Abstract
Virus infection can inflict significant damage on cardiomyocytes through direct injury and secondary immune reactions, leading to myocarditis and dilated cardiomyopathy. While viral myocarditis or cardiomyopathy is a complication of systemic infection of cardiotropic viruses, most individuals infected with the viruses do not develop significant cardiac disease. However, some individuals proceed to develop severe virus-mediated heart disease. Recent studies have shown that viral infection of cardiomyocytes is required for the development of myocarditis and subsequent cardiomyopathy. This suggests that viral infection of cardiomyocytes can be an important step that determines the pathogenesis of viral myocarditis during systemic infection. Accordingly, this article focuses on potential defense mechanisms within the cardiomyocyte against virus infection. Understanding of the cardiomyocyte defense against invading viruses may give us novel insights into the pathophysiology of viral myocarditis, and enable us to develop innovative strategies of diagnosis and treatment for this challenging clinical entity.
Keywords: apoptosis, autophagy, cardiomyopathy, coxsackievirus B3, CVB3, myocarditis, innate immunity, interferon, proteasome, RIG-I-like receptor, SOCS, suppressor of cytokine signaling, Toll-like receptor
Virus infection of the heart can cause severe damage to cardiomyocytes through multiple mechanisms. Direct virus-mediated injury and secondary immune reactions, including inflammatory and autoimmune responses, have been reported both in animal models and in humans [1,2]. Since viral infection of the heart is almost exclusively accompanied by histological inflammation of heart muscles, ‘viral myocarditis’ has become standard terminology to express virus-mediated cardiomyopathy.
The epidemiology of viral myocarditis is poorly understood because of its wide spectrum of clinical presentations including asymptomatic cases and a lack of non-invasive markers for diagnosis [3,4]. The Dallas criteria, a histopathological criteria requiring endomyocardial biopsy, has been used as a gold standard for diagnosis; however, the sensitivity of diagnosis for viral myocarditis is not sufficient to warrant the use of invasive procedures as a routine [5]. In a patient population diagnosed as having myocarditis based on multiple criteria including clinical and histopathological observations, the 5-year survival rate has been reported to be 56–70% [6,7]. In contrast, the long-term survival rate of patients with fulminant myocarditis is surprisingly high (93% at 11 years) once they are successfully treated for fatal outcomes. These data imply that fulminant myocarditis is not merely a severe form of acute myocarditis [7]. In patients with dilated cardiomyopathy (DCM), various viral genomes can be identified in endomyocardial biopsy samples [8,9]. However, the impact of the presence of viral genomes on cardiac function and clinical outcome in humans is still controversial [10,11]. To date, despite evidence that viral myocarditis can lead to cardiomyopathy and heart failure [7,12], it is still unclear as to what percentage of idiopathic DCMs is developed from viral myocarditis.
A variety of viruses have been identified in patients with myocarditis by serological analysis, PCR, immunohistochemistry, in situ hybridization and electron microscopy. Among the various cardiotropic viruses, adenovirus and enterovirus have long been considered the most important viruses leading to myocarditis [13]. In a recent US multicenter analysis of histologically identified myocarditis, adenovirus, enterovirus and cytomegalovirus were the viruses most commonly identified by PCR from endomyocardial biopsy samples [8]. In studies performed in Germany, a high prevalence of parvovirus B19 has been found in patients with a history of clinically suspected myocarditis or idiopathic DCM [9,11,14], while parvovirus B19 has also been identified in patients who do not have myocarditis [15–17]. The geographical difference in viruses identified from endomyocardial biopsy samples suggests the need for future worldwide multicenter studies with consistent inclusion criteria to verify these results. In spite of the involvement of many viruses, the cellular and molecular mechanisms of viral myocarditis have been most thoroughly investigated in murine models with coxsackievirus B3 (CVB3) infection. Therefore, the mechanistic experiments described in this article focus primarily, but not exclusively, on CVB3-infected murine models. It is important to note that CVB3 are members of the Picornaviridae family, genus enterovirus.
Pathogenesis of CVB3 myocarditis
It has been presumed that CVB3 infection can lead to viral myocarditis and subsequent cardiomyopathy through three phases; acute, subacute and chronic [18,19]. In the acute phase, direct injury of cardiomyocytes due to the virus infection can be observed with minimal mono-nuclear cell infiltration. In response to viral infection, innate immune cells such as dendritic cells (DCs), natural killer (NK) cells and macrophages migrate into the heart and limit viral propagation until the specific, adaptive immunity can respond. The critical protective role of NK cells against viral myocarditis has been confirmed in studies using NK-cell-depleted mice [20]. Once the virus has been recognized by innate antigen-presenting cells such as DCs, the fragmented viral antigens are presented by MHC class II with costimulatory molecules, resulting in the full activation of antigen-specific T cells. A recent study indicated that T-cell priming via CD4−/CD8+ DCs is an important factor that determines susceptibility to CVB3 myocarditis [21].
In the subacute phase, inflammatory cell infiltration composed of antigen-specific T lymphocytes becomes prominent in the heart. The CD8+ cytotoxic T cells specifically recognize the virus-infected cardiomyocytes presenting the viral antigens via MHC class I on their surface. These cells proceed to lyse infected cardiomyocytes. Antibody production against the virus also peaks during this period. Although these immune responses are crucial for clearing the virus, overly activated immune reactions through cytotoxic T lymphocytes or the appearance of autoimmunity against cardiac proteins can cause further damage to salvaged cardiomyocytes. It is generally believed that these factors (direct viral injury and host innate and adaptive immune responses) determine the severity of the acute to subacute phases of viral myocarditis.
In the chronic phase, while active viral infection and immune reactions disappear, an auto-immune response and/or low-grade inflammation of an unknown mechanism seem to last for years. This can be a detrimental factor for the development of subsequent DCM in combination with the extent of cardiac remodeling due to the loss of cardiomyocytes in the acute and subacute phases. A low level of persistent CVB3 genomes can also be found in the heart despite the absence of replication-competent virus particle in this phase [22,23]. Although a number of mechanisms have been suggested for persistent viral infection [24], the mechanism by which long-term coxsackievirus persistence occurs in the presence of an intact immune system remains unclear. While the presence of replication-incompetent CVB3 genome is sufficient to induce cardiomyopathy in a mouse model [25], the pathological effects of the viral genomes on cardiac function and clinical outcome in humans is still controversial [10,11]. Further exploration of the molecular mechanisms underlying the persistence of virus genomes in the heart and viral genome-mediated cardiomyopathy must await future study.
