Estrogen Receptor Activation Reduces Stroke Risk and Injury
Gender differences showing a lower prevalence and better outcome after ischemic stroke in women have been described, differences that are abrogated by natural or surgical menopause. 1,2 High levels of endogenous estrogens in premenopausal women have been associated with reduced risk for a number of diseases, such as hypertension, diabetes, obesity, vascular disease, and stroke.2 The growing number of postmenopausal women due to shifts in world demographics also requires special action for the prevention and treatment of these conditions.2 Clinical and preclinical studies indicate that natural estrogens such as 17β-estradiol exerts profound protective effects in the adult and the aging brain.1,3 Three proteins have been identified to mediate the effects of estrogens: ERα, ERβ, and GPER.2,4 While expression and function of ERα and ERβ, have been well studied under physiological conditions, information about their function and expression under disease conditions – particularly in stroke 1 - is still scarce.2,4
Interactions Between Estrogen and the Renin-Angiotensin System
Angiotensin II is an important regulator of kidney function, inflammation, vascular tone and thus cerebral perfusion.5,6 Estrogen inhibits the activity or expression of different components of the renin angiotensin system such as ACE, angiotensin II, or angiotensin AT1 receptors; 6,7 Conversely, cessation of estrogen production after menopause activates the renin-angiotensin system.6 Previous studies indirectly suggested that the AT1 receptor, the predominant cellular target of angiotensin II, interacts with the function of estrogen receptors. Using a model of surgical menopause, Chappell et al. found that the the AT1 antagonist olmesartan is as effective as 17β-estradiol to suppress hypertension due to estrogen deficiency.8 Also, Tsuda et al. reported that in mice with atherosclerosis neither low-dose 17β-estradiol nor olmesartan had an effects on its own on atherosclerotic lesion formation; however, in combination lesion formation was almost completely suppressed.7 In addition, the AT1 antagonist losartan exerts central effects on thirst and sodium appetite in rats which is inhibited by estrogen.9 Collectively, these findings indirectly suggested that both, the renin-angiotensin system and estrogen-estrogen receptor signaling might share and/or amplify common modes of action that might be relevant for pathologies such as post-menopausal hypertension, or its consequences including myocardial infarction and stroke.
Estrogen-Independent Effects on Estrogen Receptor Signaling
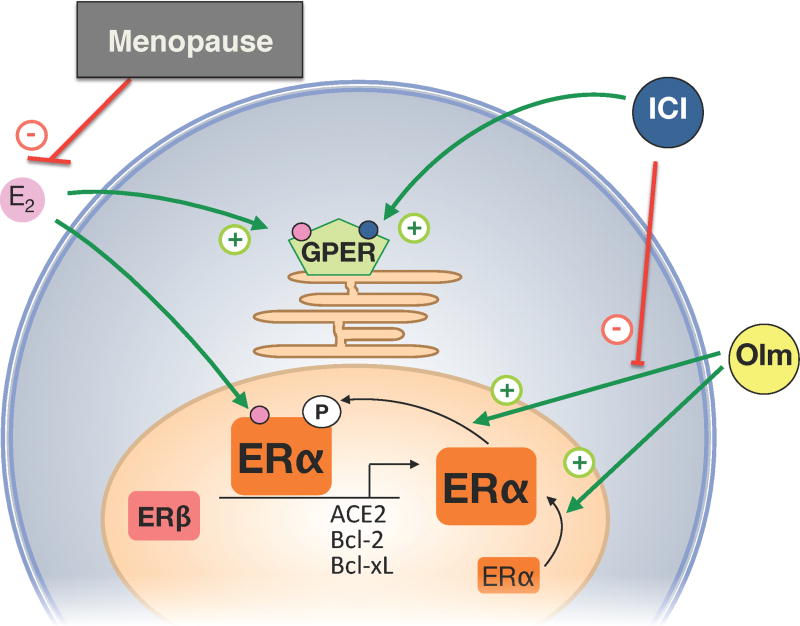
In the present issue of Hypertension, Shimada et al. have now taken this issue a step further.10 They assessed directly - using a model of surgical menopause - the potential involvement of estrogen receptors in the protective effects of the AT1 antagonist olmesartan on cerebral infarct size and the cellular changes associated with it. Besides, this comprehensive study reports several novel findings on the role of the brain renin-angiotensin system and regulation of estrogen receptors following ischemic stroke. The investigators found that in the brain, ACE2 is expressed at higher levels than ACE1, and that all three estrogen receptors, ERα, ERβ, and GPER are dectectable. Ovariectomy had very distinct effects on stroke-induced changes: it increased infarct size and cerebral angiotensin II and AT1 receptor expression, but reduced expression of ERα, ACE2, and the AT2 receptor. On the other hand, the expression levels of ACE1, or ERβ or GPER remained unaffected by menopause or stroke. These findings are important since they demonstrate that neither components of the RAS nor estrogen receptors are regulated uniformly under the same conditions (i.e. physiological processes such as menopause, or diseases such as ischemic stroke). The most important findings of the study by Shimada et al. 10 was that olmesartan treatment not only reduced infarct size, but that these effects of olmesartan were estrogen receptor-dependent, i.e. olmesartan increased ERα in the peri-infarct zone, an effect that was blocked by the ER-antagonist ICI 182780 (Figure). Expression of ERβ and GPER remained unaffected by stroke or olmesartan treatment. Having discovered this new and important effects of a non-estrogenic drug on ERα signaling, the investigators went on to study whether olmesartan regulates ERα function, and found that olmesartan stimulates both expression and phosphorylation of ERα, but only its phosphorylation was sensitive to ERα blockade by ICI182,780. Given the previous observations of interactions between the RAS and estrogen,7–9 these important data are the first to demonstrate a direct interaction between an AT1 antagonist and ERα, compatible with the concept that ERα-dependent activation, molecular regulation, and organ protection may occur even in the absence of estrogen.
