Abstract
Serotonin (5-hydroxytryptamine, 5-HT) was one of the first neurotransmitters for which a role in development was identified. Pharmacological and gene knockout studies have revealed a critical role for 5-HT in numerous processes, including cell division, neuronal migration, differentiation and synaptogenesis. An excess in brain 5-HT appears to be mechanistically linked to abnormal brain development, which in turn is associated with neurological disorders. Ambient levels of 5-HT are controlled by a vast orchestra of proteins, including a multiplicity of pre- and post-synaptic 5-HT receptors, heteroreceptors, enzymes and transporters. The 5-HT transporter (SERT, 5-HTT) is arguably the most powerful regulator of ambient extracellular 5-HT. SERT is the high-affinity uptake mechanism for 5-HT and exerts tight control over the strength and duration of serotonergic neurotransmission. Perturbation of its expression level or function has been implicated in many diseases, prominent among them are psychiatric disorders. This review synthesizes existing information on the ontogeny of SERT during embryonic and early postnatal development though adolescence, along with factors that influence its expression and function during these critical developmental windows. We integrate this knowledge to emphasize how inappropriate SERT expression or its dysregulation may be linked to the pathophysiology of psychiatric, cardiovascular and gastrointestinal diseases.
Keywords: serotonin transporter, gene variants, antidepressants, ontogeny, psychiatric disease, cardiovascular and gastrointestinal disease
1. Introduction
Serotonin (5-HT) is a neuromodulatory neurotransmitter serving a wide array of physiological and behavioral functions. It has become clear over recent decades that a particularly salient role of 5-HT is as a developmental signal, important for cueing proper wiring of neural circuits (see Gaspar et al., 2003 for review). It is not surprising then, that a growing literature is focused on understanding the consequences of inappropriate ambient levels of 5-HT during development for the adult phenotype. Indeed, abnormal levels of 5-HT result in aberrant morphology and wiring of the nervous system across species, ranging from Drosophila (Sykes and Condron, 2005; Daubert et al., 2010;), to Aplysia (Castellucci et al., 1970; Brunelli et al., 1976) to mammals (see Gaspar et al., 2003 for review). Ambient levels of 5-HT are controlled by a number of factors including rate of 5-HT synthesis and amount available for release, rate of release, rate of enzymatic breakdown, rate of diffusion through the extracellular milieu and active uptake. The serotonin transporter (SERT) is the high-affinity uptake mechanism for 5-HT. In the mature animal it is located perisynaptically on presynaptic 5-HT nerve terminals as well as on axons and 5-HT cell bodies in the raphe (Rudnick and Clark, 1993; Barker and Blakely, 1995; Blakely et al., 1998). SERTs are also expressed on non-neuronal cells including platelets (Rudnick, 1977; Hranilovic et al., 1996), lymphoblasts (Khan et al., 1996; Faraj et al., 1997), monocytes (Yang et al., 2007), enterochromaffin cells, endothelial cells (Gershon, 1999 & 2003; Wheatcroft et al., 2005) and placental syncitiotrophoblasts (Balkovetz et al, 1989). In brain, SERT is arguably the predominant mechanism controlling the strength and duration of serotonergic neurotransmission. As such, factors that perturb SERT expression and/or function during critical periods early in development might be expected to have detrimental consequences in later life. This forms the basis of the present review, which describes what is known about development of SERT in brain under normal conditions, what intrinsic and extrinsic factors can influence SERT expression and function and importantly, how perturbation of SERT early in life can contribute to psychiatric and physiological disease states in later life.
2. Developmental profile of SERT gene and protein expression
To begin, it is necessary to first summarize what is known about “normal” SERT development. Cortical neurogenesis in humans occurs between gestational week (GW) 6 and 16 (Sidman and Rakic, 1973) with neuronal migration leading to distinguishable cortical layers occurring between GW24 and GW26, an event coinciding with synaptogenesis (Huttenlocher and de Courten, 1993). With the advent of SERT specific antibodies (Qian et al., 1995) it became possible to map SERT development in human brain. In fetal brain, SERT antibodies revealed SERT-positive, thick, varicose fibers emerging from the raphe, and reaching the cortical analae at GW8, the subplate at GW10, arriving at the cortical plate at GW13 (Verney et al., 2002). These findings are consistent with those in non-human primates, where serotonergic axons have been detected in entorhinal cortex in early embryonic life of rhesus macques (Berger et al., 1993) and where the 5-HT terminal network has been shown to mature very rapidly during early postnatal life (Lambe et al., 2000; Verney 2003).
In addition to SERT-positive fibers arising from the raphe, a second type of SERT-positive fiber was detected that did not resemble classical catecholaminergic fibers (Verney et al., 2002). These non-monoaminergic SERT-positive fibers differ in several respects from those emerging from the raphe, including their anatomic location, the internal capsule, an unusual localization for monoaminergic fibers, and their transient expression between GW12 and GW14. These non-monoaminergic neurons send projections from the anterior and posterior ends of the interior capsule, which lies between the caudate and putamen, and project toward the cerebral and temporal cortex. That these fibers appear at GW12 and disappear by GW14 indicate that 5-HT may play a role in the trophic organization of the somatosensory cortex at this time (Verney et al., 2002).
One of the markers used to define fibers as monoaminergic in these studies was the presence of immunoreactivity for the vesicular monoamine transporter-2 (VMAT2). VMAT2 is responsible for taking up monoamines (including 5-HT, dopamine (DA), norepinephrine (NE)) from the intracellular fluid and storing them in synaptic vesicles, and so is a useful marker of monoaminergic processes (Henry et al., 1998). Importantly, VMAT2 serves to store 5-HT into synaptic vesicles and protect it from degradation. While SERT and VMAT2 expression do not always go hand in hand in adult brain, for example, in certain neuronal populations within the cerebral cortex (Lebrand et al., 1998), the implications for this discourse during transient phases of early embryonic development remain unclear. Given the important trophic role of 5-HT during early development, and that excess 5-HT during these critical phases can have negative implications for the adult, it is possible that this transient period of SERT expression on non-VMAT2 expressing processes may be important for keeping 5-HT levels in check (for review see Gaspar et al., 2003).
In rodents in situ hybridization (Lebrand et al., 1996; Hansson et al., 1998, 1999), autoradiographic binding (D’Amato et al., 1987; Bennett-Clarke et al., 1997) and immunocytochemical (Lebrand et al., 1998; Zhou et al., 2000) approaches also reveal transient SERT gene and protein expression in brain during early development. Now, with the genetic tractability of mouse models, important insight into the physiological implications for these transient periods of SERT expression in the developing brain are being uncovered. For example, studies using SERT knockout (KO) mice have demonstrated a clear need for SERT in the normal development of thalamic projections (Persico et al., 2001; Salichon et al., 2001).
It is now well established that SERT is expressed much more broadly during development than in adulthood, and is expressed in non-5-HT neurons as well as neural crest derivatives. However, to further interrogate the expression pattern of SERT during development Narboux-Nême and co-workers (2008) inserted Cre recombinase into the SERT gene (SERTcre) of two reporter mouse lines; (1) the ROSA26R mouse, which drives LacZ expression in the cell’s cytoplasm regardless of its lineage (Soriano, 1999; Narboux-Nême et al., 2008) and (2) the TaumGFP mouse which is specific to neurons (Hippenmeyer et al., 2005; Narboux-Nême et al., 2008). This enabled a fate mate for SERT expressing cells to be created. The results, as anticipated, revealed remarkably broad SERT expression in non-5-HT cells during development (Narboux-Nême et al., 2008). SERT is particularly precocious in non-neural cells being robustly expressed in heart and liver at ED10.5 (Narboux-Nême et al., 2008). These results from SERTcre mice complement and extend earlier reports using in situ hybridization approaches (Lebrand et al., 1998; Hansson et al., 1998). Moreover, they reveal that brain regions, including CA1–CA2 of hippocampus, dentate gyrus, striatum, piriform cortex and amygdala, previously thought to transiently express SERT, (based on in situ hybridization or immunocytochemistry), (Cases et al., 1998; Hansson et al., 1998; Lebrand, et al., 1998; Zhou et al., 2000) do not appear to express the SERT gene at any time during development.
What is the fate of SERT after it first appears embryonically? Does SERT protein expression remain static or does its expression wax and wane? As just decribed, SERT protein measured by quanitative autoradiography, is first expressed during prenatal brain development on ED12 in mice, and by ED18 it is found in all of the same brain regions where 5-HT is localized immunohistochemically (Brüning et al., 1997). During the course of embryonic development, in brain regions such as the thalamus and somatosensory cortex, SERT density peaks and subsequently declines by PD7 (Brüning & Liangos 1997). During critical stages of brain development 5-HT levels can influence the expression or function of key mediators of 5-HT neurotransmission, including SERT. In the juvenile brain, SERT binding measured using a SERT selective radioligand in homogenates prepared from rodent frontal cortex, increases steadily from weaning until late adulthood (Moll et al., 2000). However, in other brain regions such as the dorsal raphe and parietal cortex, SERT density measured using quantitative autoradiography peaks and declines prior to PD20, possibly in response to a peak in extracellular 5-HT in those regions during brain development and maturation in rats (Galineau et al., 2004). In agreement with these findings in rats, Sidor et al. (2010) more recently reported that mRNA for SERT in several regions of the dorsal raphe was highest at PD14, the youngest age examined in their study, and declined to relatively stable expression levels across the age range PD17-28 in adolescent mice.
Thus, SERT gene and protein expression is transient in some brain regions, peaks and wanes in other regions and follows a steady incline to adult levels in others. However, as discussed in the following sections, many factors can influence both total SERT protein expression as well as the plasma membrane distribution of SERT (section 3), which can be linked to negative consequences for mental and physical health (section 4).
3. Factors regulating SERT expression and function in early development
SERT expression and function is dictated by a large and diverse array of intrinsic and extrinsic factors. Studies in adult laboratory animals have revealed not only genetic variants that influence SERT activity (Murphy et al., 2004), but also auto- and hetero-receptors, via a number of kinases and phosphatases (e.g. Blakely et al., 1998; Daws et al., 2000; Ansah et al., 2003; Zhu et al., 2004, 2005, 2007), cytokines (e.g. Zhu et al., 2010), integrins (e.g. Carneiro et al., 2008), neurotrophic factors such as BDNF (e.g. Daws et al., 2007) as well as hormones (e.g. Slotkin et al., 1996a,b, 2006; Baganz et al., 2010). Extrinsic factors such as diet, environmental stressors and drugs can also have prominent effects on SERT expression and function. Less is known about how these factors might come into play during prenatal and postnatal development. The following sections review what we do know and the implications for regulation of ambient levels of extracellular 5-HT during early developmental periods.
3.1 Intrinsic factors (Genes)
The human SERT is coded by a single gene (SLC6A4) mapped to chromosome 17q11.2. It comprises 14 exons that span ~ 40 kB. Based on our current understanding, SERT is predicted to contain 630 amino acids with 12 transmembrane spanning domains. Since the human SERT was cloned in 1993 (Ramamoorthy et al., 1993), numerous gene variants have been identified that affect the expression and/or function of SERT. These have been reviewed in detail elsewhere (Murphy et al., 2004; Serretti et al., 2006; Murphy and Lesch, 2008) and so will be described only briefly here.
3.1.1. Serotonin transporter gene linked polymorphic region (5-HTTLPR)
The most well-described and common SERT variant is the SERT gene-linked polymorphic region (5-HTTLPR), originally described as a 44 base pair insertion/deletion in the promoter region which results in a short (S) or long (L) form, but now identified as a 43 base pair insertion/deletion (Heils et al., 1996; Nakamura et al., 2000; Kraft et al., 2005). The frequency of this polymorphism varies with ethnicity with the S allele generally being more prevalent in Asian populations (range 72–81%) than in Caucasian populations (ranges from 38–57%) (Lesch et al., 1996; Gelernter et al., 1997; Noshkova et al., 2008). As discussed later (see section 4), this variant has been the focus of a large and growing number of association studies in clinical populations. While the results have been mixed, there is a considerable body of evidence linking this variant to numerous psychiatric diseases.
The 5-HTTLPR affects the amount of SERT protein transcribed. Most studies measuring SERT protein expression in lymphoblasts and platelets show that carriers of the S allele express approximately half as many SERTs than individuals homozygous for the L allele (Lesch et al., 1996; Stoltenberg et al., 2002; for reviews see Murphy et al., 2004; Murphy and Lesch, 2008), but some studies have failed to replicate this finding (Preuss et al., 2000). Likewise, imaging studies of human brain have yielded mixed results, with some reporting that carriers of the S allele have reduced SERT relative to homozyotes for the L allele (Heinz et al., 2000; Kalbitzer et al., 2010), and others reporting no difference in SERT expression among genotypes (Willeit et al., 2001; Shioe et al., 2003; Van Dyck et al., 2004; Parsey et al., 2006). Binding studies in post-mortem tissue have also failed to identify variation in SERT expression among 5-HTTLPR genotypes (Mann et al., 2000; Arango et al., 2003).
However, there are many caveats that make interpretation of data obtained from human studies often difficult, a subject recently reviewed by Willeit and Praschak-Rieder (2010). For example, many studies do not account for additional single nucleotide polymorphisms (SNPs) that occur in the SERT gene, including the promoter region (Nakamura et al., 2000; Wendland et al., 2008). One SNP occurs in the area of the L-allele, where an alanine is substituted by a guanine. This SNP is commonly referred to as the triallelic 5-HTTLPR variant and is denoted LA/LA, LA/LG and LG/LG. Studies which have taken into account this triallelic stratification have revealed greater SERT binding in homozyote LA carriers than in S allele carriers, whereas LG carriers share similar SERT expression levels as carriers of the S allele (Praschak-Rieder et al., 2007; Reimold et al., 2007; Kalbitzer et al., 2010). Along similar veins, others have reported that a significant fraction of L alleles in the 5-HTTLPR are in fact low-expressing due to interaction with another SNP, the rs25531 (Hu et al., 2006). Together with work from Wendland et al., (2008) who identified an additional SNP, rs25532, it is now clear that the traditional view of S and L alleles as low- and high-expressing, respectively, is over simplistic and must be considered in terms of the modulating effects of rs25521 and rs25532 (Wendland et al., 2008), as well as the triallelic SNP.
Selectivity of ligand can also be problematic. For example, [123I]-2-β-carbomethoxy-3-b-(4-iodophenyl)tropane ([123I]β-CIT) binds not only SERT, but also to the dopamine transporter (DAT) and has some affinity for the norepinephrine transporter (NET) as well. Studies using this ligand have failed to detect differences in SERT expression as a function of 5-HTTLPR genotype. In contrast, more selective ligands for SERT, such as [11C]-3-amino-4-(2-dimethylaminomethylphenylthio)benzonitrile ([11C]DASB), routinely reveal greater SERT expression in LA homozygotes than in carriers of the S allele (for review see Willeit and Praschak-Rieder, 2010).
In addition, there are a multiplicity of endogenous and extrinsic factors which can influence SERT expression (see comment by Ordway, 2000) and which cannot be fully controlled in humans. For example, psychoactive drugs that target the SERT, such as selective serotonin reuptake inhibitors (SSRIs), as well as “recreational” drugs such as 3,4-methylenedioxymethamphetamine (MDMA, or “Ecstasy”) and cocaine can cause both acute effects on SERT plasma membrane expression, as well as long term effects on total SERT protein levels (e.g. Ramamoorthy and Blakely, 1999; for review see Luellen et al., 2010). Similarly, age (e.g. Pirker et al., 2000), seasonal variation (Buchert et al., 2006; Praschak-Rieder et al., 2008; Ruhe et al., 2009 Kalbitzer et al., 2010) and psychiatric condition (for reviews see Stockmeier, 2003; Meyer, 2007) are but a few of the many factors that can exert marked effects on SERT expression levels that could potentially serve to mask 5-HTTLPR genotype-dependent variations in SERT expression.
Layered onto these potentially confounding variables, is also the possibility that ligands used to measure SERT protein cannot adequately distinguish between SERT located in the plasma membrane and that in the cytosol. This raises the possibility that the inability to detect reduced SERT expression in carriers of the S allele is due to differences in plasma membrane expression of SERT. For example, carriers of the S allele may have 50% less SERT protein, but the majority of SERT may be located in the plasma membrane domain most of the time, with little, if any residing in the cytosolic compartment. This is certainly a conceivable scenario, for example, as an adaptive mechanism to maintain relatively normal 5-HT homeostasis in the face of constitutively reduced SERT protein. In contrast, it is possible that homozygotes for the L allele may express only half of total SERT in the plasma membrane the majority of the time. This would enable L/L individuals more dynamic control over 5-HT uptake, with the ability to recruit additional SERT from the cytosolic compartment to the plasma membrane upon demand. On the other hand, carriers of the S allele would be severely limited because, according to this scenario, the majority of their SERT armamentarium would already be positioned in the plasma membrane. Of course, this scenario remains to be empirically tested, but still, provides another potential explanation for the inability of human imaging studies to consistently find reduced SERT expression in carriers of the S allele.