CVB3 infection of the heart is required for the development of myocarditis
While viral myocarditis is a complication of systemic infection of cardiotropic viruses, most individuals infected with the viruses do not develop significant cardiac disease. However, some individuals proceed to develop severe virus-mediated heart disease. Interestingly, there is no evidence that the latter individuals are more susceptible to infectious diseases, in general, than the former. It has recently been reported that infection of cardiac-specific coxsackievirus–adenovirus receptor (CAR) knockout (KO) mice with CVB3 results in few or no infections of cardiomyocytes [26,27]. Given that CAR is an established receptor for CVB3 infection, this result seems to be obvious. However, importantly, common findings such as infiltration of mononuclear cells, necrosis and fibrosis are also minimal or absent in the CAR KO heart while other organs are similarly infected with CVB3 compared with wild-type mice. These data indicate that infection of the cardiomyocyte is required for the induction of subsequent histological and functional abnormalities in the heart when systemically infected with CVB3. From the point of view of pathogenesis in viral myocarditis, these data also suggest that virus infection of the cardiomyocyte may be an important step that determines the severity of myocarditis and subsequent development of DCM. While our understanding of the pathogenesis of cardiomyopathy post-virus infection of the heart has improved, relatively little is known about factors that determine susceptibility of the cardiomyocyte to viral infection. Recent studies have indicated the presence of susceptibility factors to CVB3 infection within the cardiomyocyte using cardiac-specific transgenic mouse models [28,29]. The fact that the cardiomyocyte itself plays an important role in protecting against viral myocarditis sheds light on the previously underestimated defense mechanisms within the cardiomyocyte. Accordingly, this article focuses on the recently reported antiviral mechanisms in various host cells that may also play an important role within the cardiomyocyte. It should be noted that unlike CVB3, some viruses found in human endomyocardial biopsy samples do not always infect cardiomyocytes [30]. Thus, we do not exclude the possibility that these viruses can induce myocarditis in the absence of infection of the cardiomyocyte.
Innate immunity
The immune system of higher vertebrates is divided into two broad categories, innate and adaptive immunity. Innate immunity is a defense mechanism that mounts a counterattack immediately after a pathogen enters the body by recognizing pathogen-associated molecular patterns (PAMPs). The innate immune system is evolutionarily conserved. Moreover, it is the first line of the defense against microbial pathogens until the antigen-specific, adaptive immunity becomes fully active. In an animal model of CVB3 myocarditis using Balb/c mice, few mononuclear cell infiltrates are observed in the heart during the first 4–5 days post-viral infection, while a substantial virus infection of cardiomyocytes starts from 3 days post-infection [29]. Furthermore, it has been reported that an increase in virus-specific IgG is detectable from 7 days post-CVB3 infection in both Balb/c and C57B6 mice [31]. This indicates that innate immunity takes a major role in antiviral defense at least in the first 5–7 days post-CVB3 infection in mice.
Role of cytokines signaling within the cardiomyocyte
The expression of various cytokines and chemokines such as IL-1, IL-2, IL-6, IL-10, TNF-α, IFN-γ and CXCL10 can be detected in the heart during the acute phase of viral infection [20,32]. Given that these cytokines are elevated prior to inflammatory cell infiltration, cells intrinsic to the myocardium such as cardiomyocytes, endothelial cells and fibroblasts as well as innate immune cells are thought to play central role in their expression. While these cytokines are crucial for the recruitment and activation of immune cells, the endocrine, paracrine and autocrine effects of cytokines on cardiomyocytes also act as the first line of defense against virus infection. However, because of their modulatory effects on systemic immunity, it has been difficult to evaluate the direct effect of cytokines on cardiomyocyte susceptibility to virus infection in vivo. The crucial role of cytokine-mediated cardiac protection against CVB3 infection has been shown using cardiac-specific suppressor of cytokine signaling (SOCS)-1 and -3 transgenic mice [28,29]. SOCS family proteins are a key negative-feedback regulator of JAK and STAT signaling [33]. Many cytokines are known to activate the JAK–STAT signaling pathway, which subsequently activates the transcription of cytokine-responsive genes including SOCS family genes. The induced SOCS proteins inhibit JAK-mediated phosphorylation of the cytokine receptor by binding to JAK or the receptor, thus leading to the shutoff of subsequent activation of STAT molecules. This negative-feedback regulation via SOCS tightly regulates the duration and intensity of cytokine-induced JAK–STAT signaling. CVB3 infection is associated with activation of JAK–STAT signaling in the heart with the expression of SOCS1 and SOCS3 mRNA [29]. To understand the in vivo significance of JAK–STAT activation and SOCS expression in the heart during CVB3 infection, cardiac-specific transgenic mice that express SOCS1 or SOCS3 under the direction of α-myosin heavy chain promoter were infected with CVB3. Interestingly, the hearts of both the cardiac-specific SOCS1 and SOCS3 transgenic mice exhibit dramatically increased susceptibility to CVB3 infection compared with their wild-type littermates [28,29]. Given that the expression of SOCS1 or SOCS3 is limited to cardiomyocytes, the increased cardiac susceptibility is thought to be independent of alterations in systemic immune response to CVB3 infection. These data indicate that the activation of JAK–STAT signaling through cytokines during CVB3 infection can drive antiviral mechanisms within the cardiomyocyte and may be an important determinant of the initial infection of the heart in the very early stages of CVB3 infection. Although the nonspecific effects caused by transgenic expression of SOCS molecules need to be considered, these findings suggest that the innate mechanisms within the host cardiomyocyte could be a novel target for future diagnostic and therapeutic strategies against viral myocarditis.
Do interferons play a key role in cardiac antiviral defense?
The significant effect of JAK–STAT signaling within the cardiomyocyte against CVB3 infection raises the question of whether interferons (IFNs) play an important role in cardiac antiviral defense. This is critical given that IFNs are potent and pivotal antiviral cytokines in mammals. To date, three types of IFNs have been identified [34]; these include type I IFN (mainly α/β), type II IFN (γ) and type III IFN (λ). Of the IFNs, type I IFN is known to play central role in innate antiviral immunity. Indeed, most of the innate mechanisms sensing PAMPs eventually induce the production of type I IFNs. In innate immune cells, the plasmacytoid DC mainly produces type I IFNs in response to viral infection [35]. Interestingly, in isolated adult mouse cardiomyocytes, stimulation of type II IFN, IFN-γ, induces no antiviral effects against CVB3 infection. Conversely, type I IFN, IFN-β, significantly inhibits virus infection [28]. This suggests the importance of type I IFN signaling rather than type II IFN signaling in adult cardiac antiviral defense. The protective effect of type I IFN administration against CVB3 myocarditis has also been confirmed in Balb/c mice [36]. However, it has yet to be determined whether endogenous type I IFN production is required for limiting CVB3 infection of the heart. The expression of IFN-β mRNA becomes detectable in the heart from 3 to 4 days post-CVB3 infection [31,37]; however, it is well known that there are already significant numbers of infected cardiomyocytes at these time points. To examine the role of activation of endogenous type I IFN signaling in the heart, mice lacking type I IFN receptor were infected with CVB3. These mice showed markedly increased viral replication in the liver with a significantly earlier mortality as compared with wild-type mice. However, there was no significant increase in the viral RNA in the type I IFN receptor-deficient heart when estimated by RNA in situ hybridization. This indicates that the increased mortality of the KO mice may have nothing to do with viral infection of the heart. Furthermore, these data cast doubt on the functional contribution of endogenous type I IFN signaling against CVB3 infection in the heart [38]. Similarly, an absence of IFN-β causes a marked increase in mortality after CVB3 infection, but there is no significant increase in CVB3 titer in the IFN-β-deficient heart compared with wild-type heart [39]. These results demonstrate that type I IFNs are essential for limiting systemic viral replication; however, whether endogenous type I IFNs can efficiently prevent CVB3 from infecting and spreading in the heart remains unclear. Given the impact of viral infection of the heart on the development of myocarditis, and the potential importance of persistent infection by enteroviruses in the pathogenesis of cardiomyopathy, the innate antiviral mechanisms within the cardiomyocyte, including the effect of type I IFNs, should be thoroughly elucidated in the future.