Figure 1.
Estrogen receptor-dependent protective effects of olmesartan in ischemia-induced brain injury in estrogen (E2)-deficient states such as menopause (natural or following ovariectomy). The angiotensin AT1 receptor antagonist olmesartan (Olm) increases expression and posphorylation of ERα. This results in up-regulation of the ACE2, Bcl-2 and Bcl-xL genes in an E2-independent manner, an effect that is blocked by the ERα antagonist ICI 182,780 (ICI). ICI also acts as an agonist of GPER. +, activation; -, inhibition.
Implications and Perspectives
The findings presented by Shimada et al.10 leave us with several questions. First, and perhaps most important, does activation of ERα by an AT1 antagonist represent a class effect or is it it simply due to the structural properties of this particular drug? Functional similarities with estrogen have been previously reported for other vasoprotective drugs such as the β1 receptor-antagonist nebivolol.11 Second, olmesartan attenuates atherosclerosis progression,12 , a disease sensitive to ERα-activation;13 thus, the question remains how much of these olmesartan effects are mediated through ERα. In addition, novel estrogen receptors such as GPER may also affect olmesartan’s cellular target.14 Indeed, olmesartan was recently shown to reduce intimal angiogenesis12, an effect that could be explained through anti-angiogenic action of GPER activation.15 Finally, and most important, is the question of whether ERα-dependent effects of olmesartan are present and required for olmesartan’s effects in patients. Although olmesartan potentiates the anti-hypertensive effect of 17β-estradiol in postmenopausal women,16 most recently the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trial reported excess cardiovascular events and cardiovascular deaths in diabetics with cardiovascular disease.17 It can only be speculated whether the increased risk involved ERα-dependent effects of olmesartan. Also, whether the increased risk attributed to olmesartan17 is equally present in men and women is not known since no gender-dependent subanalysis of this study is available. In any case, the work presented by Shimada et al.10 provides surprising and important new pieces to the puzzle how estrogen receptors and the renin angiotensin system interact. Whether olmesartan (or other ARBs) also causes ERα activation in humans or in diseases distinct from ischemic stroke should be addressed in future studies.
Acknowledgments
Sources of Funding
Supported by Swiss National Science Foundations grants 108258 and 122504 (to M.B) and PBZHP3-135874 (to M.R.M.), and NIH grants CA116662, CA18743, and CA12773 (to E.R.P.).
Footnotes
Conflicts of Interest
None
References
- 1.Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women's Health Initiative. Endocr Rev. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- 2.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 3.Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol Reprod. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- 4.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 5.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17:2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 6.Shenoy V, Grobe JL, Qi Y, Ferreira AJ, Fraga-Silva RA, Collamat G, Bruce E, Katovich MJ. 17beta-estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides. 2009;30:2309–2315. doi: 10.1016/j.peptides.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda M, Iwai M, Li JM, Li HS, Min LJ, Ide A, Okumura M, Suzuki J, Mogi M, Suzuki H, Horiuchi M. Inhibitory effects of AT1 receptor blocker, olmesartan, and estrogen on atherosclerosis via anti-oxidative stress. Hypertension. 2005;45:545–551. doi: 10.1161/01.HYP.0000157409.88971.fc. [DOI] [PubMed] [Google Scholar]
- 8.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2. Lewis rat. Hypertension. 2003;42:781–786. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- 9.Mecawi AS, Lepletier A, Araujo IG, Fonseca FV, Reis LC. Oestrogenic influence on brain AT1 receptor signalling on the thirst and sodium appetite in osmotically stimulated and sodium-depleted female rats. Exp Physiol. 2008;93:1002–1010. doi: 10.1113/expphysiol.2008.042713. [DOI] [PubMed] [Google Scholar]
- 10.Shimada K, Kitazato KT, Kinouchi T, Yagi K, Tada Y, Satomi J, Kageji T, Nagahiro S. Activation of estrogen receptor α and of ACE2 suppresses ischemic brain damage in oophorectomized rats. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.167650. In press. [DOI] [PubMed] [Google Scholar]
- 11.Garban HJ, Buga GM, Ignarro LJ. Estrogen receptor-mediated vascular responsiveness to nebivolol: a novel endothelium-related mechanism of therapeutic vasorelaxation. J Cardiovasc Pharmacol. 2004;43:638–644. doi: 10.1097/00005344-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cheng XW, Song H, Sasaki T, Hu L, Inoue A, Bando YK, Shi GP, Kuzuya M, Okumura K, Murohara T. Angiotensin type 1 receptor blocker reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.168385. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol's atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey SH, Bhat M, Aileru A, Chappell M. GPR30 attenuates AT1 receptor expression in rat mesenteric smooth muscle cells. FASEB J. 2011;25:1088.1088. [Google Scholar]
- 15.Holm A, Baldetorp B, Olde B, Leeb-Lundberg LM, Nilsson BO. The GPER1 agonist G-1 attenuates endothelial cell proliferation by inhibiting DNA synthesis and accumulating cells in the S and G2 phases of the cell cycle. J Vasc Res. 2011;48:327–335. doi: 10.1159/000322578. [DOI] [PubMed] [Google Scholar]
- 16.Mirza FS, Ong P, Collins P, Okamura K, Gerhard-Herman M, Williams GH, Seely EW. Effects of estradiol and the angiotensin II receptor blocker irbesartan on vascular function in postmenopausal women. Menopause. 2008;15:44–50. doi: 10.1097/gme.0b013e318150d13e. [DOI] [PubMed] [Google Scholar]
- 17.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]