3.1.2. Other serotonin transporter variants, common and rare
In addition to the much studied 5-HTTLPR polymorphism, another relatively common SERT gene variant occurs as a variable number of tandem repeats in the second intron (VNTR-2) (Lesch et al., 1996; Ogilvie et al., 1996). VNTR-2 contains nine, ten or twelve copies of a sixteen- or seventeen-base pair repeat. VNTR-2 appears to be a transcriptional regulator of gene expression at this locus, but the functionality of VNTR-2 depends on which individual repeat elements are present as well as the tissue in which it is expressed (Fiskerstrand et al., 1999; MacKenzie and Quinn 1999; Lovejoy et al., 2003; for review see Murphy et al., 2004). Generally however, the VNTR-2 is associated with enhancer activity to increase SERT gene transcription.
Several other SNPs have been identified that serve to change the structure or function of the human SERT. A gain of function mutation was first reported by Kilic and co-workers (2003). This variant, in which isoleucine at position 425 is replaced by valine (I425V), has dramatically increased SERT function. This increased function could be attributed to both an increase in the maximal velocity (Vmax) for 5-HT uptake as well as increased affinity (KM) of the SERT for 5-HT – i.e. smaller KM value. In 2005, the Blakely group performed a comprehensive functional analysis of ten nonsynonymous SNPs that occur in the human SERT gene (Prasad et al., 2005). They found four of the SNPs to be gain-of-function mutations, including I425V and G56A, whereas the P339L SNP resulted in a striking loss-of-function mutation. Five of the SNPs were completely insensitive to activators of protein kinase G (PKG) or p38 mitogen activate protein kinase (MAPK), two major signaling pathways regulating plasma membrane expression of SERT and its intrinsic activity (Ramamoorthy et al., 1998; Ramamoorthy and Blakely, 1999; Zhu et al., 2004; Samuvel et al., 2005; Zhu et al., 2005; Zhu et al., 2007). These and other variants have been reviewed by Murphy and colleagues (2008) so will not be discussed further here.
An interesting caveat based on the discovery of these gene variants is that because SERT density is often measured by SERT selective radiolabeled ligands in autoradiography and imaging of rodent and human brain, SNPs within SERT that alter its affinity for SSRIs and other synthetic ligands, without impeding the capacity of SERT to take up 5-HT could also introduce confounds for such measurements. For example, two SNP haplotypes (Glu39Gly and Arg152Lys) have been identified among C57 and 129S mouse lineages that impair 5-HT uptake capacity in C57BL/6 and C57BL/10 mice and reduce the [3H]-labeled SSRI paroxetine binding in 129S-derived strains (Carneiro et al. 2009). As discussed earlier, SERT gene variants, if gone undetected, may lead to erroneous interpretation of binding studies in rodents and humans.
3.1.3. Serotonin transporter gene variants: Implications
The discovery of these SERT variants has two fundamentally important implications. First, when investigating the influence of a particular gene variant on SERT expression and function, the existence of all variants need to be considered when interpreting data. Wendland and co-workers (2006) make this point most elegantly, and in efforts to enable more comprehensive genotyping, refined existing PCR protocols to provide a method to simultaneously genotype an individual for 5-HTTLPR, rs25531, VNTR-2 and Ile425 variants. As reviewed by Murphy and Lesch (2008), expression of SERT as a function of 5-HTTLPR genotype can be further modified by the co-existence of SNPs, some of which were described earlier. This knowledge is essential for furthering our understanding of how genetic variants are linked to behavioral and physiological abnormalities.
The second major implication, and one that is central to the theme of this review, is that because SERT appears early in development, these genetically encoded variants could have dramatic consequences for the ability of SERT to regulate ambient levels of extracellular 5-HT during critical developmental windows. Indeed, inappropriate regulation of extracellular 5-HT during critical stages of early development can have a profound negative effect on mood and behavior in adulthood (Gaspar et al., 2003). This will be reviewed in section 4.
3.2 Extrinsic factors (Environment)
As just discussed, precise quantitation of SERT expression and function in humans can be difficult. Interpretation of human data is also difficult because of the numerous variables that can influence SERT expression and function. However, we have gleaned tremendous insight into how intrinsic and extrinsic factors can influence SERT by using animal models, where these factors can be tightly controlled. What we have learned in terms of the developing animal is discussed in the following sections.
3.2.1. Nutritional Status and Birth Weight
Prenatal malnourishment impinges heavily on the serotonergic system in brain (Resnick and Morgane, 1984; Blatt et al., 1994; Mokler et al., 1999; Mokler et al., 2003). These results include diminished growth and arborization of 5-HT neurons in the dorsal raphe nucleus (DRN) (Díaz-Cintra et al., 1981; Blatt et al., 1994; Cintra et al., 1997a,b) and decreased 5-HT nerve terminals in hippocampus as indexed by reduced 5-HT1A receptor expression (Blatt et al., 1994).
SERT expression is, not surprisingly, affected by nutritional status during fetal and early postnatal development. For example, prenatally protein malnourished rats, fostered to dams fed a standard protein diet (25% casein), exhibited a 15–25% reduction in [3H]citalopram binding to SERT in the dentate gyrus, CA1 and CA3 regions of hippocampus in adulthood (Blatt et al., 1994). Similarly, rats born to dams fed a standard chow diet but permitted access to only half that of free feeding rats, expressed dramatically reduced levels of SERT immunostaining in thalamo-cortical fibers at PD3 and PD7 (Medina-Aguirre et al., 2008). Interestingly, at 4 months of age, pups born to similarly undernourished dams showed increased SERT expression in hypothalamus (Pôrto et al., 2009), suggesting that prenatal malnourishment has brain region and/or age specific effects on SERT expression. In the case of rats fed iron deficient diets from weaning to adulthood, SERT expression was increased or decreased depending on brain region and gender. In males, SERT expression was decreased by 20–30% in several regions, including the nucleus accumbens, olfactory tubercle and colliculus, whereas in females there was a 15–25% increase in SERT expression in the olfactory tubercle, zona incerta, anteroventral thalamic nucleus and vestibular nucleus (Burhans et al., 2005).
SERT expression in later life also varies according to birth weight. It is well known that rat pups from the same litter exhibit a range of body sizes. Dependent on their position in the uterus, pups obtain differing levels of blood supply and hence nutrition; the smallest pups within a litter receive the lowest blood supply, and the largest pups the greatest (Himpel et al., 2006). Although these early postnatal differences in birth weight disappear in adulthood, SERT expression remains correlated with birth weight. In adult (PD90) rats, SERT expression was 20% lower in the frontal cortex of those with lower birth weight compared to those with higher birth weight. In contrast, there was no difference in brain stem SERT expression. These findings suggest that low birth weight may be a risk for abnormal development of the serotonergic system in limbic regions of brain (Himpel et al., 2006).
While it is clear that prenatal or early postnatal under nutrition and birth weight can affect SERT expression, there are no studies to date that have directly studied how these extrinsic factors might influence SERT function. These studies will lend important insight into whether or not changes in SERT expression translate to an impaired ability to regulate ambient levels of extracellular 5-HT, or whether compensatory factors regulating extracellular 5-HT can overcome under or over expression of SERT protein during early development.
3.2.2. Stress
Nutrition, as just discussed, is a form of stressor, leading to increased activation of the hypothalamic-pituitary-adrenal (HPA) axis and release of stress hormones, such as corticosterone (cortisol in humans) (Lesage et al., 2006; Vieau et al., 2007). In addition to under nutrition a wide variety of stressors (or activators of the HPA axis) during early pre- and postnatal development exert profound effects on the 5-HT system and SERT expression, which may be linked to altered brain structure and wiring (Calabrese et al., 2009; Cottrell and Seckl, 2009; McEwen 2010).
When the HPA axis is activated, glucocorticoids are released, in humans this is mostly cortisol and in laboratory rodents, corticosterone. Dexamethasome is a synthetic glucocorticoid with a wide array of therapeutic uses, including treatment for preterm labor occurring between the 24th and 34th weeks of gestation (Gilstrap et al., 1995). While not associated with increased rates of neonatal death, it is associated with lower birth weight (Bloom et al., 2001). Studies investigating the effects of dexamethasone in prenatal rats reveal a striking effect on SERT. For example, maternal administration of dexamethasone during gestation (gestational day (GD) 17–19) in rats produces persistent increases in [3H]paroxetine binding to SERT in brainstem and cortex, even at low doses, below those used therapeutically. Similarly, rats administered dexamethasone on PD1-3 or PD7-9, also showed marked increases in [3H]paroxetine binding (Slotkin et al., 2006). In contrast, dexamethasone administered to mature animals failed to produce similar increases (Slotkin et al., 1996 a & b). Interestingly, the increase in SERT expression in rats given dexamethasone early in development occurred regardless of the effect on tissue 5-HT. At GD17-19 dexamethasone did not affect 5-HT levels in rats, but 5-HT levels were increased when given at PD1-3 and decreased when given at PD7-9 (Slotkin et al., 2006). These data support the idea that the effects of dexamethasone on SERT expression are not soley driven by altered presynaptic activity but likely reflect a loss of post-receptor-signaling capabilities or structural “miswiring”, where neural projections form inappropriate connections with postsynaptic cells (Kreider et al., 2006; Slotkin et al., 2006).
Similarly, other forms of stress, such maternal separation (Vicentic et al., 2006), serve to influence SERT expression. The duration of maternal separation is a prominent factor in determining the direction of effect on SERT expression. For example, relative to non-handled and animal facility handled control rats, pups separated from their mother for 15 min daily from PD2-14 had 15–50% greater SERT expression in amygdaloid nuclei (Vicentic et al., 2006), but no change in other regions, including the cortex, nucleus accumbens, hypothalamus and hippocampus. In contrast, pups separated from their mother for 180 min daily from PD2-14 did not produce any effect on SERT expression relative to control rats in any brain region studied. One obvious caveat with maternal separation is whether the “stress” of maternal separation is overcome by the increased parenting that follows (e.g. increased licking and grooming of pups) and raises the possibility that maternal separation is not an especially robust stressor (Millstein and Holmes, 2007). Non-the-less, a particularly interesting finding from these studies came from the “non-handled” group. Like the animal facility handled controls, these rat pups were never separated from their dam. However, whereas the animal facility handled pups were transferred to clean cages four times during PD2-14, the “non-handled” group was only touched once, on PD11, for a single cage change. The relative lack of human handling and perhaps stress associated with being housed in a soiled environment for a longer period, produced the most striking changes in SERT expression. SERT protein, as determined by quantitative autoradiograpy using [125I]3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester (RTI-55), was decreased by approximately 30–45% in hypothalamus, 20–25% in amygdala and 35% in CA3 region of hippocampus, compared to the animal facility handled control rats (Vicentic et al., 2006).
Together, these findings suggest that activation of the HPA axis early in neonatal and postnatal development may have lasting effects on SERT expression and serotonergic neurotransmission that might account for later life adverse neurobehavioral consequences. These effects however, appear to be “dose-dependent”. For example, Macrì and co-workers recently showed that resilience and vulnerability to cognitive dysfunction in adulthood were related to circulating levels of corticosterone experienced during neonatal development (Macrì et al., 2009). During the first 10 days of life, mice were given low (33 mg/l) or high (100 mg/l) corticosterone supplements via maternal drinking water. This resulted in significantly increased plasma corticosterone levels in dams at both day 5 and day 10 for high corticosterone and at day 10 for the low corticosterone condition, compared to control dams. In pups, plasma corticosterone levels were significantly higher than control for both low and high corticosterone conditions at PD5, but significantly higher only for the high corticosterone condition at PD10. The consequences of these manipulations for SERT expression in adulthood (5 months) were assayed by measuring SERT expression in blood serum. SERT in blood serum was measured using peptide fragments of SERT as antigens to detect serum natural autoantibody levels by a modified enzyme-linked immunosorbent assay (Macrì et al., 2009). In mice subjected to the low corticosterone condition there was a 2-fold increase in blood serum SERT natural antibody expression. In contrast, the high corticosterone condition had no significant effect on SERT natural antibody expression. These findings correlated with improved cognitive performance in adulthood in mice exposed to the low corticosterone condition, whereas those exposed to the higher corticosterone condition did not show any change in cognitive performance compared to control mice (Macrì et al., 2009). Indeed others have reported that “mild” neonatal stress may reduce adult stress and fear responses and increase cognitive abilities in adulthood (Catalini et al., 1993; Bredy et al., 2004; Coutellier et al., 2009) while others show that more robust neonatal stressors are linked to increased levels of anxiety and fear behavior (Romeo et al., 2003; Wei et al., 2010), although effects were often gender specific and likely strain dependent (for review see Millstein and Holmes, 2007; Mozhui et al., 2010). These and other ramifications of early life stress on risk for disease, and the potential role of SERT are discussed further in section 4.
3.2.3. Immune system activation
Immune challenge in early development is yet another stressor that serves to regulate SERT expression, at least at the level of mRNA. A recent study by Sidor and co-workers (2010) examined the effect of lipopolysaccharide (LPS, 0.05 mg/kg, i.p.) given to mouse pups at PD3 and PD5 on SERT mRNA in raphe nuclei. They found both regional and transient effects. At PD14 SERT mRNA was decreased relative to saline injected control pups in the dorsal part of the DRN. In contrast, SERT mRNA was increased in ventral and lateral parts of the DRN at PD17, while there was no effect on SERT mRNA in the median raphe nucleus (MRN) (Sidor et al., 2010). More recently, maternal immune system activation in response to intrauterine infection in rabbit dams was reported to produce decreases in 5-HT immunoreactive fiber expression in the somatosensory cortex, reduced cortical SERT mRNA expression, and decreased 5-HT levels and tryptophan metabolism in 1-day old kits (Kannan et al., 2010). While the functional ramifications of these effects on SERT mRNA and protein expression remain to be elucidated, it is likely that activation of the immune system early in life could have dramatic effects on SERT function and subsequent control of ambient levels of extracellular 5-HT.
A rapidly growing body of literature documents prominent effects of immune function on SERT in cell systems and in adult animals. For example, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) increase SERT mRNA and protein in cultured cells (Ramamoorthy et al., 1995; Mössner et al., 1998). Zhu and co-workers (2006) went on to show, using raphe neuron derived RN46A cells, that both IL-1β and TNF-α cause rapid catalytic activation of SERT, which was p38 MAPK dependent. Given p38MAPK has an important role in sustaining SERT expression at the plasma membrane (Samuvel et al., 2005), immune factors that operate via this signaling mechanism could have marked effects on serotonergic neurotransmission. Indeed, Zhu et al., (2010) recently showed that LPS, via interleukin-1 receptor activation and p38MAPK dependent mechanisms, stimulated SERT activity in vivo and produced pro-depressive effects in adult mice.
3.2.4. Pharmacological Interventions
The behavioral effects of pharmacological blockade of SERT early in development, either by administration of SSRIs to pregnant dams or directly to pups early postnatally, has received much attention (Hiliakivi et al., 1986; Yanelli et al., 1999; Ansorge et al., 2004; Ansorge et al., 2008; Forcelli and Heinrichs, 2008; Popa et al., 2008; and see Table 2 for review in Narboux-Nême et al., 2008). Unfortunately, and in contrast to the vast number of studies that have investigated the effects of SSRIs on SERT expression and function in adult animals (for review, see Luellen et al., 2010), relatively few studies have explored effects of SSRIs on SERT early in development. While reports from adult rodents are mixed, a common finding is that chronic SSRI treatment decreases SERT expression in mice (Hirano et al., 2005; Mirza et al., 2007) and rats (e.g. Brunello et al., 1987; Kovachich et al., 1992; Wanatabe et al., 1993; Pineyro et al., 1994; Benmansour et al., 1999, 2002; Horschitz et al., 2001; Gould et al., 2006, 2007; Rossi et al., 2008). Among the limited reports of the effect of chronic SSRI treatment early in development, the results appear to be compatible with those in adults. For example, rats given fluoxetine (10 mg/kg either via minipup or s.c.) from ED14-PD7 (Forcelli and Heinrichs, 2008) or ED13-20 (Cabrera-Vera and Battaglia, 1998) had reduced SERT expression in raphe, hypothalamus, hippocampus and amygdala in later life. Similarly, rats given citalopram (5 mg/kg/12 hr, s.c.) from PD8-21 displayed reduced SERT in cortex (Maciag et al., 2006). In another study, Bock and colleagues (2005) administered the SSRI fluoxetine (5 mg/kg per day s.c.) or the NET blocker reboxetine (10 mg/kg per day s.c.) to rat pups from PD2 to PD5 and then assessed the consequences for cortical SERT and NET expression in adulthood (PD90). Fluoxetine treatment resulted in a 10% increase in SERT expression in frontal cortex, but remarkably, reboxetine produced an even greater increase (20%) in SERT expression compared to saline injected control rats. Interestingly, reboxetine treatment PD2-5 had no consequence for NET expression (Bock et al., 2005). Thus, modulation of the NE system during brain development can have long term effects on SERT and the serotonergic system.