Toll-like receptors
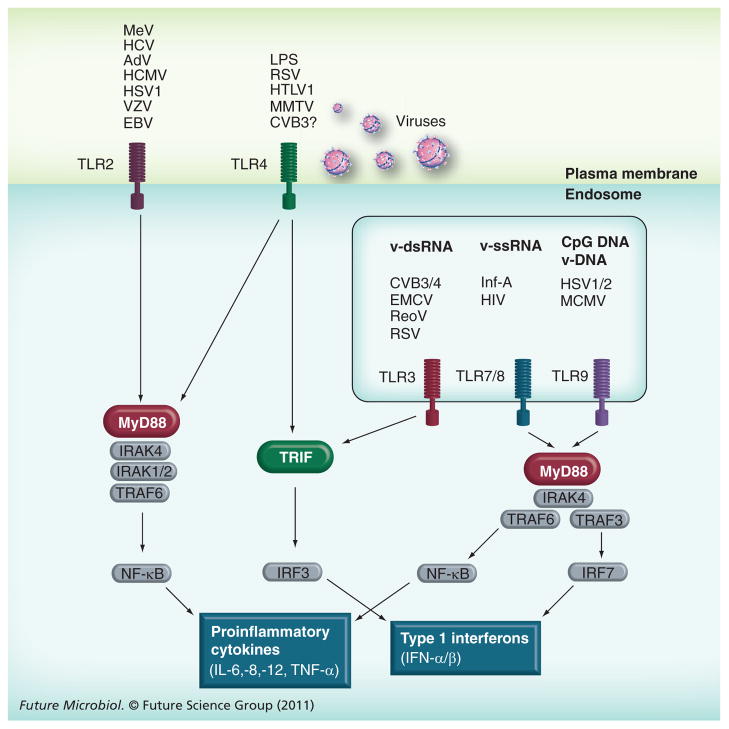
Toll-like receptors (TLRs) are a hallmark of innate immunity that activate defense mechanisms against microbial pathogens by recognizing PAMPs. To date, 10 and 13 TLRs have been identified in humans and mice, respectively [40]. Although TLRs are ubiquitously expressed at low levels in nonimmune cells, relatively high-level expression can be observed in innate immune cells such as macrophages and DCs. TLR signaling is activated by a variety of ligands that are generally associated with infectious pathogens. Of the TLRs, TLR2, TLR3, TLR4, TLR7, TLR8 and TLR9 are reported to activate antiviral effects by inducing type I IFNs (Figure 1) [30,41]. In the process of enterovirus infection of the heart, TLR3 and TLR7/8 are thought to be mainly involved in the virus sensing mechanisms since these receptors can recognize dsRNA and ssRNA, respectively. The enterovirus genome is positive ssRNA and serves as a template for the generation of negative-stranded viral RNA during replication. In this process, the formation of dsRNA composed of the positive and negative stranded RNA, termed replication intermediates, can be observed. These viral ssRNAs and dsRNAs can potentially be ligands for TLR3 and TLR7/8.
Figure 1. Toll-like receptors and their signaling against virus infection.
While TLR2 and 4 recognize viral pathogens on the plasma membrane, TLR3, 7/8 and 9 recognize viral RNA or DNA in the endosome. For the induction of type I IFNs, there are two major pathways downstream of TLRs, MyD88-dependent and TRIF-dependent pathways. Based on data from animal models, the TLR3–TRIF pathway seems to play an important role against CVB3 myocarditis. Further study will be necessary for other cardiotropic virus-mediated myocarditis. Moreover, the significance of each TLR signaling pathway in the cardiomyocyte remains to be elucidated.
AdV: Adenovirus; EBV: Epstein–Barr virus; HCMV: Human cytomegalovirus; HSV: Herpes simplex virus; HTLV: Human T-lymphotropic virus; Inf-A: Influenza virus A; IRF: Interferon regulatory factor; LPS: Lipopolysaccharide; MCMV: Mouse cytomegalovirus; MeV: Measles virus; ReoV: Reovirus; RSV: Human respiratory syncytial virus; VZV: Varicella zoster virus.
It has been reported that germ-line KO of TLR3 markedly increases CVB3 and CVB4 infection of the heart [42–44]. Intriguingly, the disruption of TLR3 has no significant effect on type I IFN mRNA expression in the heart, whereas TLR3-deficiency abolishes type I IFN expression in DCs post-CVB3 infection [42,44]. Furthermore, adoptive transfer of wild-type macrophages partially rescues antiviral effects in TLR3 KO mice following CVB4 infection [43]. This suggests a crucial role of TLR3 signaling in innate immune cells against Coxsackievirus infection; however, the role for TLR3 signaling in the cardiomyocyte is still obscure. Infection of TLR3 KO mice with encephalomyocarditis virus (EMCV), another picornavirus that causes myocarditis, also leads to significantly earlier mortality in association with increased viral replication and myocardial injury [45]. Unexpectedly, there was a significant increase in IFN-β in the heart even in the absence of TLR3 after EMCV infection. This suggests that TLR3 may not be a key factor for the IFN-β induction in the heart. Moreover, the discrepancy between IFN-β level and the virus titer in the heart implied a relatively minor role of IFN-β in cardiac antiviral defense. Recently, it has been reported that transgenic expression of TLR3 under the direction of the β-actin promoter can rescue the phenotype of type I IFN receptor KO mice following CVB3 infection, indicating the presence of a novel TLR3-mediated antiviral signaling pathway that does not require type I IFN induction [42]. Nevertheless, while there is no doubt about the protective role of TLR3 signaling in systemic antiviral defense, it is still uncertain how much TLR3 signaling contributes to defense mechanisms within the cardiomyocyte during CVB3 infection. Future study should clarify whether TLR induction of type I IFN signaling in the cardiomyocyte is likely to have a significant effect on viral infection of the heart or whether other TLR downstream signaling mechanisms might be important.
