked “+”), or anti-p50(NFκB) antibody (lanes marked “κB”). (C) Quantitative Comparison of NFκB versus T-bet binding to the IFN-γ promoter in naive and memory CD4 T cells. ChIP analysis was performed on resting and activated naive (N) and memory (M) CD4 T cells as in (A) and (B), except real-time qPCR was used for amplification. Graph shows the average fold induction over negative control immunoprecipitates from 4 independent experiments.
For NFκB ChIP, we used anti-p50 antibodies to immunoprecipitate NFκB, and amplify out a 388bp fragment corresponding to the NFκB binding site of the IFN-γ promoter (16). By ChIP analysis, we detected a 388bp band in anti-p50 IPs in memory CD4 T cells which were stimulated for 6–48hrs, but not in resting memory CD4 T cells (Fig. 5B). We further analyzed quantitative differences in the temporal binding of NFκB p50 versus T-bet on the IFN-γ promoter in naive and memory CD4 T cells, using real time qPCR analysis of NFκB p50 or T-bet ChIP (Fig. 5C). In naive CD4 T cells, both NFκB p50 and T-bet showed maximal association to the IFN-γ promoter after 48hrs of TCR stimulation (Fig. 5C). In memory CD4 T cells, there was increased engagement of NFκB p50, but not T-bet, on the IFN-γ promoter 6hrs after TCR stimulation, while after 48hrs of TCR stimulation both NFκB and T-bet bound to the IFN-γ promoter (Fig. 5C). These quantitative ChIP results reveal two important insights into transcriptional control of rapid recall in memory T cells: First, they demonstrate that NFκB is bound to the IFN-γ promoter at early times (6hrs) post-stimulation only in memory and not in naive CD4 T cells, despite the presence of nuclear p50 in 6hr-activated naive cells as detected by western blot (Fig. 4). Second, the ChIP results show that mobilization of NFκB on the IFN-γ promoter occurs earlier than T-bet in memory CD4 T cells.
NFκB activity and p50 translocation is required for rapid recall of memory CD4 T cells
To determine a functional requirement for NFκB in memory CD4 T cell recall that was suggested by the molecular analyses above, we selectively inhibited NFκB activity and assayed its effect on memory T cell recall and signaling. Activation of OVA-specific memory CD4 T cells with antigen (OVA/APC) in the presence of the pharmacologic NFκB inhibitor PDTC (21–22) resulted in near complete inhibition of early IFN-γ production at 6 and 24hrs post-antigen stimulation (Fig. 6A). In addition, PDTC also inhibited antigen-driven CD25 upregulation (right), indicating that NFκB is required for early recall responses by memory CD4 T cells.
FIGURE 6. NFκB transcriptional activity and p50 translocation is required for rapid recall of memory CD4 T cells.

(A) OVA-specific memory CD4 T cells were stimulated with OVA/APC for 6 and 24hrs in the presence of PDTC (25μM) or PBS control, and T-bet expression, IFN-γ production and CD25 induction were analyzed by flow cytometry gated on CD4+KJ1-26+ T cells. Results are representative of five experiments. (B) OVA-specific memory CD4 T cells were activated for 6hrs with anti-CD3/anti-CD28 antibodies in the presence of media alone, or media plus cell permeable control peptide or p50 inhibitor peptide(see methods) and IFN-γ production was assessed. Results in are representative of three independent experiments with the average inhibition by p50 =55±11%. (C) Memory CD4 T cells activated as in (B), showing downregulation of antigen-specific TCR expression (Clonotype KJ1-26) and upregulation of CD25 expression after 6hrs of activation with OVA/APC in the presence of control or P50 inhibitor peptide.
To rule out secondary effects of PDTC on TCR-mediated events, we also used a cell-permeable, peptide-specific inhibitor of NFκB p50 translocation (see methods) to determine whether activation of NFκB p50 was required for memory CD4 T cell signaling and function. As shown in Fig. 6B, activation of memory CD4 T cells for 6 hrs resulted in IFN-γ production that was unperturbed by the presence of a control cell-permeable peptide, but reduced by >50% in the presence of a similar concentration of p50 inhibitor peptide (Fig. 6B). Importantly, the p50 inhibitor peptide also inhibited other early TCR-driven events in memory T cell activation including TCR downregulation, measured by surface expression of the KJ1-26 clonotype, and CD25 upregulation (Fig. 6C). These results demonstrate regulation of early memory CD4 T cell signaling and effector function via NFκB p50 translocation.