A major question that has been, and continues to be the focus of intense research, is whether these antidepressant-induced changes in SERT expression during embryonic and early postnatal periods have detrimental behavioral and physiological consequences in the long term? Data from human studies are mixed, but suggest an increased risk for serotonin withdrawal syndrome (Sanz et al., 2005) or pulmonary hypertension (Chambers et al., 2006). As discussed in section 4.2, it is clear from rodent studies that developmental effects of SSRI administration during gestation or early postnatal life are linked to a wide range of behavioral and physiological dysfunctions. These studies in rodents suggest that even modest changes in SERT expression during these criticial developmental periods can have profound consequences later in life.
Another common drug encountered during gestational and early neonatal development is nicotine. Its effects on various neurotransmitter systems have been widely studied (Ernst et al., 2001; Slotkin 2004, 2008; Slotkin et al., 2007a,b; Dwyer et al., 2008; Herrman et al., 2008; Pauly and Slotkin, 2008), but less is known about its direct effects on SERT. Recent work from the Slotkin group studied the effect of nicotine given to pregnant rats to simulate plasma levels equivalent to those found in human smokers (Slotkin and Seidler, 2010). They found that nicotine by itself elicited modest increases or had no effect on SERT expression in cortex, midbrain and brainstem in offspring aged PD30-150 (Slotkin and Seidler, 2010). However, nicotine augmented the effects of dexamethasone on SERT expression. Marked increases in SERT expression were found in cortex, midbrain and brainstem, and decreased SERT expression in striatum of male offspring. In contrast, SERT expression remained relatively static in female offspring. Interestingly, in male offspring, increased SERT expression in midbrain and brainstem were especially prominent at PD30 and PD60, whereas the effects on cortical and striatal SERT expression were strongest at PD100 (Slotkin and Seidler, 2010). Serotonin concentration was unchanged at PD2 but was subsequently decreased, and 5-HT turnover likewise showed no initial change but increased later in development. Together with measures of 5-HT receptor expression as indices of pre- and post-synaptic activity, the authors concluded that exposure to these drugs early in life serve to change the trajectory of synaptic development. Thus, even after a period of relatively normal development, abnormalities emerge later in development (Slotkin and Seidler, 2010 and references therein). Given that smoking during pregnancy increases the risk for preterm delivery, and that glucocorticoids are given to prevent preterm labor, this pharmacological combination, in part via its effects on SERT expression, likely exacerbates risk for abnormal neural function and behavior in later life, particularly in males.
3.2.5. Epigenetic Regulation
As discussed in section 3.1, gene variants affect SERT expression and function. Further regulation of SERT function can manifest via structural modifications of chromosomal regions, which serve to change gene activity without changing the nucleotide sequence, a phenomenon known as epigenetic regulation. In this regard, the rhesus macaque, which carries an orthologue of the human 5-HTTLPR (rh5-HTTLPR) has provided a valuable resource for researchers. Rhesus monkeys have been used to study the effects of early life stress by several groups (Barr et al., 2004a,b; Spinelli et al., 2007; Kinnally et al., 2008, 2010a,b). The most commonly used stressor is that of maternal separation. Infants are instead nursery reared. The Capitanio group showed that stressful experiences during infancy could influence peripheral blood mononuclear cell (PBMC) SERT mRNA expression (Kinnally et al., 2008, 2010a) and recently Kinnally and co-workers (2010c) used similar approaches to provide evidence for epigenetic regulation of SERT by early life stress. Cytosine-phosphate-guanosine (CpG) islands are regions of the genome rich in cytosine and guanine (CG) nucleotides and often located in or near promoter regions (Bird, 1986). Increased methylation of cytosines within CpG islands is linked with decreased gene transcription (Jones and Takai, 2001). In humans and rhesus macaques, an 800 bp CpG island is positioned about 200 bp downstream of the 5-HTTLPR and overlaps with the transcription initiation start site of the SERT gene. This CpG island can regulate SERT expression; for example, greater methylation of this CpG island is associated with reduced SERT expression in human lymphoblast cell lines (Philibert et al., 2007). Consistent with this finding, Kinnally and co-workers (2010c) showed that rhesus macaques carrying the short allele of the rh5-HTTLPR had, on average, higher levels of CpG methylation than carriers of the long allele, and this was associated with lower PBMC SERT expression. Of note, higher SERT CpG methylation, but not SERT genotype, was associated with an increased ability of early life stress to produce behavioral stress reactivity in infants (Kinnally et al., 2010c). Results such as these point to CpG methylation as an important regulator of SERT expression and that epigenetic modulation of SERT during critical developmental windows might be linked to increased risk for psychiatric disease in later life.
3.2.6. Implications
Of course this is by no means an exhaustive list of factors that can affect SERT expression or function. There is evidence that other hormones such as estrogen (Maswood et al., 1999; Krajnak et al., 2003; Wihlbäck et al., 2004; Koldzic-Zivanovic et al., 2004; Bertrand et al., 2005; Sumner et al., 2007; Benmansour et al., 2009), testosterone (McQueen et al., 1999) and insulin (Figlewicz, 1999; France et al., 2009; Ramakrishnan et al., 2009; Abraham et al., 2010) as well as growth factors such as brain derived neurotrophic factor (BDNF) (Daws et al., 2007; Deltheil et al., 2008; Molteni et al., 2010) can all regulate SERT expression and/or activity in adult animals. However, to date reports that demonstrate direct effects of these factors on SERT expression and function in early development are lacking. As discussed in the following section, dysregulation of ambient 5-HT levels during critical developmental periods can have profound detrimental effects in adulthood manifesting as disorders ranging from psychiatric disease to cardiovascular dysfunction. Studies interrogating factors that can regulate the activity state of the primary mechanism controlling extracellular 5-HT, the SERT, during early developmental periods are clearly needed.
4. Abnormal SERT development and disease
So far we have reviewed factors that influence SERT expression and/or activity, including both intrinsic (heritable) and extrinsic (not heritable), during important stages of early development. However, how this relates to negative behavioral outcomes and disease states in later life remains an area of intense research and debate. The following section reviews existing literature as it relates to two key psychiatric disorders, depression and autism, as well as cardiovascular and gastrointestinal diseases.
4.1 SERT gene variants
As discussed in section 3.1, numerous variants of the SERT gene exist. Many of these have been linked to disease states, prominent among them psychiatric disorders. Not surprisingly there are thousands of reports in the literature describing various interactions and associations of these SERT gene variants and disease, and more than 75 reviews articles have been published so far. Here we focus on the relationship between SERT gene variants and some of the most widely studied disorders.
4.1.1. Depression, anxiety and related emotional disorders
Since the landmark study of Caspi and coworkers (2003) reporting an association between the 5-HTTLPR and stressful events early in life, this has been one of the most studied gene variants. The key finding was that carriers of the S allele, when exposed to early life stressful events, had a dramatically increased risk for suffering depression in later life (Caspi et al., 2003). Since this time there has been much controversy and debate, with some groups able to replicate this finding and others not (for reviews see Vergne and Nemeroff, 2006; Caspi et al., 2010; Kato and Serretti, 2010). Primary reasons for the inability to replicate this finding appear to relate to different subject populations and less stringent methodologies. For example, the majority of studies that failed to replicate relied on brief, self-report measures of stress. In contrast, studies that were able to replicate used objective indicators or face-to-face interviews to determine stress exposure (Caspi et al., 2010). Caspi and co-authors (2010) provide a detailed synopsis of the current status of research in this field and highlight the need to adopt an inclusive approach to the literature on SERT and stress sensitivity, rather than an exclusive analysis of the literature that has attempted to replicate the original findings of Caspi and co-workers (2003) using methods that can only approximate those used in the original study. Using this criterion, there is overwhelming consensus from human studies that the S allele moderates the influence of stress on depression. This gene by environment interaction has been further cemented by use of animal models, including rhesus monkeys and rodents.
Rhesus monkeys have been an invaluable resource in this regard. As discussed earlier, they have an orthologue of the human 5-HTTLPR, which similarly, is associated with decreased transcriptional activity (Lesch et al., 1996; Barr et al., 2003; Soumi, 2006). The influence of early life stress in rhesus monkeys has been studied by separating infants from their mothers and rearing them with other infants. Monkeys carrying the S allele that have been separated from their mother show increased anxiety, agitation, stereotypies and exaggerated HPA axis responses (Barr et al., 2004; Spinelli et al., 2007), which persist into later life (Barr et al., 2004). The stress-linked S carrier phenotype in rhesus monkeys also parallels human findings in terms of brain morphology. These monkeys have abnormal cortico-limbic structure and function and reduced gray matter volume in amygdala, medial prefrontal cortex, orbitofrontal cortex and pulvinar (Jedema et al., 2010). More recently, elegant studies using boron-doped diamond microelectrodes to measure 5-HT uptake in lymphocytes from rhesus monkeys showed that 5-HT uptake was slower in carriers of the S allele, consistent with reduced SERT function in these animals (Singh et al., 2010).
Although there is no rodent orthologue of the 5-HTTLPR, genetic modification of the SERT gene in rodents has afforded a model in which experimental conditions can be very tightly controlled, recently reviewed by Kalueff et al., (2010). Mice or rats in which the SERT has been functionally excised, either by targeted mutation or chemical mutagenesis, exhibit increased anxiety-like behavior, impaired fear extinction and exaggerated HPA-axis response to acute stress (Holmes et al., 2003a,b; Homberg et al., 2007; Kalueff et al., 2010). SERT KO rats spend more time immobile in the forced swim test, a behavioral assay for negative emotion or depressed-like behavior (Olivier et al., 2008). This “depression-like” phenotype is less clear in SERT KO mice, and is largely contingent upon the background strain (reviewed in Kalueff et al., 2010). That said, there is evidence that SERT KO mice exposed to conditions known to activate the HPA axis (e.g. repeated swim, tail suspension) develop behavioral despair, a depression-like phenotype (Wellman et al., 2007). Taken together there is general agreement that SERT KO rodents display behaviors that parallel increased anxiety and depression. The relationship between SERT expression level and behavior is further exemplified by the finding that SERT overexpressing mice are less anxious (Jennings et al., 2006; Line et al., 2010), highlighting a reciprocal relationship between SERT expression and level of anxiety.
Taken together, findings in humans, non-human primates and rodents support the idea that constitutive variation in SERT expression is linked to anxiety and related behaviors. It will be interesting in future studies, using the tractability of mouse genetics, to determine if there are critical developmental windows during which inherent differences in SERT expression map adult behavior (e.g. using conditional SERT KO early developmentally), or whether persistent differences in SERT expression throughout life are necessary to support behavioral phenotypes.
4.1.2. Autism and related developmental disorders
Autism and other related developmental psychiatric disorders, extending from Asperger’s to pervasive developmental disorders not otherwise specified (PDD-NOS), with a broad range of symptoms and severity, are complex disorders that are frequently marked by abnormalities in 5-HT metabolism and neurotransmission (Santangelo and Tsatsanis 2005; Lam et al., 2006). Blood hyperserotonemia is a hallmark of most autistic individuals and is likely due to high platelet 5-HT stores resulting from increased SERT-mediated 5-HT uptake. Blood hyperserotonemia is consistent with reports of reduced extracellular 5-HT availability and/or serotonergic neurotransmission in brain, again likely due to enhanced 5-HT uptake via SERT (Anderson et al., 1990; Chugani et al., 1999; McDougle et al., 2005; Croonenberghs et al., 2007; McNamara et al., 2008; Veenstra-VanderWeele et al., 2009). As indicated previously in this review, clinical and animal studies indicate that 5-HT plays many critical roles in regulating neuronal morphology (branching patterns, synapses, etc.) during brain development, and its perturbation may adversely alter behavior and future responsiveness to psychoactive drugs (Chandana et al., 2005; Whitaker-Azmitia 2005; Boylan et al., 2007; Murrin et al., 2007; Carola et al., 2010; Daubert and Condron 2010; Jones et al., 2010; Mulla, 2010). Since SERT is a major regulator of 5-HT neurotransmission and presynaptic 5-HT stores, it has been implicated in the etiology of autism spectrum disorders (ASDs).
Indeed, the SERT has been identified as a major candidate gene for autism in linkage and association studies for over a decade (Cook et al., 1997). Since nuanced details of how SERT expression and function can be dysregulated in autism in ethnically diverse populations are just beginning to be uncovered, controversy remains over the role played by common and rare SERT gene variants (e.g. Sakurai et al., 2008; Prasad et al., 2009). However, many studies report that function-impairing genetic polymorphisms of the SERT appear to be associated with higher risk of developing the disorder, as well as with rigid-compulsive traits, consistent with obsessive-compulsive disorder (Murphy et al., 2004; Devlin et al., 2005; Sutcliffe et al., 2005; DiCicco-Bloom et al., 2006; Moy et al., 2006). These SERT gene variants may underlie and/or contribute to some of the variability in autism phenotypes. For example, autistic carriers of the S allele of the SERT promoter polymorphism (5-HTTLPR) tend to exhibit more severe social interaction impairments, and are likely to exhibit poor response to SSRIs and antipsychotics (Tordjman et al., 2001; Murphy et al., 2004; Seretti et al., 2006; Brune et al. 2006; Dolzan et al., 2008; Henry et al., 2009). In support of reduced SERT expression contributing some role, cortical SERT density is lower in autistic children relative to age-matched controls, and SERT binding throughout many regions of brain is relatively low in autistic adult men in two independent tomography studies (Makkonen et al., 2008; Nakamura et al., 2010). However, in other reports, the long allele form of the 5-HTTLPR appears to be overtransmitted in autistic patients and/or is associated with increased aggression and repetitive behaviors (Devlin et al., 2005; Brune et al., 2006). This is consistent with findings that SSRI treatments improve aggression and social interaction in some autistic patients, while having no effect or negative effects in others (DeLong et al., 2002; West et al., 2009). Rare variants of the SERT gene may further confound interpretation of linkage and association studies for more common variants through epistatic interactions (Sutcliffe et al., 2005; Veenstra-VanderWeele et al., 2009). Some variants can also alter SERT affinity for ligands used in imaging studies, as described earlier in this review.
However, debate remains over the extent to which SERT-gene polymorphisms contribute to elevated risk for autism. A recent, global meta-analysis of familial studies found no overall association between either of the common 5-HTTLPR or VNTR polymorphisms and autism, although it did find overtransmission of the S form of the 5-HTTLPR in autistic families from US populations of mixed ethnic descent that was absent in European studies (Huang and Santangelo, 2008). The authors indicated that larger sample sizes are required for better resolution of such studies, and that a more varied suite of SERT polymorphisms or genes in or near the SERT gene should be investigated. A multiplexed variation scan study for rare coding variants in the SLC6A4 gene found no association or frequency difference among autistic patients and controls (Sakurai et al., 2008). However, in a study of five rare SERT variants that were overtransmitted in autistic patients, including Gly56Ala, four of them enhanced SERT activity to varying degrees in ChO Flip-In cells (Prasad et al., 2009). The authors concluded that rare SERT variants may act in different ways, from enhancing SERT function (Carneiro et al., 2009) to differential phosphorylation, trafficking and catabolism (Ramamoorthy et al., 2007).
Other non-SERT gene interacting factors could also be involved at the cellular level. For example, association with platelet aggregation factors (Carneiro et al., 2008), or cholesterol composition in lipid microdomains or “rafts” that regulate intracellular turnover and transport of SERT (Magnani et al., 2004; Saher et al., 2005) could alter SERT surface availability and the balance of extracellular and intracellular 5-HT levels. The Gly56Ala mutant knock-in mouse has elevated platelet 5-HT levels, and in this respect it has construct validity with hyperserotonemic autism (Veenstra-VanderWeele et al., 2009). As those authors hypothesize, it may be that optimal SERT function may be confined to a narrow middle range, such that either over or underactivity may contribute to autism susceptibility to account for the wide range of behavioral phenotypes seen in autism.