TLR7/8 recognizes synthetic imidazoquinoline-like molecules and ssRNA in endosomes [46]. It has been reported that CVB3 infection increases the expression level of TLR7/8 and induces secretion of inflammatory cytokines from cultured human cardiomyocytes, mainly in a TLR7/8-dependent manner [47]. It was also found that CVB3 ssRNA transfected with cationic lipids localizes in endosomes; however, it still remains unclear whether naturally released CVB3 ssRNA genome from the virus capsid can be incorporated into endosomes. Interestingly, a recent study has found that activation of TLR7 signaling induces autophagy [48]. Given that CVB3 infection activates formation of autophagosomes, the autophagosomes seems to serve as a platform of CVB3 replication and that the autophagosomes can fuse with endosomes before becoming autolysosomes (see the ‘Autophagy and CVB3 infection’ section), CVB3 ssRNA may be recognized by TLR7 through the fusion between autophagosomes and endosomes. In fact, other RNA viruses such as vesicular stomatitis virus, sendai virus and influenza virus are reported to be recognized by TLR7 in autolysosomes through autophagy-mediated transportation of the cytosolic viral replication intermediates [49]. Nevertheless, the mechanism underlying host cell recognition of infected CVB3 ssRNA through TLR7/8 should be elucidated in the future. In addition, it would be necessary to validate in vivo significance of TLR7/8 signaling in the innate defense against CVB3 infection using KO animals.
In addition to RNA-sensing TLRs, TLR4 deficiency is reported to increase CVB3 titer in the heart at 2 days post-infection whereas the extent of myocarditis and the virus titer eventually become lower at 12 days compared with wild-type mice [50]. This suggests the involvement of TLR4 signaling in the pathogenesis of CVB3 myocarditis; however, given that TLR4 mainly recognizes lipopolysaccharide of Gram-negative bacteria, molecular mechanisms by which CVB3 infection affects TLR4 signaling should be determined in the future.
MyD88 is an important adaptor molecule for the majority of TLRs, including TLR2, TLR4, TLR5, TLR7 and TLR9, but not TLR3. It has been reported that the infection of MyD88 KO mice with CVB3 leads to a decrease in viral titers in the heart with better survival rate compared with wild-type mice [51]. Conversely, disruption of MyD88 has been reported to have no significant effect on CVB4 infection of the heart or survival rate [43]. These rather unexpected results illustrate the complexity of TLR signaling regulation in the heart. Although we still need to clarify the role of each MyD88-dependent TLR signaling pathway in the pathogenesis of CVB3 myocarditis, based on the current experimental evidence, it is likely that the antiviral effect of the MyD88-dependent TLR signaling seems not to be as essential as TLR3. In contrast to MyD88, TLR adaptor molecule 1 (TRIF), an intracellular adaptor molecule of TLR3 and TLR4, is reported to be crucial for protection against CVB3-mediated cardiomyopathy. TRIF KO mice infected with CVB3 show increased virus titers in the heart and early mortality accompanied by histological cardiomyopathy and cardiac dysfunction [52]. Based on the CVB3 infected phenotype of TLR3 and TLR4 KO mice, it is of great interest as to whether the phenotype of TRIF KO can be explained by the inhibition of TLR3 and TLR4 signaling. Given that TLR4 signaling requires both MyD88 and TRIF, the inhibition of TLR3 may be a dominant factor in the CVB3 infected TRIF KO mice phenotype.
RIG-I-like receptors & other cytosolic viral RNA & DNA sensors
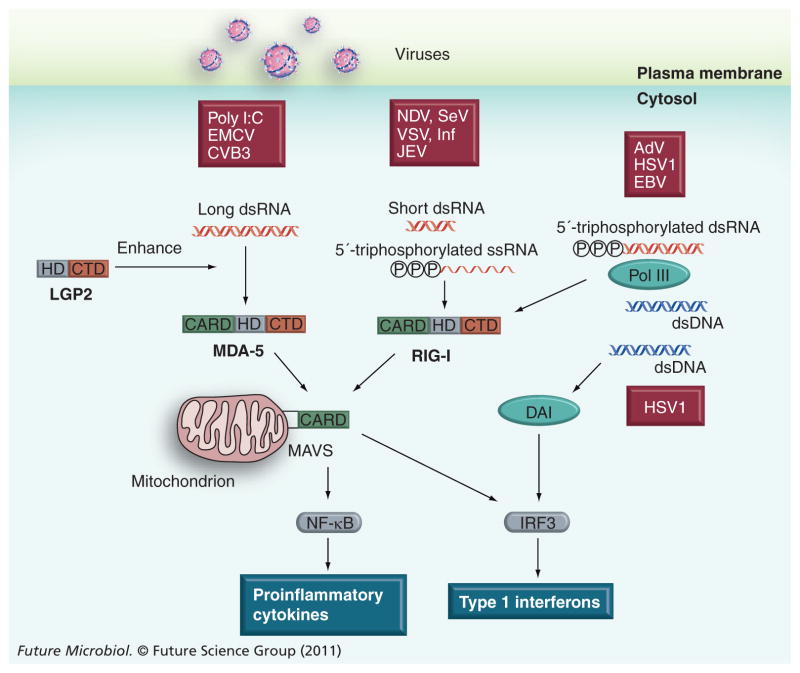
TLR3 and TLR7/8 are localized on endosomal membrane and recognize dsRNA or ssRNA in the lumen of endosomes. Given that CVB3 RNA can be found in a variety of areas in the cytosol, the TLR-mediated virus-sensing mechanisms may not be sufficient for detecting the incoming CVB3 within the cardiomyocyte [53]. Recently, it has been found that intracellular viral dsRNA and ssRNA are recognized by RNA helicases, retinoic acid-induced protein-I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5) (Figure 2) [54,55]. Furthermore, LGP2, a homolog of RIG-I and MDA-5 without the caspase recruitment domain (CARD), has been shown to enhance RIG-I and MDA-5-mediated antiviral responses, especially against EMCV [56]. Similarly, intracellular viral DNA, AT-rich dsDNA and bacterial DNA can be recognized by DNA-dependent RNA polymerase III (Pol III) in the cytosol and transcribed into 5′-triphosphorylated dsRNA. The resultant dsRNA is eventually recognized by RIG-I and activates the RIG-I-mediated antiviral response. Thus, Pol III is now regarded as a crucial cytosolic DNA virus sensor [57,58]. In addition, DNA-dependent activator of interferon regulatory factors (DAI) has also been identified as a cytosolic DNA sensor [59]. The in vivo significance of the RIG-I and MDA-5 pathway in RNA virus infection has been confirmed by using RIG-I- and MDA-5-deficient mice [60,61]. Interestingly, the affinity of each RNA helicase for a virus RNA seems to be determined based on the viral RNA structure and length. For example, the synthetic analog of viral dsRNA, polyriboinosinic:polyribocytidylic acid (poly I:C; >3 kbp) selectively activates MDA-5. By contrast, short poly I:C generated by enzyme digestion (~300 bp) is unable to activate MDA-5 but acts as a potent ligand for RIG-I [62]. This suggests that RIG-I and MDA-5 discriminate long and short dsRNA; however, the mechanism underlying the sensing of nucleotide length is unknown. RIG-I can recognize a variety of viruses, including paramyxovirus, rhabdovirus and orthomyxovirus, by interacting with not only dsRNA but also 5′-triphosphated ssRNA (5′ppp-ssRNA) [63,64]. MDA-5 mainly recognizes picornaviruses such as EMCV and CVB3 as well as poly I:C [61,65,66]. RIG-I and MDA-5 consist of three functional domains: tandem-CARDs, a DEAD box helicase-like domain, and a well-conserved C-terminal domain (CTD). The CTD plays a critical role in the specific