DISCUSSION
We present here new molecular and functional evidence for control of memory CD4 T cell recall by the transcription factor NFκB. Specifically, we demonstrate that IFN-γ production by antigen-specific and polyclonal memory CD4 T cells is associated with an increase in NFκB activity and p50 subunit expression, but not nuclear expression of the T-bet transcription factor that is required for IFN-γ production during Th1 differentiation. Moreover, there was an increase in NFκB p50, but not T-bet binding to the IFN-γ promoter in memory CD4 T cells after short-term stimulation by ChIP analysis. We further demonstrate that rapid IFN-γ production by antigen-stimulated memory CD4 T cells requires NFκB transcriptional activity, and that early memory T cell signaling and activation specifically requires nuclear translocation of the p50 subunit. Together, our findings provide a molecular basis for the anamnestic response by memory T cells.
We demonstrate that rapid IFN-γ production by memory CD4 T cells is controlled at the transcriptional level, as resting memory CD4 T cells displayed negligible expression of IFN-γ transcripts by real-time PCR, yet were substantially increased at 6hrs after stimulation. Similarly, the lack of early IFN-γ production by naive CD4 T cells was mirrored by a lack of significant IFN-γ transcription until later times after stimulation. Our results are consistent with earlier results using IFN-γ promoter-luciferase reporter mice which showed IFN-γ promoter activity (i.e. luciferase expression) only in memory CD4 T cells early after stimulation and not in naive T cells (23). Differential transcription factor usage by naive and memory CD4 T cells has also been explored in additional types of promoter-luciferase transgenic mice. These studies established that AP-1 transcriptional activity was equivalent in naive and memory CD4 T cells (24), while accumulation of NFAT activity was required at later times (after 36hrs) for IL-2 production (25). A role for NFAT or other transcription factors in early IFN-γ production was not identified in these studies, and we similarly did not find early induction of NFAT activity in memory CD4 T cells that correlated with early recall responses.
T-bet is both necessary and sufficient for Th1 differentiation and IFN-γ production resulting from stimulation of naive CD4 T cells (3, 5), and is the main controlling factor for IFN-γ production in CD4 T cells (26). We initially hypothesized that increased expression or upregulation of T-bet might control rapid IFN-γ production by memory CD4 T cells; however, results obtained through three different approaches together indicate that rapid recall by memory CD4 T cells is likely independent of T-bet transcriptional activity. First, by intracellular flow cytometry, we did not detect T-bet expression in resting or short-term activated antigen-specific or polyclonal memory CD4 T cells, nor an association with T-bet expression and rapid memory CD4 T cell IFN-γ producers. Second, by western blot analysis, we were able to detect nuclear T-bet only after sustained (24–48hr) activation of both naive and memory T cells, indicating similar delayed kinetics of nuclear T-bet accumulation for both subsets. Finally, ChIP analysis revealed a similar late kinetics of T-bet binding to the IFN-γ promoter in activated naive and memory CD4 T cells. Our results are certainly consistent with findings that terminally differentiated Th1 effector cells did not exhibit a similar requirement for T-bet for IFN-γ production compared to primary Th1 effector (27–28), due to stable T-bet-induced chromatin modifications at the IFN-γ locus (28).
Our results establish a role for NFκB transcriptional activity and in particular, translocation of the p50 subunit in rapid IFN-γ by memory CD4 T cells. In T cells, active forms of NFκB comprise heterodimers of p50/p65 subunits or homodimers of p50 that need to be liberated from the associated inhibitor molecules in the cytoplasm (IκB and p105, respectively), prior to nuclear translocation and activation (29–30). We found that translocation of p50 to the nucleus occurred at early times (from 5–60minutes) in both memory and naive CD4 T cells (data not shown) with a greater increase in nuclear p50 observed between 0–6hrs of activation of memory compared to naive CD4 T cells (Fig 4). In addition, nuclear p50 was only found associated with the IFN-γ promoter in memory CD4 T cells and not in naive CD4 T cells at the 6hr activation timepoint. Together, the western blot and ChIP analyses suggest that alterations in the IFN-γ promoter of memory CD4 T cells may facilitate access of NFκB for transcriptional activation of the IFN-γ gene. Epigenetic modifications have also been identified in memory CD8 and CD4 T cells in the promoter regions of IFN-γ and other genes that are rapidly expressed upon secondary challenge (31–33), which may account for the ability of NFκB to readily engage the IFN-γ promoter in memory, but not naïve CD4 T cells.