Carriers of SERT polymorphisms may also be more susceptible to autism only in the presence of other genetic, metabolic and/or environmental challenges during critical stages of brain development. Other genetic variants that have been linked or associated with autism susceptibility that might co-occur with common or rare SERT gene variants include, but are not limited to, scaffolding protein genes such as Deleted in schizophrenia 1 (Disc1) (Kilpinen et al., 2008), cell adhesion molecules (Wang et al., 2009), copy number variants for cell degradation markers (Glessner et al. 2009), the kinase pathway regulator PTEN (Varga et al., 2009), and the Fragile X FMR gene (Moy et al., 2009). Some of these dual polymorphisms have been replicated in inbred and/or transgenic mice and result in altered brain morphology and/or socially impaired phenotypes reminiscent of autism (Kwon et al., 2006; McFarlane et al., 2008; Moy et al., 2008; Page et al., 2009; Ayhan et al., 2010). Thus, multiple gene variants likely interact to impact brain ultrastructure and behaviors associated with autism and related disorders.
4.1.3. Hypertension and Cardiovascular Disorders
So far this review has focused only on SERT in brain as it relates to psychiatric disease. It would be remiss to exclude the fact that SERT is found in many tissues beyond the CNS, including those regulating cardiovascular and gastrointenstinal (see section 4.1.4) function. In this section we discuss what role SERT plays in blood pressure and cardiovascular control. SERT is localized in many tissues important to blood pressure and cardiovascular control, including, platelets, pulmonary and systemic arteries, heart, endothelial cells, the adrenal as well as in the nucleus tractus solitarius in brain (Schroeter et al., 1997; Mortensen et al., 1999; Eddahibi et al., 2000; Lee et al., 2001; Huang and Pickel, 2002; Armando et al., 2003; Ni et al., 2004). Moreover, SERT appears in these areas early in embryonic development. For example, using Cre recombinase β-galactosidase staining in transgenic SERT conditional reporter mice, SERT-expressing cardiac filamentous cells in the outflow tract, arterial trunk, major heart valves and right ventricle were observed as early as ED10-18 (Pavone et al., 2008). Physiological function of SERT in these tissues is currently best understood in the pulmonary vasculature where uptake of 5-HT via SERT is necessary for 5-HT to function as a mitogen. SERT also appears to be important for vessel contractility and normal platelet function (for review see Ni and Watts, 2006). There is a rapidly growing literature showing that polymorphisms of the SERT or certain pharmacological manipulations of the SERT can have adverse implications that are associated with hypertension, thrombosis and heart disorders (Ni and Watts, 2006).
Among the most widely publicized examples is that of heart valve disease that occurred in adults treated for long periods (several months to years) with the successful weight-loss drug “fenphen” (fenfluramine plus phentermine). “Fenfen” was found to produce heart valve disease via a 5-HT-induced fibrotic reaction, in which collagen fiber overgrowth, coats and thickens the heart valves leading to cardiac malfunction from valve leakage and regurgitation (Connolly et al., 1997; Gardin et al., 2000; Jick 2000; Soler-Soler and Galve, 2000). The related drug, dexfenfluramine, as well as MDMA have also been associated with heart valve dysfunction by the same mechanism (Bhattacharyya et al., 2009). This 5-HT induced fibrotic overgrowth of heart valves has been replicated in rodent studies. For example, when 20 mg/kg 5-HT was administered to adult rats daily for 3 months, similar collagen-rich carcinoid plaques developed (Gustafsson et al., 2005). A common feature of these drugs is that they are all substrates for SERT and increase circulating 5-HT levels by causing the release of 5-HT via SERT. Given that such severe heart valve dysfunction can develop within a few months in adults in response to SERT mediated elevatations in systemic 5-HT levels, it is not surprising that SERT dysfunction or blockade can produce cardiac abnormalities during embryonic and postnatal development and growth (Yavarone et al., 1993; Sari and Zhou, 2003).
Related to this, a subject of some debate is whether administration of SSRI antidepressants to women during pregnancy and early postpartum can contribute to cardiac dysfunction in offspring. As discussed previously, SSRIs work by increasing extracellular 5-HT by inhibiting its uptake via SERT. Some clinical studies indicate administration of SSRIs such as citalopram, fluoxetine, sertraline, or paroxetine during pregnancy may double the risk of minor cardiac defects in offsping (Diav-Citrin et al., 2008; Pedersen et al., 2009). Others suggest that risks of neonatal cardiac malformations from SSRI exposure during pregnancy are negligible and/or transient (Costa et al., 2004; O’Brien et al., 2008; Klinger and Merlob, 2009). Recent rodent studies, where experimental conditions can be tightly controlled, show that prenatal and neonatal SSRI exposure can have lifelong detrimental effects on cardiac function (Fornaro et al., 2007; Noorlander et al., 2008). While no firm conclusion can be drawn regarding prenatal SSRI exposure in humans and persistent cardiovascular disease, it appears from rodent studies that maternal SSRI use may pose a significant risk, warranting further studies in this area.
While early pharmacological blockade of SERT may adversely affect cardiac function in later life, polymorphisms of SERT also contribute certain risk. For example, among carriers of polymorphisms impairing SERT function, there is an increased risk for heart disease (Serretti et al., 2006; Otte et al., 2007). Indeed, histological investigation of the heart valves of SERT KO mice revealed collagen fibrosis of both heart valves and myocardial tissue that was significantly greater than that in wildtype mice. In addition, the left ventricles of KO mice were dilated and functioned less efficiently (Mekontso-Dessap et al., 2006). In SERT KO rats, systolic blood pressure does not differ from wildtype rats at baseline, however in SERT+/− rats blood pressure is slightly elevated at baseline and remains so under hypertensive conditions (Homberg et al., 2006 as in Monassier et al., 2010). Such findings in rodents imply that function-impairing SERT gene polymorphisms should be considered a major risk factor for cardiac dysfunction.
On the other hand, pulmonary hypertension has been linked to SERT overexpression. For example, hypertension resulting from overgrowth of smooth muscle cells in the pulmonary artery is reportedly more common in carriers homozygous for the L allele of the 5-HTTLPR polymorphism. (Eddahibi et al., 2001). A subsequent study by the same research group confirmed that primary pulmonary hypertension was associated with overexpression of the SERT in pulmonary artery smooth muscle and lung cells (Marcos et al., 2004). Consistent with these findings, in SERT knock-in (overexpressing) mice, pulmonary hypertension is also observed (MacLean et al., 2004). Likewise, SERT density is higher in spontaneously hypertensive rats compared to control Wistar Kyoto rats (Roessner et al., 2009). In other rodent studies, pulmonary hypertension induced by monocrotaline (MCT) challenge to rats also increases SERT expression. Interestingly, atorvastatin (Lipitor), which is used clinically to reduce risk for heart attack or stroke, is effective in reversing both outcomes (pulmonary hypertension and SERT overexpression) of MCT treatment in rats (Laudi et al., 2007). Similarly, MCT-induced pulmonary artery hypertension in rats was reversed by 12 weeks of fluoxetine administration, however it returned after fluoxetine withdrawal (Zhu et al. 2009).
In sum, it appears that deficiencies in SERT expression or function leading to an excess in circulating 5-HT may lead to fibrotic overgrowths producing heart valve malformations, while overexpression of SERT in pulmonary smooth muscle can lead to pulmonary hypertension. Taken together with potentially unhealthy diet and behavior often exhibited by patients with depression and related psychiatric disorders, which are also associated with polymorphisms of the SERT, the effects of SERT dysfunction (above or below an ideal “normal level”) can compound the risk of cardiovascular disease (Otte et al., 2007; Williams et al. 2008). With the rising prevalence of childhood hypertension, related in part to the rapidly growing incidence of childhood obesity (Feber and Ahmed, 2010), the consequences for abnormal development of serotonergic (and other systems) as a result warrant deeper investigation. These data suggest that hypertension in children might also contribute to 5-HT and SERT linked pathologies in later life.
4.1.4. Gastrointestinal disorders
The gut contains 95% of all 5-HT in the body and extracellular levels are regulated by SERT, which is found on all mucosal epithelia cells (Gershon, 1999). Enterochromaffin cells in the gut release copious amounts of 5-HT, which serves to help regulate gastrointestinal (GI) fluid secretion and gut motility (Ormsbee and Fondacaro, 1985; Gershon, 1999). It is no surprise then that dysfunction of SERT in the gut could have adverse effects.
The relationship between irritable bowel syndrome (IBS) and SERT polymorphisms has been among the most studied. While the results have been mixed, three case controlled studies indicate a positive association between the 5-HTTLPR SS genotype and the diarrhea-predominent subgroup of IBS sufferers, whereas others find no association (Van Kerhoven et al., 2007; Colucci et al., 2008). Still others find associations between carriers of the LL genotype and improved treatment response to the 5-HT3 receptor antagonist, alosetron (Camilleri, 2004). Certainly the importance of considering the complexities of IBS, including not only multiple genetic factors, but also multiple environmental variables, must be considered and likely factor into these conflicting reports (see Colucci et al., 2008). Of interest however, is the finding that SERT KO mice have increased GI motility and diarrhea with occasional constipation reminiscent of an IBS-like clinical syndrome (Chen et al., 2001).
There are few studies addressing IBS or other GI abnormalities as a consequence of early developmental perturbations to SERT. Of those, a recent study reported higher intenstinal 5-HT levels and lower SERT mRNA in pediatric patients presenting with IBS (Faure et al., 2010). In addition, there is evidence to suggest that inflammation of the gut, via inflammatory mediators including interferon-γ and tumor necrosis factor α, reduces SERT expression and function (Mawe et al., 2006; Linden et al., 2003 & 2005; Wheatcroft et al., 2005; Foley et al., 2007). Together, these reports raise the possibility that early life infections, particularly of the gut, might lead to chronic GI diseases later in life.
4.1.5. Other disorders
Studies of SERT KO mice and rats have been informative, not only about the disorders discussed so far in this review, but have also afforded insight into the role of SERT in many others (Murphy et al., 2008; Kalueff et al., 2010). For example, SERT KO and heterozygote mice have distrupted sleep patterns (Wisor et al., 2003) and increased susceptibility to pentylenetetrazole-induced seizures (Fox et al., 2007). Sensory function is reduced in SERT KO mice (Vogel et al., 2003; Ren-Patterson et al., 2005), and in female SERT KOs bladder responses to stretching are reduced (Cornelissen et al., 2005). SERT KO mice also appear to have reduced muscle strength (Holmes et al., 2002) and reduced bone weight, thickness and structural resistance to fracture, where the most marked changes occur in weight-bearing bones (Bliziotes et al., 2002; Warden et al., 2005; Sample et al., 2008). Related to obesity, diabetes and other metabolic disorders these mice also exhibit decreased glucose tolerance and insulin sensitivity and increased body weight, particularly with age (reviewed in Murphy et al., 2008). Unfortunately, while extracellular and tissue levels of 5-HT in adult SERT heterozygote and KO mice have been well documented (Bengel et al., 1998; Mathews et al., 2004; Shen et al., 2004; Kim et al., 2005; Fox et al., 2008), we know little about how 5-HT levels are changed early in development of rodents or primates with impaired SERT function. It may be that extracellular levels of 5-HT do not mirror those of adults, but may be exacerbated (or not), contingent upon the developmental profiles of other transporters capable of 5-HT uptake and which might compensate (or not) for impaired SERT function (see Daws, 2009). Clearly there is much to be learned about the factors controlling extracellular 5-HT during embryonic and early postnatal development. Importantly, understanding how overcoming abnormal levels of 5-HT at these early developmental stages will be critical for curbing, or preventing, long term behavioral and physiological consequences.
4.2 Behavior and disease linked to pharmacological blockade of SERT during childhood or adolescent periods
4.2.1 Prenatal and early postnatal SSRI exposure effects
A recent review of safety and efficacy of SSRI administration to pregnant women and adolescents indicates potential adverse effects (Olivier et al., 2010). Acute SSRI withdrawal symptoms such as agitation are reported in infants, however delayed psychomotor development, altered pain response, cardiovascular abnormalities and increased risk of depressive disorders later in life are potential long term side effects of pre or postnatal SSRI exposure (Casper et al., 2003; Andersen and Navalta, 2004; Oberlander et al., 2005; De las Cuevas and Sanz 2006; Tuccori et al., 2009; Alwan and Friedman, 2009; Gentile, 2010).
Similar adverse physiological, neuroanatomical and behavioral effects have been observed in rodent models of early-juvenile and prenatal SSRI or tricyclic antidepressant exposure. In these rodent models dendritic spine formation and neuronal branching are permanently reduced; anxiety is increased, shock avoidance is decreased, cocaine-seeking behavior is increased, and other impairments of 5-HT function occur, some of which persist into adulthood (Andersen and Navalta, 2004; Borue et al., 2007; Forcelli and Heinrichs, 2008). In rats, prenatal (GD13-20) exposure to the SSRI fluoxetine (10 mg/kg sc daily injection of dam) decreased 5-HT content in the frontal cortex by 28% in juvenile (PD26) but not in adult (PD70) males. Only in midbrain did low 5-HT levels persist through to adulthood (Cabrera-Vera et al., 1997). Exposure to fluoxetine from PD0-6 resulted in reduced dendritic spine growth and fewer dendritic projections which altered tactile and thermal responsivity and locomotor activity in adolescence (Lee et al., 2009). In guinea pig offspring from dams administered fluoxetine (10 mg/kg/d) by osmotic minipump throughout their pregnancy, there were no adverse effects on weight gain and no higher incidence of still births, but pain threshold was lower in offspring exposed in utero to fluoxetine (Vartazarmian et al., 2005). In swiss and C57BL/6 mice administered fluoxetine from 2 to 6 weeks of age (for the first week by subcutaneous osmotic minipump and then via fluoxetine in the drining water) there was an apparent anxiogenic effect of fluoxetine in a battery of behavioral tests that were conducted beginning at 5.5 weeks of age (Oh, et al., 2009). Other effects of early juvenile SSRI exposures, prior to PD21 in rodents, which affect behavior persistently into adulthood include altered 5-HT receptor densities, abnormal cortical neuron structure, increased anxiety and stress responses, decreased aggression and altered REM sleep patterns, as reviewed in Narboux-Nême et al. (2008 see Table 2 within) and in Homberg et al., (2010). Of particular interest, the SSRI, fluoxetine, but not the NE reuptake inhibitor, desipramine, given to mice from PD4-21, exposure affected emotional behavior in adulthood, producing reduced adult exploration in the elevated plus maze and open field tests and increased the latency to feed or escape shock in new environments (Ansorge et al., 2008). The timing of such exposures in rodents corresponds with late gestational development in humans (Rice and Barone, 2000; Brent, 2004) suggesting that fetal exposure to SSRIs may produce long lasting effects on emotional behavior (Oberlander et al., 2009).
4.2.2. Effects of SSRIs during juvenile and adolescent brain development
In contrast to prenatal or early-life exposures to SSRIs under conditions of maternal treatment, treatment of children or adolescents with SSRIs can be better titrated; however, the long-term implications of SERT blockade during these developmental periods is not well understood and potential behavioral and long term effects of SSRIs on developing brains remain a major concern (Andersen and Navalta, 2004; Murrin et al., 2007; Alwan and Friedman, 2009; Homberg et al., 2010; Mulla 2010).
In 2003 the British Department of Health and in 2004 the Food and Drug Administration (FDA) mandated warning labels for all SSRIs indicating use in children may increase risk of suicide, due to reports of suicidal attempts or thoughts among children treated with SSRIs in the 1990s (Vitiello and Swedo, 2004; Olfson et al., 2006). A more recent comparative assessment of SSRIs and tricyclic antidepressants administered to children and adolescents indicated that all of these drugs increased risk of suicide to a similar extent (Schneeweiss et al., 2010). After the warning, SSRI treatment in children declined, yet suicide rates remained the same, and no alternative drug has filled the void left by SSRIs for children with psychiatric disorders (Katz et al., 2008). A new clinical trial of the S-enantiomer citalopram, which is the second SSRI (following fluoxetine) approved by the FDA for treatment of depression in children, indicated that it did not differ from placebo in the number of suicides attempted (Yang and Scott, 2010). Together, these results suggest that suicidality may not be related to SSRI treatment per se, but a risk associated with the disease itself. None-the-less, given the prevelance of polymorphisms in SERT and variance in metabolism of SSRIs, exploring alternative 5-HT uptake mechanisms (described in section 5), may be a useful direction for future investigations in efforts to identify new targets for the treatment of these disorders in children and adolescents.