recognition of dsRNA and 5′ppp-ssRNA, while the DEAD box helicase cooperatively enhances affinity to dsRNA through a conformational change [67,68]. The tandem CARDs are essential to transduce the signal via CARD–CARD interaction with a downstream CARD-containing adaptor molecule, MAVS/IPS-1/VISA/Cardif [69–72]. Recently MAVS was also shown to localize on the peroxisome [73]. Of the RLRs, the molecular mechanism underlying the interaction between RIG-I and MAVS is better characterized than MDA-5 or LGP2. The specific binding of viral RNA to CTD induces a domain rearrangement, which stabilizes the viral RNA/RIG-I complex and exposes the tandem CARDs, suggesting that RIG-I becomes an open conformation to interact with MAVS through CARDs [68,74]. The interaction of viral RNA/RIG-I or MDA-5 complex with MAVS induces the activation of transcriptional factors such as NF-κB and IFN regulatory factors 3 and 7, which eventually leads to various innate immune and inflammatory reactions including type I IFN expression [75]. The essential role of the RIG-I/MDA-5–MAVS pathway in the innate immune response to a variety of RNA viruses has subsequently been confirmed with MAVS KO mice [76,77]. It has been found that EMCV titer increases by approximately 1000-fold in the heart of MDA-5 or MAVS KO mice 48 h after infection [61,76]. This indicates a pivotal role of the MDA-5–MAVS pathway in sensing EMCV infection in the heart. Recently, two independent groups have also confirmed the crucial role of the MDA-5–MAVS pathway in systemic CVB3 infection [78,79]. Interestingly, while both groups found a decrease in IFN-α induction in the serum 48 h post-CVB3 infection, Huhn et al. detected a fairly high level of IFN-β and IFN-responsive gene (OAS1a and CXCL10) expression in the pancreas and liver in MDA-5 KO mice [78]. This suggests that MDA-5 may be dispensable for the induction of type I IFN during CVB3 infection. Since the cardiac phenotype of MDA-5 KO or MAVS KO mice post-CVB3 infection has not been reported yet, the role of the MDA-5–MAVS pathway in combatting CVB3 myocarditis is still unclear and should be examined in the future.
Figure 2. Recently discovered cytosolic viral RNA and DNA sensors and their substrates.
The recognition of viral RNA or DNA in the host cytosol activates downstream signaling, resulting in the production of type I IFNs and proinflammatory cytokines to limit viral replication and propagation. While the importance of the MDA-5–MAVS pathway against systemic CVB3 infection has been confirmed, the impact of the pathway on CVB3 infection of the heart and subsequent myocarditis is not fully understood.
AdV: Adenovirus; CBV B3: Coxsackievirus B3; CTD: C-terminal domain; DAI: DNA-dependent activator of interferon regulatory factors; EBV: Epstein–Barr virus; EMCV: Encephalomyocarditis virus; HD: DEAD box helicase-like domain; HSV: Herpes simplex virus; JEV: Japanese encephalitis virus; NDV: Newcastle disease virus; RIG-I: Retinoic acid-induced protein-I; SeV: Sendai virus; VSV: Vesicular stomatitis virus.
It is well known that two IFN-responsive genes, 2–5 (A) synthetase and dsRNA-activated protein kinase (PKR), drive antiviral effects by sensing dsRNA. The binding of dsRNA to 2–5 (A) synthetase activates the polymerization of ATP into 2′-5′-linked oligoadenylates of various lengths. The synthesized 2–5 (A), in turn, activates a latent ribonuclease, RNAse L, leading to RNAse L-mediated ssRNA cleavage. Although the importance of 2–5 (A) synthetase/RNAse pathway against picornavirus replication is fairly well characterized in cultured cells, the in vivo significance of the pathway in the pathogenesis of viral myocarditis has not been examined yet. Interaction of PKR with viral dsRNA or long dsRNA induces autophosphorylation of PKR. The phosphorylated PKR subsequently phosphorylates eIF2α, which blocks construction of the smaller ribosomal subunit, thereby preventing viral and host protein synthesis. Recently, PKR was found to also recognize ssRNA in a 5′-triphosphate-dependent fashion [80]. Therefore, the role of the PKR-mediated ssRNA sensing mechanism should be reconsidered as an alternative mechanism to RIG-I signaling for certain RNA viruses There have been no reports as to whether PKR can recognize CVB3 RNA. Since the picornavirus RNA carries a small protein (virion protein, genome; VPg) covalently attached to its 5′ end [81], if PKR can interact with CVB3 RNA, the dsRNA replication intermediate may serve as its ligand. This should be explored in the future experiments.
Protein degradation system & CVB3 infection
Autophagy & CVB3 infection
Autophagy is an evolutionarily conserved ‘eating of self’ process involving the degradation of long-lived cytoplasmic proteins and damaged organelles by lysosomal hydrolyases [82]. There are three known forms of autophagy; macroautophagy, microautophagy and chaperone-mediated autophagy, of which macroautophagy is the most common in mammalian cells, and will be henceforth referred to as autophagy. The histological hallmark of autophagy is the presence of cytoplasmic double-membrane vesicles called autophagosomes in which cytoplasmic constituents are sequestered. Matured autophagosomes eventually become autolysosomes by fusing with lysosomes, leading to the degradation and/or recycling of the trapped constituents. This autophagy-mediated clearance and/or reuse of cellular proteins plays a crucial role in maintaining cellular homeostasis, thus affecting a broad range of health and disease states including aging, development, neurodegenerative diseases, cardiovascular diseases and cancer [83–86]. Recent studies have also shown that autophagy functions in coordination with innate and adaptive immunity in response to bacterial, parasitic and virus infection [87,88]. Interestingly, while autophagy is largely involved in defense mechanisms against pathogen infections, some viruses such as enterovirus and coronavirus seem to use autophagosomes as a platform for their replication, facilitating progeny virus production [89].