Our finding that IFN-γ transcripts were not detected in resting memory T cells, and that TCR-mediated NFκB p50 activation was required for rapid IFN-γ production by memory CD4 T cells together support TCR-mediated signaling control of early memory T cell recall. We previously showed in a model of TCR-mediated signaling ablation in memory T cells, that deletion of the key TCR-coupled linker/adapter molecule in memory T cells abrogated TCR-mediated rapid recall (34). Here, we show that TCR-mediated triggering in wildtype memory T cells exhibits a preferential downstream requirement for p50 translocation to initiate early IFN-γ production. A role for p50 activation and translocation in memory T cells is also suggested by studies in gene-targeted mutant mice. Mice expressing a degradation-resistant p105 inhibitor subunit (preventing p50 translocation) exhibited profound defects in the development of memory T cells (35), and conversely, mice lacking p105, but expressing active p50, exhibited increased proportions of memory CD4 T cells (36). Furthermore, mice deficient in both p50/p105 also exhibited defects in T cell-mediated protective responses to an intracellular pathogen (37). Given the multiple immune abnormalities associated with global manipulation of NFκB expression (6, 37), a precise analysis of the in vivo role of NFκB subunits in memory responses in future studies will require manipulation of NFκB expression specifically in memory CD4 T cells using targeted ablation approaches we have previously employed (34).
Our results provide new insights into the molecular control of anamnestic immune responses. We reveal at least two pathways controlling memory T cell responses: a T-bet independent pathway for immediate recall regulated by NFκB, and a T-bet-dependent pathway for IFN-γ production at later times (38). Given the ubiquitous role of NFκB in promoting rapid inflammatory cytokine production in innate immune cells, we propose that memory CD4 T cells have co-opted an innate type of signaling pathway for their rapid effector responses through the TCR. The efficient mobilization of NFκB to relevant accessible promoters in memory T cells may therefore regulate immediate recall, with implications for memory modulation in vaccines and autoimmune diseases.
Supplementary Material
Footnotes
The project was supported by NIH AI42092 awarded to DLF. A.D.K. was supported by NIHAI059775 and AI038985.
References
- 1.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 2.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 4.Juedes AE, Rodrigo E, Togher L, Glimcher LH, von Herrath MG. T-bet controls autoaggressive CD8 lymphocyte responses in type 1 diabetes. J Exp Med. 2004;199:1153–1162. doi: 10.1084/jem.20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 6.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 8.Corn RA, Aronica MA, Zhang F, Tong Y, Stanley SA, Kim SR, Stephenson L, Enerson B, McCarthy S, Mora A, Boothby M. T cell-intrinsic requirement for NF-kappa B induction in postdifferentiation IFN-gamma production and clonal expansion in a Th1 response. J Immunol. 2003;171:1816–1824. doi: 10.4049/jimmunol.171.4.1816. [DOI] [PubMed] [Google Scholar]
- 9.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 10.Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J Immunol. 2007;179:3689–3698. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- 11.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of Memory CD4 T Cell Recall by the CD28/B7 Costimulatory Pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 12.Bingaman AW, Patke DS, Mane VR, Ahmadzadeh M, Ndejembi M, Bartlett ST, Farber DL. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35:3173–3186. doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 13.Sener A, Tang AL, Farber DL. Memory T-cell predominance following T-cell depletional therapy derives from homeostatic expansion of naive T cells. Am J Transplant. 2009;9:2615–2623. doi: 10.1111/j.1600-6143.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 14.Patke DS, Farber DL. Modulation of memory CD4 T cell function and survival potential by altering the strength of the recall stimulus. J Immunol. 2005;174:5433–5443. doi: 10.4049/jimmunol.174.9.5433. [DOI] [PubMed] [Google Scholar]