Child and adolescent neurotransmitter systems may not respond to SSRIs as adult systems do, in part because some mature sooner than others (serotonergic vs. noradrenergic), and expression and function of receptors and transporters differs during the course of juvenile development (Bylund and Reed, 2007; Liberzon and George, 2010; Murrin et al., 2007; Mulla 2010). Baseline extracellular neurotransmitter levels above an ideal normative range may produce hyperactive behavior, but further drug-induced increases may have the opposite effect, assuming a U-shaped dose-effect curve, which is why psychostimulants such as the amphetamines administered to children with attention deficit hyperactivity disorder (ADHD) can effectively reduce hyperactivity (Andersen, 2005).
Studies examining mid to late juvenile and adolescent exposures to SSRIs in rats and mice have yielded mixed results. For example, while adolescent fluoxetine exposure has been reported to have no lasting adverse effects on anxiety or stress response in adulthood in C57BL/6 and BALB/c mice (Norcross et al., 2008), other studies report age and strain dependent differences in response to the acute effects of SSRIs. For example, fluoxetine failed to produce antidepressant-like effects in the forced swim and tail suspension tests in juveniles of any strain (BALB/c, C57, and a C57/129S hybrid strain), but was effective in adult mice of C57 and C57/129S strains, but not in BALB/c mice (Mason et al., 2009). One mechanism by which SSRIs may exert their beneficial therapeutic effects is through neurogenesis, but again, rodent studies have revealed that this neurogenic effect is age and strain dependent. For example, in BALB/c and C57BL/6 mice, adult hippocampal neurogenesis only occurred when treatment commenced in adolescence (PD35), and this effect was abolished by stress (Navailles et al., 2008). A gender by age comparison of peri-pubescent and adult rats, showed that fluoxetine did not enhance hippocampal neurogenesis in either male or female adolescents to the same extent as it did in adults (Hodges et al., 2009). In sum, it appears that long term effects of SSRI treatments in juvenile rodents exert subtle effects on the adolescent brain, and further studies are needed to assess whether some SSRIs may have better risk/benefit profiles than others for the developing brain. Studies such as these, geared toward identifying developmental (age) and genetic (strain and genes) factors that influence response to antidepressant drugs will be important for optimizing treatments for specific age groups and genetic background.
4.2.3. Implications
Rodent studies of SSRI treatment early in development clearly show that the effects on behavior and physiology only occur when SSRIs are given in these early periods. These effects are not recapitulated when SSRIs are given later in development (Ansorge et al., 2004 & 2008; see also Table 2 in Narboux-Nême et al. 2008 and review by Oberlander et al., 2009). Likewise, as discussed earlier in this review, there is a strong interaction between stressful life events early in life and increased probability of suffering from depression, anxiety and related disorders, which do not occur when stress occurs later in life (Caspi et al., 2010; Oberlander et al., 2009). Preclinical studies in rodents and clinical studies in humans reveal striking parallels in the behavioral and physiological ramifications following these extrinsic early life perturbations of SERT (see Oberlander et al., 2009). Together with the fact that intrinsic factors, such as genetic reduction in SERT function, also increases risk for psychiatric and other disorders in later life, it is clear that SERT exerts a major influence on the developing brain, (as well as other organs and tissues). In this regard, finding ways to “normalize” extracellular levels of 5-HT in these critical early developmental periods is an important area for continued research. One such avenue of research is to explore alternative mechanisms for 5-HT uptake.
5. Alternative transporters for serotonin: Role in disease and treatment response
So far we have discussed SERT in relative isolation, as the only mechanism for 5-HT uptake in brain. However, while SERT may be the transporter with the highest affinity for 5-HT, there is a rapidly growing body of literature reporting the presence of non-SERT 5-HT transporters that can play a significant role in mediating 5-HT neurotransmission (reviewed in Daws, 2009). These include the well-known NET and DAT, but also the organic cation transporter (OCT), particularly the OCT3 subtype, and plasma membrane monoamine transporter (PMAT), for which a role in biogenic amine neurotransmission has only recently been realized. It stands to reason, therefore, that these transporters might act to compensate for reduced SERT expression or function, particularly early in development. While there is evidence from rodent studies showing that, in adult mice, DAT (Pan et al., 2001; Zhou et al.,2002) and OCT3 (Baganz et al., 2008) can compensate, at least in part, for 5-HT uptake, nothing is known about the role of non-SERT 5-HT transporters in regulating ambient 5-HT during development. Likewise, how such ectopic uptake of 5-HT might predispose to disorders early in life or later in adulthood, as well as influence subsequent treatment response, remains unclear. Using antidepressant response as an example, the following section summarizes what little we do know and ends by proposing a hypothesis through which this might occur.
5.1 Ontogenic profiles of biogenic amine transporters and antidepressant response: What do we know?
Remarkably little is the answer, and even more so when one narrows the developmental window to “adolescent”. As discussed in section 4.1. there is now considerable evidence that the S allele of the 5-HTTLPR is linked to several disorders, primary among them depression and related psychiatric disorders, and often these individuals do not respond well to currently available antidepressant medications (Rausch, 2005; Serretti et al., 2007). However, age is an even stronger predictor of antidepressant response. Children and adolescents respond poorly compared with adults (Bylund and Reed, 2007). The SSRI, fluoxetine (Prozac) is currently the only available treatment for depression approved by the FDA for children and adolescents up to 18 years old and escitalopram (Lexapro) is approved for adolescents age 12 and older. The lack of therapeutic options for this age group is compounded further by a recent report that treatment response to the SSRI, citalopram, in children and young adults carrying the S allele, was especially poor (Kronenberg et al., 2007). Given the high prevalence of adolescent depression, affecting 4–8% of the population with an incidence of 25% by the end of adolescence (Kessler et al., 2001) and, of major concern, the particularly high prevalence of suicide in this young population, (the third leading cause of death in the 15 to 19 year age group) (Reed et al., 2008), it is clear there is an urgent need to understand the neural mechanisms accounting for these differences between adolescents and adults, with the hope to uncover more effective treatments.
Again, rodent studies are lending a hand. In rodents, PD21 is considered juvenile and PD28, early adolescent (Bylund and Reed, 2007; Hefner and Holmes, 2007; Pechnick et al., 2008; Reed et al., 2008). Consistent with clinical responses in humans, SSRIs are effective in reducing the time spent immobile in the forced swim test (FST), an index of antidepressant-like activity, in rats as young as PD21 as well as in adults, whereas NET blockers, such as the tricyclic, desipramine (DMI), are ineffective in the FST in rats younger than PD28 (Pechnick et al., 2008; Reed et al., 2008). The mechanistic basis for these findings remains to be determined, but is generally thought attributable to delayed maturation of the NE neurotransmitter system relative to 5-HT. In terms of the actual drug targets themselves, SERT and NET, ontogenic profiles for their expression during this developmental window are sparse. Galineau and co-workers (2004) reported a triphasic profile for SERT. In terminal regions such as amygdala and hypothalamus, expression peaked around PD21, decreased at PD28 and plateaued through PD70, the oldest age tested. For NET, Sanders and co-workers (2005) reported that NET expression in the CA3 region of hippocampus was 3-fold less in rats aged PD20 compared to PD25 and adults. Thus, based on studies in rats, expression of SERT and NET appears to positively correlate with the emergence of an antidepressant-like response to SSRIs and NET blockers. The ontogeny of DAT more closely resembles that of NET, with expression increasing steadily until plateauing at adult levels at P28 (Galineau et al., 2004).
What about OCT3? To our knowledge there are no published reports of its ontogeny in brain or even in peripheral organs. For its closely related subtype, OCT1, mRNA expression reaches adult levels by P15 in kidney and by P22 in liver (Alnouti et al., 2006). Although difficult to translate these findings to those which may occur in brain, these data support the idea that in juveniles, OCT3 may reach adult levels earlier than SERT and NET. Of note, in adults, OCT3 is widely expressed in SERT rich brain regions, anatomically positioning OCT3 to take up 5-HT (Gasser et al., 2009).
What about PMAT? Likewise, there are no published reports of its ontogeny in brain, or in periphery, perhaps not surprising since it was identified and cloned relatively recently, in the mid-2000s (Engel et al., 2004; Engel and Wang, 2005). Like OCT3, in adult mice PMAT is a widely distributed, neuronally-expressed transporter, which is co-expressed in many regions containing SERT, supporting a role for PMAT in 5-HT uptake, at least under certain conditions (Dahlin et al., 2007).
Taken together the possibility is raised that non-SERT mediated 5-HT uptake during childhood and adolescence may account for poor treatment response to SSRIs by buffering the increase in extracellular 5-HT that is believed to be the first step to initiating the cascade of events that ultimately lead to therapeutic response (see Daws 2009). This could be further compounded by developmental windows where there is disproportionately high expression (and/or function) of non-SERT transporters for 5-HT compared to SERT. While this remains to be determined empirically, it provides one plausible mechanism for the relative lack of therapeutic benefit of SSRIs in children and adolescents.
5.2. The corticosterone-sensitive organic cation transporter 3: Linking early life stress and 5-HTTLPR behavioral phenotypes
Corticosterone is a potent antagonist of OCT3, but not other biogenic amine transporters including SERT and PMAT (Engel et al., 2004; Gasser et al., 2006; Koepsell et al., 2007) and is ~100-fold more selective for OCT3 than OCT1 or OCT2 (Hayer-Zillgen et al., 2002). This suggests that corticosterone, released in response to stress, may tamper with 5-HT neurotransmission during development by blocking 5-HT uptake via OCT3. Indeed evidence that OCT3 function is modulated by stress in adult animals comes from reports that in dorsomedial hypothalamus, a site where stress and glucocorticoids acutely modulate 5-HT neurotransmission, stress or injections of corticosterone rapidly increase extracellular 5-HT (Feng et al., 2005). Of note, the increase in 5-HT following corticosterone was greater than an equivalent dose of an SSRI (Feng et al., 2005; see also Feng et al., 2009, 2010). Elegant in vitro studies by Gasser and co-workers (2006) showed that this increase in 5-HT was likely mediated by OCT3. Consistent with the increase in extracellular 5-HT following corticosterone, reduction of OCT3 function using either antisense or pharmacological approaches reveal an antidepressant-like response in rodents (Kitaichi et al., 2005; Baganz et al., 2008 & 2010).
We recently found that expression and function of OCT3 is significantly increased in SERT heterozygote mice, which have reduced SERT expression, and provide a model for human carriers of the S allele (Baganz et al., 2008). Moreover, blockade of OCT3 elicited antidepressant-like effects in SERT mutant mice but not wildtype mice (Baganz et al., 2008). Since OCT3 can take up 5-HT with high capacity and the stress hormone, corticosterone, is a potent blocker of OCT3, the intriguing possibility that OCT3 might be an important mechanism contributing to dysfunction of serotonergic neurotransmission during early life stress, is raised. It is well known that abnormally high levels of extracellular 5-HT during critical periods in brain development can modify wiring of brain circuitry and cause long lasting changes in adult behavior (Gaspar et al., 2003). Extracellular 5-HT is already increased 5-fold in SERT+/− mice and 9-fold in mice lacking the SERT compared to wildtype mice (SERT+/+) (Shen et al., 2004; Mathews et al., 2004). Corticosterone released in response to early life stress, by blocking OCT3, may lead to even greater levels of extracellular 5-HT in brain of SERT+/− and SERT KO mice and exacerbate the deleterious behavioral phenotypes that can emerge in adulthood. Indeed, such a possibility may help to explain why early life stress increases the probability of suffering depressive illness in later life in carriers of the S allele of the 5-HTLLPR (Caspi et al., 2003 & 2010; Serreti et al., 2006). Put another way, low SERT activity (S carriers) may create risk in the face of stress because there is not enough SERT function to compensate for the loss of OCT3 function that results from its blockade by corticosterone released in response to stress during development. This will be an exciting line for future research that may allow us to glean new insight into the underlying etiology of this gene × stress interaction and identify new targets for preventative or therapeutic intervention.
6. Concluding remarks
It is clear from the literature that there are an overwhelming number of intrinsic and extrinsic factors that can influence SERT expression and/or activity from early embryonic periods through adolescence. While these factors continue to affect SERT expression and function throughout adulthood, it seems that such modulation of SERT during early periods in development are especially important for ensuring “normal” development. Given the complex interplay between neurotransmitter systems, this extends beyond “normal” development of the serotonergic system, but also to the myriad of neurotransmitter systems that are affected by 5-HT, which combined, orchestrate appropriate wiring of brain circuitry. Figure 1 provides a summary cartoon of what has been covered in this review of the literature, which highlights some of the vast array of factors that can influence SERT expression and/or activity. In the case of early developmental windows, embryonic through adolescence, the majority of studies have focused on the influence of these factors on SERT expression. At this time, essentially nothing is known in terms of how these factors influence SERT activity, for example, by catalytic activation or trafficking to and from the plasma membrane, during these critical developmental windows. We do know that extracellular levels of 5-HT peak and wane during early development. However, we are yet to establish how increases or decreases in SERT expression and/or activity may be mechanistically linked to mistiming of these peaks and troughs in extracellular 5-HT during development. What has emerged from the literature is a clear link between abberant SERT expression and a host of disorders ranging from psychiatric (e.g. depression, ASD) to physiological (e.g. cardiovascular, gastrointestinal). There is much yet to be understood about the mechanics of this relationship, which is and will continue to be the subject of intense research for many years to come. Ultimately, the hope is that such lines of research will help to elucidate the etiological bases for these disorders and subsequently the design of improved therapeutics or preventative interventions.
Figure 1.
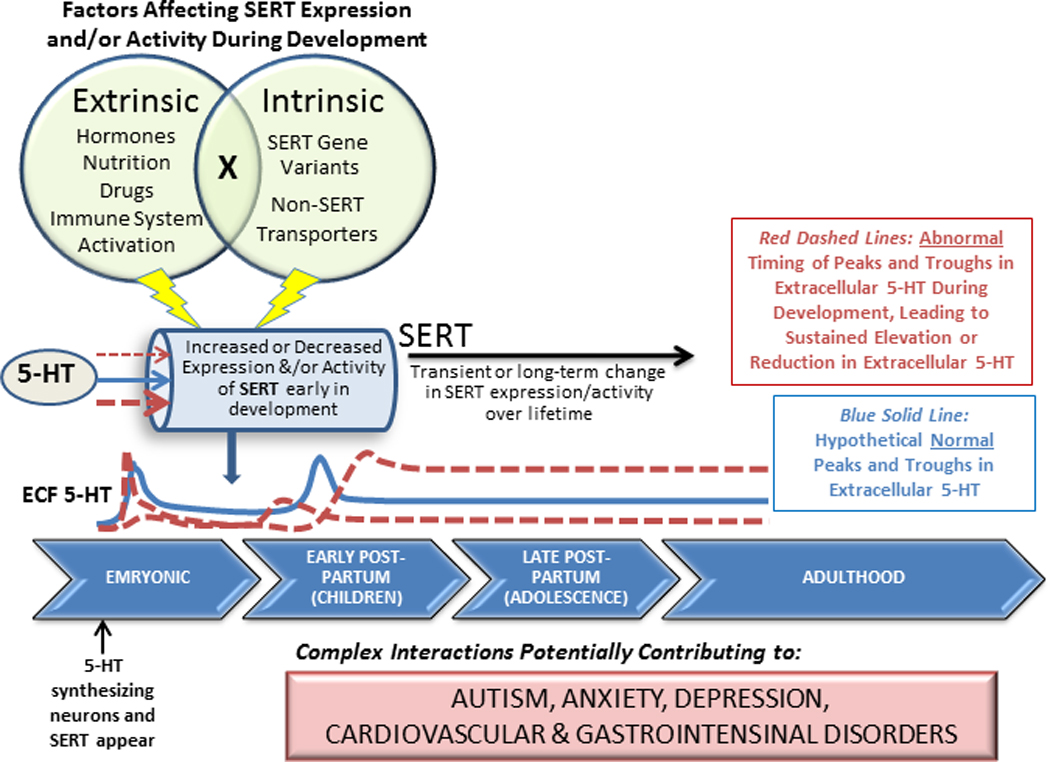
A complex web of factors can affect SERT expression and/or activity during embryonic and early postnatal development. SERT is the primary, high-affinity uptake mechanism for 5-HT and plays a major role in regulating levels of extracellular 5-HT. While 5-HT levels normally peak and wane during development, mistiming, or inappropriate magnitudes of these peaks may contribute to a host of 5-HT-linked pathologies in later life. Abnormal SERT function at critical times during development might contribute to the manifestation of inappropriate 5-HT levels.