It has been reported that CVB3 infection increases autophagosome formation in HEK293 cells in vitro and in pancreatic acinar cells in vivo, accompanied by an increase in the LC3-II/LC3-I ratio, a marker for the activation of autophagy [90,91]. Although the underlying molecular mechanisms are not fully understood, the expression of viral proteinases such as 2BC and 3A, and activation of host eIF2α or calpain seem to be involved in virus-mediated autophagosome formation [91–93]. It should be noted that virus-induced vesicles are sometimes called ‘autophagy-like vesicles’ because of the differences in average diameter (autophagy-like vesicles; 200–400 nm, classical autophagosome; 800 nm) and autophagic flux. However, given that these two types of vesicles share many common characteristics such as double membranes, cytoplasmic localization, co-expression of LC3 and lysosomal-associated membrane protein (LAMP)-1, and increased LC3-II formation [94], here we simply call virus-induced vesicles ‘autophagosomes’. While the activation of autophagy is not beneficial for many viruses, enteroviruses seem to have evolved to subvert this host antiviral mechanism. In fact, the treatment of HEK293 or primary neurons with 3-MA, an inhibitor of autophagosome formation, decreases the replication of CVB3 or CVB4, respectively. This indicates that coxsackieviruses utilize host autophagy machinery for progeny production. Interestingly, despite the upregulation of autophagosome formation in CVB3-infected cells, protein degradation through autophagic machinery is inhibited when estimated by the accumulation of p62, a specific substrate that is degraded by autophagy [90,91,95]. It has been reported that several viruses such as influenza A, HIV and HCV have unique mechanisms to increase their replication by reducing host autophagic flux, a dynamic process comprising the formation of the autophagosome, amphisome (autophagic vacuoles formed by the fusion of an autophagosome with an endosome) and autolysosome (autophagic vacuoles formed by the fusion of an autophagosome with a lysosome). These viruses prevent the formation of amphisomes or autolysosomes by inhibiting the fusion of autophagosomes with endosomes and/or lysosomes, resulting in a decrease in host autophagic flux [96–98]. In the case of CVB3-infected pancreatic acinar cells, amphisome formation is intact whereas autolysosome formation is inhibited, suggesting that CVB3 limits the fusion of autophagosomes and/or amphisomes with lysosomes [90]. The beneficial effect of inhibition of autolysosome formation on CVB3 replication has also been confirmed by knocking down LAMP-2, a lysosomal membrane protein critical for autolysosome formation [91]. These data suggest that CVB3 infection induces autophagosome formation while inhibiting subsequent autolysosome formation, leading to the accumulation of autophagosomes in host cells. Given that inhibition of autophagosome formation decreases viral replication, and the induction of autophagosome formation or prevention of subsequent autolysosome formation increases viral replication, autophagosomes may be a crucial platform for CVB3 replication [89].
The cardiomyocyte is a terminally differentiated, nondividing cell in which basal autophagy is likely to be important in quality control of endogenous proteins and cellular organelles. Indeed, the disruption of autophagy machinery in the adult heart by knocking out of autophagy related 5 homolog (ATG5) results in cardiac hypertrophy and contractile dysfunction that is accompanied by increased levels of ubiquitinated proteins and ultrastructural abnormalities in sarcomere and mitochondria [86]. In addition, autophagy seems to constitute an important physiological or pathophysiological response to cardiac stresses such as ischemia or acute pressure overload with the accumulation of autophagosomes both in humans and animal models [83,84,99]. It has been reported that the ATG5–ATG12 conjugate, a key regulator of the autophagic process, plays an important role in innate antiviral responses via inhibition of type I IFN production by directly interacting with RIG-I and MAVS [100]. Moreover, the transportation of viral ssRNA by autophagosome into lysosomes has been reported to be crucial for TLR7-mediated ssRNA sensing [49]. Although there are still many pieces missing from a comprehensive understanding, these recent findings imply that autophagy and the innate immune system may be cooperatively working against a variety of stresses including virus infection within the cardiomyocyte. While the decreased autophagic flux in CVB3-infected cells suggests the involvement of abnormalities in autophagy in the pathogenesis of CVB3-mediated cardiomyopathy, there has been no experimental evidence linking autophagy to CVB3-mediated cardiomyopathy to date.
The proteasome & CVB3 infection
The ubiquitin–proteasome system (UPS) is a pivotal protein degradation mechanism in eukaryotic cells. UPS rapidly eliminates abnormal proteins and/or controls the expression level of short-lived regulatory proteins. Inhibition of proteasomal protein degradation has been reported to induce cardiac dysfunction in humans, indicating the essential function of UPS in cardiomyocytes [101–103]. It has been demonstrated that viruses have evolved a variety of mechanisms to escape from host innate and adaptive immunity by modulating host UPS. In addition, some viruses utilize host UPS to facilitate their replication, maturation and exit from the host [104]. CVB3 infection has been reported to increase protein poly-ubiquitination and decreases free ubiquitin in HeLa cells. By contrast, the treatment of HeLa cells with the proteasome inhibitor, MG132, or lactacystin markedly decreases CVB3 replication [105]. Consistently, systemic administration of the proteasome inhibitor, MLN353, attenuates CVB3-induced myocardial damage in mice at 9 days post-infection [106]. Recently, the involvement of ubiquitin-independent proteasome system in CVB3 replication has also been reported [107]. These data indicate that the activation of the host proteasome system is beneficial for CVB3 replication in the cell. It has been reported that proteasome subunit α type 7 (PSMA7), a subunit of the 20S proteasome, negatively regulates the MAVS-mediated innate immune response [108]. Given that PSMA7 is located on the outer ring of the proteasome complex and plays a key role in proteasomal activity regulation, the inhibition of the MAVS pathway via PSMA7 may be involved in the mechanisms by which activation of the proteasome increases CVB3 replication. The interaction between UPS and innate immune mechanisms within the cardiomyocyte would be an interesting topic for future research.