- 15.Lai W, Chang CH, Farber DL. Gene transfection and expression in resting and activated murine CD4 T cell subsets. J Immunol Methods. 2003;282:93–102. doi: 10.1016/j.jim.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. Embo J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem. 2006;281:11992–12000. doi: 10.1074/jbc.M513613200. [DOI] [PubMed] [Google Scholar]
- 18.Yu M, Moreno JL, Stains JP, Keegan AD. Complex regulation of tartrate-resistant acid phosphatase (TRAP) expression by interleukin 4 (IL-4): IL-4 indirectly suppresses receptor activator of NF-kappaB ligand (RANKL)-mediated TRAP expression but modestly induces its expression directly. J Biol Chem. 2009;284:32968–32979. doi: 10.1074/jbc.M109.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 20.Rincon M, Flavell RA. Transcription mediated by NFAT is highly inducible in effector CD4+ T helper 2 (Th2) cells but not in Th1 cells. Mol Cell Biol. 1997;17:1522–1534. doi: 10.1128/mcb.17.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 22.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aune TM, Penix LA, Rincon MR, Flavell RA. Differential transcription directed by discrete gamma interferon promoter elements in naive and memory (effector) CD4 T cells and CD8 T cells. Mol Cell Biol. 1997;17:199–208. doi: 10.1128/mcb.17.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincon M, Flavell RA. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dienz O, Eaton SM, Krahl TJ, Diehl S, Charland C, Dodge J, Swain SL, Budd RC, Haynes L, Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc Natl Acad Sci U S A. 2007;104:7175–7180. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda JL, George TC, Hagman J, Gapin L. Temporal dissection of T-bet functions. J Immunol. 2007;178:3457–3465. doi: 10.4049/jimmunol.178.6.3457. [DOI] [PubMed] [Google Scholar]
- 28.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 29.Costello R, Cerdan C, Lipcey C, Algarte M, Martin Y, Baeuerle PA, Olive D, Imbert J. The role of NF-kappa B1 (p50/p105) gene expression in activation of human blood T-lymphocytes via CD2 and CD28 adhesion molecules. Cell Growth Differ. 1993;4:947–954. [PubMed] [Google Scholar]
- 30.McCaffrey PG, Kim PK, Valge-Archer VE, Sen R, Rao A. Cyclosporin A sensitivity of the NF-kappa B site of the IL2R alpha promoter in untransformed murine T cells. Nucleic Acids Res. 1994;22:2134–2142. doi: 10.1093/nar/22.11.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fann M, Godlove JM, Catalfamo M, Wood WH, 3rd, Chrest FJ, Chun N, Granger L, Wersto R, Madara K, Becker K, Henkart PA, Weng NP. Histone acetylation is associated with differential gene expression in the rapid and robust memory CD8(+) T-cell response. Blood. 2006;108:3363–3370. doi: 10.1182/blood-2006-02-005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong J, Ivascu C, Chang HD, Wu P, Angeli R, Maggi L, Eckhardt F, Tykocinski L, Haefliger C, Mowes B, Sieper J, Radbruch A, Annunziato F, Thiel A. IL-10 is excluded from the functional cytokine memory of human CD4+ memory T lymphocytes. J Immunol. 2007;179:2389–2396. doi: 10.4049/jimmunol.179.4.2389. [DOI] [PubMed] [Google Scholar]
- 33.Araki Y, Wang Z, Zang C, Wood WH, 3rd, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, Becker KG, Zhao K, Weng NP. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Nat’l Acad Sci USA. 2010;107:827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriskantharajah S, Belich MP, Papoutsopoulou S, Janzen J, Tybulewicz V, Seddon B, Ley SC. Proteolysis of NF-kappaB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- 36.Chang M, Lee AJ, Fitzpatrick L, Zhang M, Sun SC. NF-kappa B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J Immunol. 2009;182:3131–3138. doi: 10.4049/jimmunol.0803637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 38.Farber DL. Biochemical signaling pathways for memory T cell recall. Semin Immunol. 2009;21:84–91. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