Acknowledgements
Supported in part from USPHS grant MH64489 (LCD), MH064489-07S1, (LCD), NARSAD (LCD) and the San Antonio Area Foundation (GGG).
Abbreviations
- CpG
Cytosine-phosphate-guanosine
- DA
dopamine
- DAT
dopamine transporter
- DRN
dorsal raphe nucleus
- ED
embryonic day
- GD
gestational day
- GW
gestational week
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- KO
knockout
- MAPK
mitogen activate protein kinase
- MDMA
3,4-methylenedioxymethamphetamine
- MCT
monocrotaline
- NE
norepinephrine
- NET
norepinephrine transporter
- OCT
organic cation transporter
- PMAT
plasma monoamine transporter
- PD
postnatal day
- SSRI
selective serotonin reuptake inhibitor
- SNP
single nucleotide polymorphisms
- VNTR-2
variable number of tandem repeats in the second intron
- VMAT2
vesicular monoamine transporter-2
- 5-HT
serotonin, 5-hydroxytrytamine
- SERT/5-HTT
serotonin transporter
- 5-HTTLPR
serotonin transporter linked polymorphic region
- +/+
wildtype
- +/−
heterozygote
- −/−
null; knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynette C. Daws, Email: daws@uthscsa.edu.
Georgianna G. Gould, Email: gouldg@uthscsa.edu.
References
- Abraham PM, Kuruvilla KP, Mathew J, Malat A, Joy S, Paulose CS. Alterations in hippocampal serotonergic and INSR function in streptozotocin induced diabetic rats exposed to stress: Neuroprotective role of pyridoxine and Aegle marmelose. J Biomed Sci. 2010;17(78):1–15. doi: 10.1186/1423-0127-17-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Disposition. 2006;34:477–482. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- Alwan S, Friedman JM. Safety of selective serotonin reuptake inhibitors in pregnancy. CNS Drugs. 2009;23:493–509. doi: 10.2165/00023210-200923060-00004. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann N Y Acad Sci. 1990;600:331–340. doi: 10.1111/j.1749-6632.1990.tb16893.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Ansah TA, Ramamoorthy S, Montañez S, Daws LC, Blakely RD. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304) J Pharmacol Exp Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behaviour in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. Journal of Neuroscience. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango V, Huang YY, Underwood MD, Mann JJ. Genetics of the serotonin system in suicidal behavior. J Psychiatr Res. 2003;37:375–386. doi: 10.1016/s0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Armando I, Tjurmina OA, Li Q, Murphy DL, Saavedra JM. The serotonin transporter is required for stress-evoked increases in adrenal catecholamine synthesis and angiotensin II AT(2) receptor expression. Neuroendocrinology. 2003;78:217–225. doi: 10.1159/000073705. [DOI] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.144. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin transporter deficient mice. Proc Nat Acad Sci USA. 2008;105:18976–18781. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Martin KP, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: Organic cation transporter 3, the smoking gun. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.2740-10.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Barker EL, Blakely RD. Norepinephrine and serotonin transporters: Molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 321–333. [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Soumi SJ, Higley JD. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004a;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell S, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA. 2004b;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Piotrowski JP, Altamirano AV, Frazer A. Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine. Neuropsychopharmacology. 2009;34:555–564. doi: 10.1038/npp.2008.23. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Rhoades RW. Contributions of raphe-cortical and thalamocortical axons to the transient somatotopic pattern of serotonin immunoreactivity in rat cortex. Somatosens Mot Res. 1997;14:27–33. doi: 10.1080/08990229771196. [DOI] [PubMed] [Google Scholar]
- Berger B, Alvarez C, Goldman-Rakic PS. Neurochemical development of the hippocampal region in the fetal rhesus monkey. I. Early appearance of peptides, calcium-binding proteins, DARPP-32, and monoamine innervation in the entorhinal cortex during the first half of gestation (E47 to E90) Hippocampus. 1993;3:279–305. doi: 10.1002/hipo.450030305. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Paranavitane UT, Chavez C, Gogos A, Jones M, van den Buuse M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: A behavioral and electrochemical study. Brain Res. 2005;1064:10–20. doi: 10.1016/j.brainres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Schapira AH, Mikhailidis DP, Davar J. Drug-induced fibrotic valvular heart disease. Lancet. 2009;374:577–585. doi: 10.1016/S0140-6736(09)60252-X. [DOI] [PubMed] [Google Scholar]
- Bird A. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Chen JC, Rosene DL, Volicer L, Galler JR. Prenatal protein malnutrition effects on the serotonergic system in the hippocampal formation: an immunocytochemical, ligand binding, and neurochemical study. Brain Res Bull. 1994;34:507–518. doi: 10.1016/0361-9230(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bliziotes M, Gunness M, Eshleman A, Wiren K. The role of dopamine and serotonin in regulating bone mass and strength: Studies on dopamine and serotonin transporter null mice. J Musculskelet Neuronal Interact. 2002;2:291–295. [PubMed] [Google Scholar]
- Bloom SL, Sheffield JS, McIntire DD, Leveno KJ. Antenatal dexamethasone and decreased birth weight. Obstet Gynecol. 2001;97:485–490. doi: 10.1016/s0029-7844(00)01206-0. [DOI] [PubMed] [Google Scholar]
- Bock N, Quentin DJ, Hüther G, Moll GH, Banaschewski T, Rothenberger A. Very early treatment with fluoxetine and reboxetine causing long-lasting change of the serotonin but not the noradrenaline transporter in frontal cortex of rats. World J Biol Psychiatry. 2005;6:107–112. doi: 10.1080/15622970510029731. [DOI] [PubMed] [Google Scholar]
- Borue X, Chen J, Condron BG. Developmental effects of SSRIs: Lessons learned from animal studies. Int J Dev Neurosci. 2007;25:341–347. doi: 10.1016/j.ijdevneu.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan CB, Blue ME, Hohmann CF. Modeling early cortical serotonergic deficits in autism. Behav Brain Res. 2007;176:94–108. doi: 10.1016/j.bbr.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous Californian mouse (Peromyscus californicus) Horm Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Brent RL. Utilization of juvenile animal studies to determine the human effects and risks of environmental toxicants during postnatal developmental stages. Birth Defects Res B Dev Reprod Toxicol. 2004;71:303–320. doi: 10.1002/bdrb.20020. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents with Autism. Am J Psychiatry. 2006;163:2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Brunello N, Riva M, Volterra A, Racagni G. Effect of some tricyclic and nontricyclic antidepressants on [3H]imipramine binding and serotonin uptake in rat cerebral cortex after prolonged treatment. Fundam Clin Pharmacol. 1987;1:327–333. doi: 10.1111/j.1472-8206.1987.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Brüning G, Liangos O, Baumgarten HG. Prenatal development of the serotonin transporter in mouse brain. Cell Tissue Res. 1997;289:211–221. doi: 10.1007/s004410050868. [DOI] [PubMed] [Google Scholar]
- Brüning G, Liangos O. Transient expression of the serotonin transporter in the developing mouse thalamocortical system. Acta Histochem. 1997;99:117–121. doi: 10.1016/S0065-1281(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Buchert R, Schulze O, Wilke F, Berding G, Thomasius R, Peterson K, Brenner W, Clausen M. Is correction for age necessary in SPECT or PET of the central serotonin transporter in young, healthy adults? J Nucl Med. 2006;47:38–42. [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: Differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Reed AL. Childhood and adolescent depression: Why to children and adults respond differently to antidepressant drugs? Neurochem Int. 2007;51:246–253. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Garcia F, Pinto W, Battaglia G. Effect of prenatal fluoxetine (Prozac) exposure on brain serotonin neurons in prepubescent and adult male rat offspring. J Pharmacol Exp Ther. 1997;280:138–145. [PubMed] [Google Scholar]
- Cabrera-Vera TM, Battaglia G. Prenatal exposure to fluoxetine (Prozac) produces site-specific and age-dependent alterations in brain serotonin transporters in rat progeny: Evidence from autoradiographic studies. J Pharmacol Exp Ther. 1998;286:1474–1481. [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva M. Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology. 2009;345:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Is there a SERT-ain association with IBS? Gut. 2004;53:1396–1399. doi: 10.1136/gut.2004.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci. 2009;106:2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, Pascucci T, Puglisi-Allegra S, Cabib S, Gross C. Effect of the interaction between the serotonin transporter gene and maternal environment on developing mouse brain. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.10.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knockouts. J Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Catalini A, Marinelli M, Scaccianoce Sm, Nicolai R, Muscolo LA, Porcu A, Koranyi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New Engl J Med. 2006;354:579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel R, Mangner TJ, Chakraborty PK, Chugani HT, Chugani DC. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cintra L, Granados L, Aguilar A, Kemper T, DeBassio W, Galler J, Morgane P, Durán P, Díaz-Cintra S. Effects of prenatal protein malnutrition on mossy fibers of the hippocampal formation in rats of four age groups. Hippocampus. 1997a;7:184–191. doi: 10.1002/(SICI)1098-1063(1997)7:2<184::AID-HIPO5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Cintra L, Aguilar A, Granados L, Galván A, Kemper T, DeBassio W, Galler J, Morgane P, Durán P, Díaz-Cintra S. Effects of prenatal protein malnutrition on hippocampal CA1 pyramidal cells in rats of four age groups. Hippocampus. 1997b;7:192–203. doi: 10.1002/(SICI)1098-1063(1997)7:2<192::AID-HIPO6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Colucci R, Blandizzi C, Bellini M, Ghisu N, Tonini M, Del Tacca M. The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med. 2008;14:295–304. doi: 10.1016/j.molmed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997;2:247–250. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- Cornelissen LL, Brooks DP, Wibberley A. Female, but not male, serotonin transporter (5-HTT) knockout mice exhibit bladder instability. Auton Neurosci. 2005;122:107–110. doi: 10.1016/j.autneu.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Costa LG, Steardo L, Cuomo V. Structural effects and neurofunctional sequelae of developmental exposure to psychotherapeutic drugs: experimental and clinical aspects. Pharm Rev. 2004;56:103–147. doi: 10.1124/pr.56.1.5. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:1–9. doi: 10.3389/neuro.08.019.2009. article 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutellier L, Friedrich AC, Failing K, Marashi V, Wurbel H. Effects of foraging demand on maternal behavior and adult offspring anxiety and stress response in C57BL/6 mice. Behav Brain Res. 2009;196:192–199. doi: 10.1016/j.bbr.2008.08.042. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Wauters A, Deboutte D, Verkerk R, Scharpe S, Maes M. Central serotonergic hypofunction in autism: results of the 5-hydroxy-tryptophan challenge test. Neuro Endocrinol Lett. 2007;28:449–455. [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato RJ, Largent BL, Snowman AM, Snyder SH. Selective labeling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labeling of multiple sites by [3H]imipramine. J Pharmacol Exp Ther. 1987;242:364–371. [PubMed] [Google Scholar]
- Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010 Jun 17; doi: 10.1016/j.tins.2010.05.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Heffron DS, Mandell JW, Condron BG. Serotonergic dystrophy induced by excess serotonin. Mol Cell Neurosci. 2010;44:297–306. doi: 10.1016/j.mcn.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT1B receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem. 2007;101:641–651. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharm Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De las Cuevas C, Sanz EJ. Safety of selective serotonin reuptake inhibitors in pregnancy. Curr Drug Saf. 2006;1:17–24. doi: 10.2174/157488606775252593. [DOI] [PubMed] [Google Scholar]
- DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol. 2002;44:652–659. doi: 10.1017/s0012162201002717. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD CPEA Genetics Network. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, Di Gianantonio E, Clementi M, Weber-Schoendorfer C, Schaefer C, Ornoy A. Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol. 2008;66:695–705. doi: 10.1111/j.1365-2125.2008.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Cintra S, Cintra L, Kemper T, Resnick O, Morgane PJ. The effects of protein deprivation on the nucleus raphe dorsalis: a morphometric Golgi study in rats of three age groups. Brain Res. 1981;221:243–255. doi: 10.1016/0006-8993(81)90775-7. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzan V, Serretti A, Mandelli L, Koprivsek J, Kastelic M, Plesnicar BK. Acute antipyschotic efficacy and side effects in schizophrenia: association with serotonin transporter promoter genotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1562–1566. doi: 10.1016/j.pnpbp.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Hanoun N, Lanfoumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxia pulmonary hypertension in mice lacking the 5-HT transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. doi: 10.1124/mol.105.016832. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiat. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Faraj BA, Olkowski ZL, Jackson RT. Prevalence of high serotonin uptake in lymphocytes of abstinent alcoholics. Biochem Pharmacol. 1997;53:53–57. doi: 10.1016/s0006-2952(96)00726-5. [DOI] [PubMed] [Google Scholar]
- Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feber J, Ahmed M. Hypertension in children: New trends and challenges. Clin Sci (Lond) 2010;119:151–161. doi: 10.1042/CS20090544. [DOI] [PubMed] [Google Scholar]
- Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063:69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Feng N, Telefont M, Kelly KJ, Orchinik M, Forster GL, Renner KJ, Lowry CA. Local perfusion of corticosterone in the rat medial hypothalamus potentiates D-fenfluramine-induced elevations of extracellular 5-HT concentrations. Horm Behav. 2009;56:149–157. doi: 10.1016/j.yhbeh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Feng N, Lowry CA, Lukkes JL, Orchinik M, Forster GL, Renner KJ. Organic cation transporter inhibition increases medial hypothalamic serotonin under basal conditions and during mild restraint. Brain Res. 2010;1326:105–113. doi: 10.1016/j.brainres.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP. Endocrine regulation of neurotransmitter transporters. Epilepsy Res. 1999;37:203–210. doi: 10.1016/s0920-1211(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Fiskerstrand CE, Lovejoy EA, Quinn JP. An intronic polymorphic domain often associated with susceptibility to affective disorders has allele dependent differential enhancer activity in embryonic stem cells. FEBS Lett. 1999;458:171–174. doi: 10.1016/s0014-5793(99)01150-3. [DOI] [PubMed] [Google Scholar]
- Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TFN-alpha decrease serotonin transporter function and expression in CaCO2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, Heinrichs SC. Teratogenic effects of maternal antidepressant exposure on neural substrates of drug-seeking behavior in offspring. Addiction Biology. 2008;13:52–62. doi: 10.1111/j.1369-1600.2007.00078.x. [DOI] [PubMed] [Google Scholar]
- Fornaro E, Li D, Pan J, Belik J. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am J Respir Crit Care Med. 2007;176:1035–1040. doi: 10.1164/rccm.200701-163OC. [DOI] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, French HT, Stein AR, Huang S-J, Tolliver TJ, Murphy DL. Neurochemical, behavioral and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology (Berl) 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France CP, Li JX, Owens WA, Koek W, Toney GM, Daws LC. Reduced effectiveness of escitalopram in the forced swimming test is associated with increased serotonin clearance in food-restricted rats. Int J Neuropsychopharmacol. 2009;12:731–736. doi: 10.1017/S1461145709000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galineau L, Kodas E, Guilloteau D, Vilar M-P, Chalon S. Ontogeny of the dopamine and serotonin transporters in the rat brain: An autoradiographic study. Neurosci Lett. 2004;363:266–271. doi: 10.1016/j.neulet.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. J Am Med Assoc. 2000;283:1703–1709. doi: 10.1001/jama.283.13.1703. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: Potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gentile S. On categorizing gestational, birth, and neonatal complications following late pregnancy exposure to antidepressants: the prenatal antidepressant exposure syndrome. CNS Spectr. 2010;15:167–185. doi: 10.1017/s1092852900027449. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13 Suppl 2:15–30. [PubMed] [Google Scholar]
- Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Gilstrap LC, Christensen R, Clewell WH, D'Alton ME, Davidson EC, Escobedo MB, Gjerdingen DK, Goddard-Finegold J, Goldenberg RL, Grimes DA, Hansen TN, MD, Kauffman RE, Keeler EB, Oh W, Susman EJ, Vogel MG, Avery ME, Ballard PL, Ballard RA, Crowley P, Escobedo MB, Garite T, Goldenberg RL, Hankins GDV, Jobe AH, Kauffman RE, Koppe JG, Maher JE, Merkatz IR, Shankaran S, Simpson KN, Sinclair JC, Slotkin TA, Taeusch HW, Wright LL, Alexander D, Ballard RA, Berberich MA, Bracken M, Cooper L, Culpepper L, Elliott JM, Ferguson JH, Frigoletto F, Gail DB, Gilstrap LC, Hall WH, Jobe AH, Jones MD, Medoff-Cooper B, Merenstein GB, Whalen JM, Wright LL, Ferguson JH, Lenfant C, Hinshaw AS. Effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–418. [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C, Glaberson W, Flory J, Otieno F, Garris M, Soorya L, Klei L, Piven J, Meyer KJ, Anagnostou E, Sakurai T, Game RM, Rudd DS, Zurawiecki D, McDougle CJ, Davis LK, Miller J, Posey DJ, Michaels S, Kolevzon A, Silverman JM, Bernier R, Levy SE, Schultz RT, Dawson G, Owley T, McMahon WM, Wassink TH, Sweeney JA, Nurnberger JI, Coon H, Sutcliffe JS, Minshew NJ, Grant SF, Bucan M, Cook EH, Buxbaum JD, Devlin B, Schellenberg GD, Hakonarson H. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Altamirano AV, Javors MA, Frazer A. A comparison of the chronic treatment effects of venlafaxine and other antidepressants on serotonin and norepinephrine transporters. Biol Psychiatry. 2006;59:408–414. doi: 10.1016/j.biopsych.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Gould GG, Javors MA, Frazer A. Effect of chronic administration of duloxetine on serotonin and norepinephrine binding sites in rat brain. Biol Psychiatry. 2007;61:210–215. doi: 10.1016/j.biopsych.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen I, Waldum H. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Cabrera-Vera TM, Hoffman BJ. Infraorbital nerve transection alters serotonin transporter expression in sensory pathways in early postnatal rat development. Brain Res Dev Brain Res. 1998;111:305–314. doi: 10.1016/s0165-3806(98)00148-5. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience. 1999;89:243–265. doi: 10.1016/s0306-4522(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Hayer-Zillgen M, Brüss M, Bönisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. British J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Henry CA, Shervin D, Neumeyer A, Steingard R, Spybrook J, Choueiri R, Bauman M. Retrial of selective serotonin reuptake inhibitors in children with pervasive developmental disorders: a retrospective chart review. J Child Adolesc Psychopharmacol. 2009;19:111–117. doi: 10.1089/cap.2008.037. [DOI] [PubMed] [Google Scholar]
- Henry JP, Sagné C, Botton D, Isambert MF, Gasnier B. Molecular pharmacology of the vesicular monoamine transporter. Adv Pharmacol. 1998;42:236–239. doi: 10.1016/s1054-3589(08)60736-x. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008;20:184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Sinclair JD, Hilakivi IT. Effects of neonatal treatment with clomipramine on adult ethanol related behavior in the rat. Brain Res. 1986;317:129–132. doi: 10.1016/0165-3806(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Himpel S, Bartels J, Zimdars K, Huether G, Adler L, Dawirs RR, Moll GH. Association between body weight of newborn rats and density of serotonin transporters in the frontal cortex at adulthood. J Neural Transm. 2006;113:295–302. doi: 10.1007/s00702-005-0330-4. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. Neuron. 2005;55:726–740. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Seki T, Sakai N, Kato Y, Hashimoto H, Uchida S, Yamada S. Effects of continuous administration of paroxetine on ligand binding site and expression of serotonin transporter protein in mouse brain. Brain Res. 2005;1053:154–161. doi: 10.1016/j.brainres.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Yang L, Van Kooy J, Santollo J, Shors TJ. Prozac during puberty: distinctive effects on neurogenesis as a function of age and sex. Neuroscience. 2009;163:609–617. doi: 10.1016/j.neuroscience.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biol Psychiatry. 2003a;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003b;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Homberg J, Mudde J, Braam B, Ellenbroek B, Cuppen E, Joles JA. Blood pressure in mutant rats lacking the 5-hydroxytryptamine transporter. Hypertension. 2006;48:e115–e116. doi: 10.1161/01.HYP.0000246306.61289.d8. [DOI] [PubMed] [Google Scholar]
- Homberg J, Olivier J, Smits B, Mul J, Mudde J, Verheul M, Nieuwenhuizen O, Cools A, Ronken E, Cremers T, Schoffeimeer A, Ellenbroek B, Cuppen E. Characterization of the serotonin transporter knockout rat: A selective change in functioning of the serotonergic system. Neuroscience. 2007;146:1662–1672. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Schloss P. Down-regulation of the rat serotonin transporter upon exposure to a selective serotonin reuptake inhibitor. Neuroreport. 2001;12:2181–2184. doi: 10.1097/00001756-200107200-00027. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Lesch KP, Ugarkovic D, Cicin-Sain L, Jernej B. Identification of serotonin transporter mRNA in rat platelets. J Neural Transm Gen Sect. 1996;103:957–965. doi: 10.1007/BF01291786. [DOI] [PubMed] [Google Scholar]
- Hu ZZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Santangelo SL. Autism and serotonin transporter gene polymorphisms: a systematic review and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:903–913. doi: 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- Huang J, Pickel VM. Serotonin transporters (SERTs) within the rat nucleus of the solitary tract: Subcellular distribution and relation to 5-HT2A receptors. J Neurocytol. 2002;31:667–679. doi: 10.1023/a:1025795729393. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striatal cortex of man. Hum Neurobiol. 1993;6:1–9. [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings K, Loder M, Sheward W, Pei Q, Deacon R, Benson M, Olveerman H, Hastie N, Harmar A, Shen S, Sharp T. Increased expression of the 5-HT transporter confers low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H. Heart valve disorders and appetite-suppressant drugs. J Am Med Assoc. 2000;283:1738–1740. doi: 10.1001/jama.283.13.1738. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith RM, Edwards KS, Givens B, Tilley MR, Beversdorf DQ. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. Int J Dev Neurosci. 2010;28:529–536. doi: 10.1016/j.ijdevneu.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Takai D. The role of DNA methylation in mammalian epigenics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Kalbitzer J, Erritzoe D, Holst KK, Nielsen FA, Marner L, Lehel S, Arentzen T, Jernigan TL, Knudsen GM. Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. Biol Psychiatry. 2010;67:1033–1039. doi: 10.1016/j.biopsych.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Olivier JDA, Nonkes JLP, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, Balakrishnan B, Dai H, Chakraborty PK, Janisse J, Muzik O, Romero R, Chugani DC. Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J Cereb Blood Flow Metab. 2010 Sep 8; doi: 10.1038/jcbfm.2010.156. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- Katz LY, Kozyrskyj AL, Prior HJ, Enns MW, Cox BJ, Sareen J. Effect of regulatory warnings on antidepressant prescription rates, use of health services and outcomes among children, adolescents and young adults. CMAJ. 2008;178:1005–1011. doi: 10.1503/cmaj.071265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries MK. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Khan NA, Meyniel JP, Deschaux P. Ca2+/calmodulin and protein kinase C regulation of serotonin transport in human K562 lymphocytes. Cell Immunol. 1996;172:269–274. doi: 10.1006/cimm.1996.0242. [DOI] [PubMed] [Google Scholar]
- Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, Nieminen-von Wendt T, von Wendt L, Paunio T, Peltonen L. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Lyons LA, Abel K, Mendoza S, Capitanio JP. Effects of early experience and genotype on serotonin transporter regulation in infant rhesus macaques. Genes Brain Behav. 2008;7:481–486. doi: 10.1111/j.1601-183X.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Karere GM, Mendoza SP, Lyons LA, Mason WA, Capitanio JP. Serotonin pathway gene-gene and gene-environment interactions influence behavioral response to stress in infant rhesus macaques. Dev Psychopathol. 2010a;22:35–44. doi: 10.1017/S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Tarara ER, Mason WA, Mendoza SP, Abel K, Lyons LA, Capitanio JP. Serotonin transporter expression is predicted by early life stress and is associated with disinhibited behavior in infant rhesus macaques. Genes Brain Behav. 2010b;9:45–52. doi: 10.1111/j.1601-183X.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010c;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi K, Fukuda M, Nakayama H, Aoyama N, Ito Y, Fujimoto Y, Takagi K, Takagi K, Hasegawa T. Behavioral changes following antisense oligonucleotide-induced reduction of organic cation transporter-3 in mice. Neurosci Lett. 2005;382:195–200. doi: 10.1016/j.neulet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Klinger G, Merlob P. SSRIs and heart defects in neonates. Br Med J. 2009;339:b4288. doi: 10.1136/bmj.b4288. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Koldzic-Zivanovic N, Seitz PK, Watson CS, Cunningham KA, Thomas ML. Intracellular signaling involved in estrogen regulation of serotonin uptake. Mol Cell Endocrinol. 2004;226:33–42. doi: 10.1016/j.mce.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ. Effect of repeated administration of antidepressants on serotonin uptake sites in limbic and neocortical structures of rat brain determined by quantitative autoradiography. Neuropsychopharmacology. 1992;7:317–324. [PubMed] [Google Scholar]
- Kraft JB, Slager SL, McGrath PJ, Hamilton SP. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry. 2005;58:374–381. doi: 10.1016/j.biopsych.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Kranjnak K, Rosewell KL, Duncan MJ, Wise PM. Aging, estradiol, and time of day differentially affect serotonin transporter binding in the central nervous system of female rats. Brain Res. 2003;990:87–94. doi: 10.1016/s0006-8993(03)03441-3. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: Critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- Kronenberg S, Apter A, Brent D, Schirman S, Melhem N, Pick N, Gothelf D, Carmel M, Frisch A, Weizman A. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolescent Psychopharmacol. 2007;17:741–750. doi: 10.1089/cap.2006.0144. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;2:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, Christians U, Weimann J, Kaisers U, Steudel W. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;293:L630–L638. doi: 10.1152/ajplung.00110.2006. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrlé R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lee LJ. Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory-related behaviors in adolescent rats. Neurotox Res. 2009;15:212–223. doi: 10.1007/s12640-009-9022-4. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J. 2001;15:1324–1325. doi: 10.1096/fj.00-0431fje. [DOI] [PubMed] [Google Scholar]
- Lesage J, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Breton C, Deloof S, Vieau D. Perinatal maternal undernutrition programs the offspring hypothalamo-pituitary-adrenal (HPA) axis. Stress. 2006;9:183–198. doi: 10.1080/10253890601056192. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liberzon I, George SA. SSRI-enhanced locus coeruleus activity and adolescent suicide: lessons from animal models. Neuropsychopharmacology. 2010;35:1619–1620. doi: 10.1038/npp.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in muscosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- Line SJ, Barkus C, Coyle C, Jennings KA, Deacon RM, Lesch KP, Sharp T, Bannerman DM. Opposing alterations in anxiety and species-typical behaviors in serotonin transporter overexpressor and knockout mice. Eur Neuropsychopharmacol. 2010 Sep;20 doi: 10.1016/j.euroneuro.2010.08.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy EA, Scott AC, Fiskerstrand CE, Bubb VJ, Quinn JP. The serotonin transporter intronic VNTR enhancer correlated with a pre-disposition to affective disorders has distinct regulatory elements within the domain based on the primary DNA sequence of the repeat unit. Eur J Neurosci. 2003;17:417–420. doi: 10.1046/j.1460-9568.2003.02446.x. [DOI] [PubMed] [Google Scholar]
- Luellen BA, Gillman TL, Andrews AM. Presynaptic adaptive responses to constitutive versus adult pharmacologic inhibition of serotonin uptake. In: Kalueff AV, LaPorte JL, editors. Experimental Models in Serotonin Transporter Research. Cambridge: Cambridge University Press; 2010. pp. 1–42. [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A, Quinn J. A serotonin transporter gene intron 2 polymorphic region, correlated with affective disorders, has allele-dependent differential enhancer-like properties in the mouse embryo. Proc Natl Acad Sci U S A. 1999;96 doi: 10.1073/pnas.96.26.15251. 15251-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–2155. doi: 10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- Macri S, Granstrem O, Shumilina M, dos Santos FJAG, Berry A, Saso L, Laviola G. Resilience and vulnerability are dose-dependently related to neonatal stressors in mice. Horm Behav. 2009;56:391–398. doi: 10.1016/j.yhbeh.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–597. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheimer S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94:1263–1270. doi: 10.1161/01.RES.0000126847.27660.69. [DOI] [PubMed] [Google Scholar]
- Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, Wray SP, Gerhardt B, Lanthorn TH, Savelieva KV. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur J Pharmacol. 2009;602:306–315. doi: 10.1016/j.ejphar.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Maswood S, Truitt W, Hotema M, Caldarola-Pastuszka M, Uphouse L. Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: Role of the serotonin transporter and terminal autoreceptors. Brain Res. 1999;831:146–154. doi: 10.1016/s0006-8993(99)01439-0. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Meth. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Coates MD, Moses PL. Intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. J Clin Psychiatry. 2005;66 Suppl 10:9–18. [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204 Suppl:E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008;1189:203–214. doi: 10.1016/j.brainres.2007.10.063. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Sumner BE, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in mae rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63:241–247. doi: 10.1016/s0169-328x(98)00281-2. [DOI] [PubMed] [Google Scholar]
- Medina-Aguirre I, Gutiérrez-Ospina G, Hernández-Rodríguez J, Boyzo A, Manjarrez-Gutiérrez G. Development of 5-HT(1B), SERT and thalamo-cortical afferents in early nutrionally restricted rats: an emerging explanation for delayed barrel formation. Int J Dev Neurosci. 2008;26:225–231. doi: 10.1016/j.ijdevneu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, Seif I, Benhaiem-Sigaux N, Kirsch M, Hamom M, Adnot S, Eddahibi S. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. 2006;113:81–89. doi: 10.1161/CIRCULATIONAHA.105.554667. [DOI] [PubMed] [Google Scholar]
- Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Nielesen EO, Troelsen KB. Serotonin transporter density and anxiolytic-like effects of antidepressants in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:858–866. doi: 10.1016/j.pnpbp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Bronzino JD, Galler JR, Morgane PJ. The effects of median raphé electrical stimulation on serotonin release in the dorsal hippocampal formation of prenatally protein malnourished rats. Brain Res. 1999;838:95–103. doi: 10.1016/s0006-8993(99)01677-7. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Galler JR, Morgane PJ. Modulation of 5-HT release in the hippocampus of 30-day-old rats exposed in utero to protein malnutrition. Brain Res Dev Brain Res. 2003;142:203–208. doi: 10.1016/s0165-3806(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Molteni R, Cattaneo A, Calabrese F, Macchi F, Olivier JD, Racagni G, Ellenbroek BA, Gennarelli M, Riva MA. Reduced function of the serotonin transporter is associated with decreased expression of BDNF in rodents as well as humans. Neurobiol Dis. 2010;37:747–755. doi: 10.1016/j.nbd.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Monassier L, Laplante MA, Ayadi T, Doly S, Maroteaux L. Contribution of gene-modified mice and rats to our understanding of the cardiovascular pharmacology of serotonin. Pharmacol Ther. 2010 doi: 10.1016/j.pharmthera.2010.08.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Kristensen AS, Rudnick G, Wiborg O. Molecular cloning, expression and characterization of a bovine serum serotonin transporter. Mol Brain Res. 1999;71:120–126. doi: 10.1016/s0169-328x(99)00178-3. [DOI] [PubMed] [Google Scholar]
- Mössner R, Heils A, Stöber G, Okladnova O, Daniel S, Lesch KP. Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem Int. 1998;33:251–254. doi: 10.1016/s0197-0186(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Am J Med Genet C Semin Med Genet. 2006;142C:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D'Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulla H. Understanding developmental pharmacodynamics: importance for drug development and clinical practice. Paediatr Drugs. 2010;12:223–233. doi: 10.2165/11319220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: Gene, genetics disorders, and pharmacogenetics. Mol Intervent. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being re-written by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows 10 novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Narboux-Nême N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs) Neuropharmacology. 2008;55:994–1005. doi: 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Thompson J, Northcott C, Lookingland K, Watts SW. The serotonin transporter is present and functional in peripheral atrial smooth muscle. J Cardiovasc Pharmacol. 2004;43:770–781. doi: 10.1097/00005344-200406000-00006. [DOI] [PubMed] [Google Scholar]
- Ni W, Watts SW. 5-Hydroxytryptamine in the cardiovascular system: Focus on the serotonin transporter (SERT) Clin Exp Pharmacol Physiol. 2006;33:575–583. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, Ververs FF, Nikkels PG, van Echteld CJ, Visser GH, Smidt MP. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS One. 2008;3:e2782. doi: 10.1371/journal.pone.0002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey-White J, Holmes A. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology. 2008;200:413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskova T, Pivac N, Nedic G, Kazantseva A, Gaysina D, Faskhutdinova G, Gareeva A, Khalilova Z, Khusnutdinova E, Kozaric Kovacic D, Kovacic Z, Jokic M, Muck Seler D. Ethnic differences in the serotonin transporter polymorphism (5-HTTLPR) in several European populations. Prog Neuropsychopharmacol & Biol Psychiat. 2008;32:1735–1739. doi: 10.1016/j.pnpbp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- O'Brien L, Einarson TR, Sarkar M, Einarson A, Koren G. Does paroxetine cause cardiac malformations? J Obstet Gynaecol Can. 2008;30:696–701. doi: 10.1016/S1701-2163(16)32918-8. [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–2207. doi: 10.1038/npp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Marcus S, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: A case-control study. Arch Gen Psychiatry. 2006;63:865–872. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: A review. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.09.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Olivier JD, Van der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Ordway GA. Searching for the chicken’s egg in transporter gene polymorphisms. Arch Gen Psychiatry. 2000;57:739–740. doi: 10.1001/archpsyc.57.8.739. [DOI] [PubMed] [Google Scholar]