Apoptosis
Viruses manipulate host cells to ensure their own replication and, at late stages of the viral life cycle, they kill the infected host cell to facilitate their propagation in adjacent tissues. Although virus-induced apoptosis and the underlying mechanisms are well characterized in cultured cells, virus-induced apoptosis in vivo and the role of apoptosis in virus-induced diseases are not fully understood. It is well accepted that host cell apoptosis before or during virus replication can prevent and eventually limit virus propagation. However, it is still unclear whether the apoptosis-mediated antiviral mechanism is beneficial for post-mitotic cells such as neurocytes and cardiomyocytes. CVB3 infection is reported to induce apoptosis in HeLa cells by activation of proapoptotic mediators and suppression of translation and transcription through the expression of proteinase 2A and 3C [109]. In keeping with the in vitro data, CVB3 infection of the heart can activate cardiomyocyte apoptosis in both mice and humans [110–112]. Interestingly, the proapoptotic protein, apoptosis-inducing factor (Siva), which binds to the CVB3 capsid protein VP2, is strongly upregulated in the same region where apoptosis occurs during acute CVB3-induced myocarditis [113]. However, the significance of apoptosis in the pathogenesis of myocarditis has not been determined yet. DeBiasi et al. demonstrated that reovirus infection of cardiomyocytes induces caspase-3 (CASP3) activation in virus-infected cardiomyocytes in vivo [114]. In addition, this group found a significant decrease in virus-induced myocardial injury accompanied by an improved survival rate in CASP3 KO mice. Surprisingly, despite the decrease in apoptotic response, the systemic virus titer including the heart is significantly decreased in the CASP3 KO mice [114]. This strongly suggests that cardiac apoptosis may not be beneficial in inhibiting virus replication in the heart and limit the subsequent cardiomyopathy, at least in the acute phase of infection. It has also been reported that a sarcolemmal protein, dystrophin, limits CVB3 infection of the heart by inhibiting the virus’ propagation to adjacent cardiomyocytes [115]. Given that dystrophin plays a central role in maintaining the dystroglycan complex at sarcolemma, the integrity of sarcolemmal membrane is thought to be an important factor that prevents secondary CVB3 propagation. This suggests that the activation of apoptotic signaling may increase virus propagation by disrupting the sarcolemmal barrier that can otherwise quarantine the virus within the infected cardiomyocyte. Interestingly, gp130 receptor signaling, a well-characterized antiapoptotic signaling pathway in the cardiomyocyte, also has a protective role against CVB3 infection of the heart [28]. Unlike mitotic cells, cardiomyocytes may deliberately drive anti-apoptotic mechanisms during CVB3 infection not only for their survival but also for limiting virus propagation. By contrast, CVB3 may try to activate proapoptotic mechanisms to maximize their infection in the heart. Although additional evidence is required, this may be involved in the mechanisms by which CVB3 achieves persistent infection in the cardiomyocyte. A symbiotic relationship between the cardiomyocyte and CVB3 may be formed based on the balance between proapoptotic and antiapoptotic signaling in the cardiomyocyte. It has been reported that transgenic expression of replication-incompetent CVB3 genome in the cardiomyocyte leads to DCM accompanied by excitation-contraction coupling abnormalities [25]. Interestingly, no activation of apoptosis in cardiomyocytes was found under persistent expression of the CVB3 genome, suggesting the minor effect of persistent viral genomes on cardiomyocyte apoptosis in the chronic phase of infection. Despite this, the role of cardiomyocyte apoptosis in the pathogenesis of viral myocarditis should be carefully evaluated using well-characterized CVB3-infected animal models in the future.
Future perspective
The cardiomyocyte is a terminally differentiated, nondividing cell with a minimal renewal capability. A recent study has demonstrated that there is only 1% turnover annually even at the age of 25, and fewer than 50% of cardiomyocytes are exchanged during a normal life in humans [116]. Therefore, it is clear that loss of cardiomyocytes due to virus infection cannot be made up in a timely manner and is detrimental to the maintenance of cardiac function. This indicates the importance of strategies that protect cardiomyocytes from the initial or persistent virus infection. It has been demonstrated that anti-inflammatory therapy with immunosuppressants does not have beneficial effects on cardiac function and mortality in patients with myocarditis [117]. Although this does not completely exclude the involvement of inflammation in the development of cardiac dysfunction, this also suggests the presence of other important factors that may activate the development of fatal cardiomyopathy. One important aspect not yet completely understood is the fate of cardiomyocytes after virus infection. Based on previous publications, cardiomyocytes seem to have several fates postvirus infection: cell death or apoptosis due to virus infection (direct effect of virus infection), cell necrosis due to immune cell attack (indirect effect of virus infection) and symbiosis with persistently infected viruses. While molecular mechanisms of apoptosis and necrosis have been well characterized, symbiosis with persistently infected viruses and the impact on the development of cardiomyopathy are relatively obscure. Clarification of these points is awaited in the future. The persistence of the viral genome in the cardiomyocyte raises an additional question about the fate of cardiomyocytes after virus infection; can cardiomyocytes completely eliminate viruses from the myocyte using intrinsic antiviral mechanisms and return to healthy conditions? Given the fact that the diagnosis and treatment of viral myocarditis usually starts from the subacute or chronic phase of infection except fulminant myocarditis, this would be an interesting question to be tested in the future from the therapeutic viewpoints (Figure 3). Since viral myocarditis or cardiomyopathy is a complicated infectious disease accompanied by inflammatory responses, a large portion of previous work focusing on disease mechanisms has been reported from virologists and immunologists. Therefore, we have a significant amount of solid data about properties of cardiotropic viruses including various strains and variants, and how adaptive and innate immunity work in vivo during cardiotropic virus infection. Conversely, relatively little is known about how cardiomyocytes respond against invading viruses and how the acute or chronic response affects cardiomyocyte survival and/or total cardiac function. Given that virus infection of the heart itself is a key factor that determines the development of myocarditis and cardiomyopathy, research focusing on these points is necessary for a future breakthrough in this field. Specifically, the interaction between viral RNAs and cardiomyocyte sensors should be very important topics of research in the long run. Although not addressed in this article, the effect of small RNAs such as microRNAs on the development of myocarditis will also be an attractive topic in the near future. As a practical point, we need to develop more cardiac-specific KO or transgenic animal models to understand the in vivo significance of certain responses within cardiomyocytes in the disease process. Furthermore, experiments should ideally be carried out using an inbred mouse line to exclude potential variations based on the genetic background. Although this may be an exhausting effort, it would be worthwhile for researchers to discuss if we could develop a standard experimental protocol for viral myocarditis models including virus strain, titration method, mouse strain, age and gender, given the presence of too many variations in the previous publications.
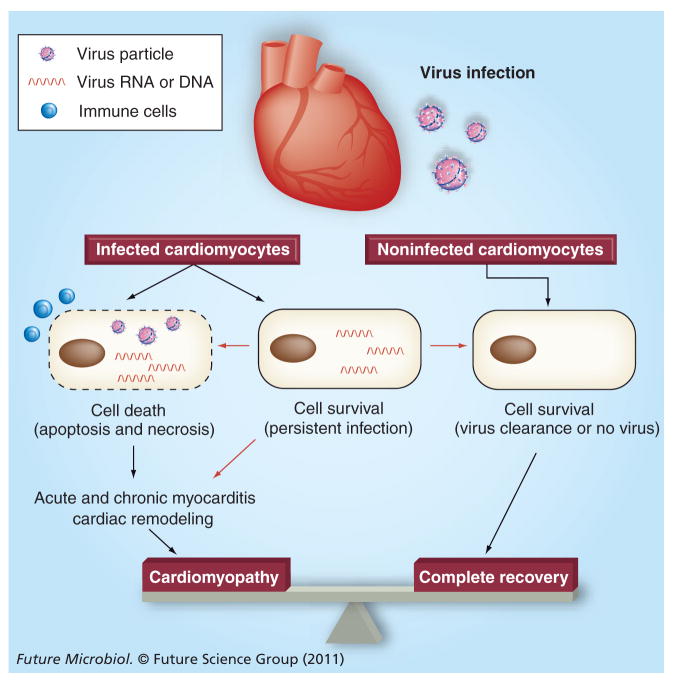
Figure 3. Potential fate of cardiomyocytes after virus infection of the heart.