- Ormsbee HS, III, Fondacaro JD. Action of serotonin on the gastrointestinal tract. Proc Soc Exp Biol Med. 1985;178:333–338. doi: 10.3181/00379727-178-42016. [DOI] [PubMed] [Google Scholar]
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: The Heart and Soul Study. Am J Psychiatry. 2007;164:1379–1384. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Gembom E, Peng W, Lesch KP, Mossner R, Simantov R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Brain Res Dev Brain Res. 2001;126:125–129. doi: 10.1016/s0165-3806(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci USA. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Ogden RT, Van Heetum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:8–11. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Pœdiatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Pavone LM, Mithbaokar P, Mastellone V, Lo Muto R, Spina A, Maharajan V, Paino G, Avallone L. Expression of the serotonin transporter (SERT) gene during mouse development. Vet Res Commun. 2008;32 Suppl 1:S167–S169. doi: 10.1007/s11259-008-9109-z. [DOI] [PubMed] [Google Scholar]
- Pechnick RN, Breese CJ, Manalo CM, Poland RE. Comparison of the effects of desmethylimipramine on behavior in the forced swim test in peripubertal and adult rats. Behav Pharmacol. 2008;19:81–84. doi: 10.1097/FBP.0b013e3282f3d07f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: Population based cohort study. Br Med J. 2009;339:b3569. doi: 10.1136/bmj.b3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert R, Madan A, Andersen A, Cadoret R, Packer H, Sandhu H. Serotonin transporter mRNA levels are associated with the methylation of an upstream CpG island. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:101–105. doi: 10.1002/ajmg.b.30414. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P, Dennis T, de Montigny C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci. 1994;14:3036–3047. doi: 10.1523/JNEUROSCI.14-05-03036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, Neumeister A, Praschak-Rieder N, Angelberger P, Brücke T. Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med. 2000;41:36–44. [PubMed] [Google Scholar]
- Popa D, Léna C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pôrto LC, Sardinha FL, Telles MM, Guimarães RB, Albuquerque KT, Andrade IS, Oyama LM, Nascimento CM, Santos OF, Ribeiro EB. Impairment of the serotonergic control of feeding in adult female rats exposed to intra-uterine malnutrition. Br J Nutr. 2009;101:1255–1261. doi: 10.1017/S0007114508061503. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Zhu C-B, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond B Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C]DASB positron emission tomography study. Biol Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry. 2008;65:1072–1078. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- Pruess UW, Soyka M, Bahlmann M, Wenzel K, Behrens S, de Jonge S, Krüger M, Bondy B. Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationship to impulsivity. Psychiatry Res. 2000;96:51–61. doi: 10.1016/s0165-1781(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Sheeladevi R, Namasivayam A. Regulation of protein kinases and coregulatory interplay of S-100beta and serotonin transporter on serotonin levels in diabetic rat brain. J Neurosci Res. 2009;87:246–259. doi: 10.1002/jnr.21833. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: Molecular cloning, expression, and chromosomal location. Proc Natl Acad Sci USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Ramamoorthy JD, Prasad PD, Bhat GK, Mahesh VB, Leibach FH, Ganapathy V. Regulation of the human serotonin transporter by interleukin-1 beta. Biochem Biophys Res Commun. 1995;216:560–567. doi: 10.1006/bbrc.1995.2659. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi D. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- Rausch JL. Initial conditions of psychotropic drug response: Studies of serotonin transporter long promoter region (5-HTTLPR), serotonin transporter efficiency, cytokine and kinase gene expression relevant to depression and antidepressant outcome. Prog Neuropsychopharmacol Biol Psychiatr. 2005;29:1046–1061. doi: 10.1016/j.pnpbp.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Reed AL, Happe HK, Petty F, Bylund DB. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology. 2008;197:433–441. doi: 10.1007/s00213-007-1052-0. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Hu XZ, Goldman D, Reischl G, Solbach C, Machulla HJ, Bares R, Heinz A. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang SJ, Tolliver T, Lesch KP, Lu B, Murphy DL. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res. 2005;79:756–771. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- Resnick O, Morganem PJ. Generational effects of protein malnutrition in the rat. Brain Res. 1984;317:219–227. doi: 10.1016/0165-3806(84)90099-3. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner V, Manzke T, Becker A, Rothenberger A, Bock N. Development of 5-HT transporter density and long-term effects of methylphenidate in an animal model of ADHD. World J Biol Psychiatry. 2009;10:581–585. doi: 10.1080/15622970802653709. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Burke TF, McCasland M, Hensler JG. Serotonin-1A receptor function in the dorsal raphe nucleus following chronic administration of the selective serotonin reuptake inhibitor sertraline. J Neurochem. 2008;105:1091–1099. doi: 10.1111/j.1471-4159.2007.05201.x. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. J Biol Chem. 1977;252:2170–2174. [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: The reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Booij J, Reitsma JB, Schene AH. Serotonin transporter binding with [I-125]beta-CIT SPECT in major depressive disorder versus controls: Effect of season and gender. Eur J Nucl Med Mol Imaging. 2009;36:841–849. doi: 10.1007/s00259-008-1057-x. [DOI] [PubMed] [Google Scholar]
- Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Reichert J, Hoffman EJ, Cai G, Jones HB, Faham M, Buxbaum JD. A large-scale screen for coding variants in SERT/SLC6A4 in autism spectrum disorders. Autism Res. 2008;1:251–257. doi: 10.1002/aur.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-HT transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample SJ, Behan M, Smith L, Oldenhoff WE, Markel MD, Kalscheur VL, Hao Z, Miletic V, Muir P. Functional adaptation to loading of a single bone is neuronally regulated and involves multiple bones. J Bone Min Res. 2008;23:1372–1381. doi: 10.1359/jbmr.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthis LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: Evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;5:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Santangelo SL, Tsatsanis K. What is known about autism: genes, brain, and behavior. Am J Pharmacogenomics. 2005;5:71–92. doi: 10.2165/00129785-200505020-00001. [DOI] [PubMed] [Google Scholar]
- Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Serotonin and its transporter on proliferation of fetal heart cells. Int J Dev Neurosci. 2003;21(8):417–424. doi: 10.1016/j.ijdevneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Patrick AR, Solomon DH, Dormuth CR, Miller M, Mehta J, Lee JC, Wang PS. Comparative safety of antidepressant agents for children and adolescents regarding suicidal acts. Pediatrics. 2010;25:876–888. doi: 10.1542/peds.2009-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter S, Levy AL, Blakely RD. Polarized expression of antidepressant sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol Cell Neurosci. 1997;9:170–184. doi: 10.1006/mcne.1997.0619. [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Mandelli L, De Ronchi D. Serotonin transporter gene variants and behavior: A comprehensive review. Curr Drug Targets. 2006;7:1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Shen H-W, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch K-P, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, Nankai M, Kanba S. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: A review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Sidor MM, Amath A, Macqueen G, Foster JA. A developmental characterization of mesolimbocortical serotonergic gene expression changes following early immune challenge. Neuroscience epub. 2010 doi: 10.1016/j.neuroscience.2010.08.060. doi: 10.1016/j.neuroscience.2010.08.060. [DOI] [PubMed] [Google Scholar]
- Singh YS, Sawarynski LE, Michael HM, Ferrell RE, Murphey-Corb MA, Swain GM, Patel BA, Andrews AM. Boron-doped diamond microelectrodes reveal reduced serotonin uptake rates in lymphocytes from adult rhesus monkeys carrying the short allele of the 5-HTTLPR. ACS Chem Neurosci. 2010;1:49–64. doi: 10.1021/cn900012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental eurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Barnes GA, McCook EC, Seidler FJ. Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Brain Res Dev Brain Res. 1996a;93:155–161. doi: 10.1016/0165-3806(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, McCook EC, Ritchie JC, Seidler FJ. Do glucocorticoids contributeto the abnormalities in serotonin transporter expression and function seen in depression? An animal model. Biol Psychiatry. 1996b;40:576–584. doi: 10.1016/0006-3223(95)00469-6. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2006;31:904–911. doi: 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at six months of age. Neuropsychopharmacology. 2007a;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Seidler FJ. Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res Bull. 2007b;74:91–103. doi: 10.1016/j.brainresbull.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: Interactions of fetal nicotine and dexamethasone on serotonin and dopamine synaptic function in adolescence and adulthood. Brain Res Bull. 2010;82:124–134. doi: 10.1016/j.brainresbull.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Soler-Soler J, Galve E. Worldwide perspective of valve disease. Heart. 2000;83:721–725. doi: 10.1136/heart.83.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lac-z expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Soumi S. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann NY Acad Sci. 2006;1994:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Schwandt M, Lindell S, Newman TK, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol. 2007;19:977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: Evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA, Little KY. Serotonin transporter promoter polymorphism, peripheral indexes of serotonin function, and personality measures in families with alcoholism. Am J Med Genet. 2002;114:230–234. doi: 10.1002/ajmg.10187. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Raloxifene blocks estradiol induction of the serotonin transporter and 5-hydroxytryptamine2A receptor in female rat brain. Neurosci Lett. 2007;417:95–99. doi: 10.1016/j.neulet.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes PA, Condron BG. Development and sensitivity to serotonin of Drosophila serotonergic varicosities in the central nervous system. Dev Biol. 2005;286:207–216. doi: 10.1016/j.ydbio.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, Carsalade V, Cohen DJ, Ferrari P, Roubertoux PL, Anderson GM. Role of the serotonin transporter gene in the behavioral expression of autism. Mol Psychiatry. 2001;6:434–439. doi: 10.1038/sj.mp.4000873. [DOI] [PubMed] [Google Scholar]
- Tuccori M, Testi A, Antonioli L, Fornai M, Montagnani S, Ghisu N, Colucci R, Corona T, Blandizzi C, Del Tacca M. Safety concerns associated with the use of serotonin reuptake inhibitors and other serotonergic/noradrenergic antidepressants during pregnancy: a review. Clin Ther. 2009;31:1426–1453. doi: 10.1016/j.clinthera.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central serotonin transporter availability measured with [123I]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry. 2004;161:525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: A functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with irritable bowel syndrome. Ailment Pharmcol Ther. 2007;26:979–986. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009;11:111–117. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- Vartazarmian R, Malik S, Baker GB, Boksa P. Long-term effects of fluoxetine or vehicle administration during pregnancy on behavioral outcomes in guinea pig offspring. Psychopharmacology. 2005;178:328–338. doi: 10.1007/s00213-004-2003-7. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Jessen TN, Thompson BJ, Carter M, Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord. 2009;1:158–171. doi: 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne DE, Nemeroff CB. The interaction of serotonin transporter gene polymorphisms and early adverse life events on vulnerability for major depression. Curr Psychiatry Rep. 2006;8:452–457. doi: 10.1007/s11920-006-0050-y. [DOI] [PubMed] [Google Scholar]
- Verney C. Phenotypic expression of monoamines and GABA in the early development of human telencephalon, transient or not transient. J Chem Neuroanat. 2003;26:283–292. doi: 10.1016/j.jchemneu.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32 Suppl 1:S16–S20. doi: 10.1016/j.psyneuen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Swedo S. Antidepressant medications in children. N Engl J Med. 2004;350:1489–1491. doi: 10.1056/NEJMp038248. [DOI] [PubMed] [Google Scholar]
- Vogel C, Mossner R, Gerlach M, Heinemann T, Murphy DL, Riederer P, Lesch KP, Sommer C. Absence of thermal hyperalgesia in serotonin transporter-deficient mice. J Neurosci. 2003;23:708–715. doi: 10.1523/JNEUROSCI.23-02-00708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanatabe Y, Sakai RR, McEwen BS, Mendelsen S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, Kim CE, Hou C, Frackelton E, Chiavacci R, Takahashi N, Sakurai T, Rappaport E, Lajonchere CM, Munson J, Estes A, Korvatska O, Piven J, Sonnenblick LI, Alvarez Retuerto AI, Herman EI, Dong H, Hutman T, Sigman M, Ozonoff S, Klin A, Owley T, Sweeney JA, Brune CW, Cantor RM, Bernier R, Gilbert JR, Cuccaro ML, McMahon WM, Miller J, State MW, Wassink TH, Coon H, Levy SE, Schultz RT, Nurnberger JI, Haines JL, Sutcliffe JS, Cook EH, Minshew NJ, Buxbaum JD, Dawson G, Grant SF, Geschwind DH, Pericak-Vance MA, Schellenberg GD, Hakonarson H. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden SJ, Bliziotes MM, Wiren KM, Eshelman AJ, Turner CH. Neural regulation of bone and the skeletal effect of serotonin (5-hydroxytryptamine) Mol Cell Endocrinol. 2005;242:1–9. doi: 10.1016/j.mce.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wei L, David A, Duman RS, Anisman H, Kaffman A. Early life stress increases anxiety-like behavior in Balbc mice despite a compensatory increase in levels of postnatal maternal care. Horm Behav. 2010;57:396–404. doi: 10.1016/j.yhbeh.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC64A, with reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive compulsive disorder. Hum Mol Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- West L, Waldrop J, Brunssen S. Pharmacologic treatment for the core deficits and associated symptoms of autism in children. J Pediatr Health Care. 2009;23:75–89. doi: 10.1016/j.pedhc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity in brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Wihlbäck AC, Sundström Poromaa PI, Bixo M, Allard P, Mjörndal T, Spigset O. Influence of menstrual cycle on platelet serotonin uptake site and serotonin 2A receptor binding. Psychoneuroendocrinology. 2004;29:757–766. doi: 10.1016/S0306-4530(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S, Tauscher J, Fuchs K, Sieghart W, Hornik K, Aschauer HN, Brücke T, Kasper S. No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biol Psychiatry. 2001;50:8–12. doi: 10.1016/s0006-3223(00)01123-9. [DOI] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: A review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. Neuroimage. 2010;53:878–892. doi: 10.1016/j.neuroimage.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Williams RB. Psychosocial and biobehavioral factors and their interplay in coronary heart disease. Annu Rev Clin Psychol. 2008;4:349–465. doi: 10.1146/annurev.clinpsy.4.022007.141237. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, Edgar DM. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. NeuroReport. 2003;14:233–238. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- Yang GB, Qiu CL, Aye P, Shao Y, Lackner AA. Expression of serotonin transporters by peripheral blood mononuclear cells of rhesus monkeys (Macaca mulatta) Cell Immunol. 2007;248:69–76. doi: 10.1016/j.cellimm.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Yang LP, Scott LJ. Escitalopram: in the treatment of major depressive disorder in adolescent patients. Paediatr Drugs. 2010;12:155–163.. doi: 10.2165/11204340-000000000-00000. Erratum in: Paediatr Drugs 12, 163. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Kargieman L, Gregoretti L, Cardinali DP. Effects of neonatal clomipramine treatment on locomotor activity, anxiety-related behavior and serotonin turnover in Syrianhamsters. Neuropsychobiology. 1999;39:200–206. doi: 10.1159/000026584. [DOI] [PubMed] [Google Scholar]
- Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology. 1993;47:573–584. doi: 10.1002/tera.1420470609. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Lesch K-P, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–119. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]
- Zhu C-B, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- Zhu C-B, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu C-B, Hewlett WA, Blakely RD. The proinflammatory cytokines interleukine-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu C-B, Steiner JA, Munn JL, Daws LC, Hewlett WA, Blakely RD. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J Pharmacol Exp Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]
- Zhu C-B, Lindler KM, Owens WA, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010 Sept 8; doi: 10.1038/npp.2010.116. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SP, Mao ZF, Huang J, Wang JY. Continuous fluoxetine administration prevents recurrence of pulmonary arterial hypertension and prolongs survival in rats. Clin Exp Pharmacol Physiol. 2009;36:e1–e5. doi: 10.1111/j.1440-1681.2009.05181.x. [DOI] [PubMed] [Google Scholar]