Infected cardiomyocytes are thought to die due to the direct effect of the infecting virus (direct injury or apoptosis) and/or the attack of immune cells such as cytotoxic T lymphocytes (necrosis or inflammation), which is usually associated with acute and chronic inflammation in the heart. The loss of functional cardiomyocytes can directly affect cardiac function or induce secondary cardiac remodeling, leading to dilated cardiomyopathy in the long run. It is also known that persistence of virus RNA can be observed in cardiomyocytes in the chronic phase of infection. However, it is still controversial whether this persistence can induce cardiomyocyte death or chronic inflammation, or affect disease progress. Interestingly, while the persistence of virus RNA in the heart is clear, the infectious particles have never been isolated from patient samples in the chronic phase of infection to date. In addition, it has not been determined whether cardiomyocytes can remove such persistently infecting virus RNA from the cell. If possible, elucidation of these mechanisms may provide novel insights into diagnostic and therapeutic strategies against viral myocarditis and subsequent cardiomyopathy. Processes that have been or not been well accepted are shown with black or red arrows, respectively.
Executive summary.
Introduction
Myocarditis is defined as histological inflammation of heart muscles.
The true epidemiology of viral myocarditis is poorly understood because of its wide spectrum of clinical presentation including asymptomatic cases and a lack of noninvasive markers for diagnosis.
The 5-year survival rate of myocarditis has been reported to be 56–70%. In contrast, the long-term survival rate of patients with fulminant myocarditis is surprisingly high (93% at 11 years) once fatal outcomes are prevented.
Adenoviruses, enteroviruses and parvovirus 19 are the most commonly identified viruses from patients with myocarditis. However, there are geographical differences in the profiles of virus genomes identified from human heart.
Coxsackievirus B3 (CVB3) infection can lead to viral myocarditis and subsequent cardiomyopathy through three phases; acute, subacute and chronic.
CVB3-mediated myocarditis has been most thoroughly investigated in murine myocarditis models. CVB3 is a member of the Picornaviridae family, genus enterovirus.
The presence of a replication-incompetent CVB3 genome is sufficient to induce cardiomyopathy in the mouse model; however, the pathological effects of the viral genomes on cardiac function and clinical outcome in humans is still controversial.
CVB3 infection of the heart is required for the development of myocarditis postsystemic infection. Thus, virus infection of the cardiomyocyte can be an important step that determines the severity of myocarditis and subsequent development of dilated cardiomyopathy.
Innate immunity
Activation of JAK–STAT signaling during CVB3 infection activates antiviral mechanisms within the cardiomyocyte.
The significance of type I interferon signaling within the cardiomyocyte should be thoroughly elucidated in the future.
The protective role of TLR3–TRIF signaling in systemic antiviral defense is clear; however, it is still uncertain exactly how much TLR3–TRIF signaling contributes to defense mechanisms within the cardiomyocyte.
It is necessary to validate the in vivo significance of TLR7/8 signaling in CVB3 infection using knockout animals. However, based on MyD88 knockout mice data, the antiviral effect does not seem to be very strong.
The MDA-5–MAVS pathway is an important sensing mechanism for systemic CVB3 infection. LGP2 may also be involved. The role of the MDA-5–MAVS pathway within the cardiomyocyte should be determined in the future.
Together with recently found cytosolic RNA sensors, re-evaluation of role of classical RNA sensors (2–5 [A] synthetase and PKR), in the pathogenesis of myocarditis is necessary.
Protein degradation system
Enteroviruses seem to use autophagosomes as a platform of replication to facilitate progeny virus production.
CVB3 infection induces autophagosome formation while inhibiting subsequent autolysosome formation, leading to the accumulation of autophagosomes in host cells.
Autophagy and the innate immune system may work cooperatively against a variety of stresses including virus infection in the cardiomyocyte.
CVB3 infection activates the host proteasome system, which appears to be beneficial for virus replication.
Apoptosis
Although CVB3 infection induces apoptosis in vitro and in vivo, the significance of apoptosis in the pathogenesis of myocarditis has not been determined.
In a retrovirus-mediated myocarditis model, despite the decrease in apoptotic response, the systemic virus titer (including the heart) is significantly decreased in caspase-3 knockout mice. This strongly suggests that cardiac apoptosis may not be beneficial in inhibiting virus replication in the heart.
Preservation of sarcolemmal integrity by dystrophin prevents CVB3 propagation.
Activation of cardiac antiapoptotic signaling through gp130 decreases CVB3 infection of the heart.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Research in T Yajima’s laboratory is supported by NIH (5R01HL092116) and by American Heart Association Scientist Development grant (0730333N). The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52(4):274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose NR. Myocarditis: infection versus autoimmunity. J Clin Immunol. 2009;29(6):730–737. doi: 10.1007/s10875-009-9339-z. [DOI] [PubMed] [Google Scholar]
- 3▪.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360(15):1526–1538. doi: 10.1056/NEJMra0800028. Comprehensive review of myocarditis from the viewpoint of clinicians. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29(17):2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113(4):593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 6.Grogan M, Redfield MM, Bailey KR, et al. Long-term outcome of patients with biopsy-proved myocarditis – comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995;26(1):80–84. doi: 10.1016/0735-1097(95)00148-s. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy RE, 3rd, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342(10):690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 8.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111(7):887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 10.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118(6):639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112(13):1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Markham DW, Drazner MH, Mammen PP. Fulminant myocarditis. Nat Clin Pract Cardiovasc Med. 2008;5(11):693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 13.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343(19):1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 14.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(15):1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 15.Corcioli F, Zakrzewska K, Rinieri A, et al. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J Med Virol. 2008;80(11):2005–2011. doi: 10.1002/jmv.21289. [DOI] [PubMed] [Google Scholar]
- 16.Lindner J, Noutsias M, Lassner D, et al. Adaptive immune responses against parvovirus B19 in patients with myocardial disease. J Clin Virol. 2009;44(1):27–32. doi: 10.1016/j.jcv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Schenk T, Enders M, Pollak S, Hahn R, Huzly D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol. 2009;47(1):106–110. doi: 10.1128/JCM.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99(8):1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- 19.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104(9):1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 20▪.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. Comprehensive review of molecular pathogenesis of coxsackievirus B3 myocarditis. [DOI] [PubMed] [Google Scholar]
- 21.Weinzierl AO, Szalay G, Wolburg H, et al. Effective chemokine secretion by dendritic cells and expansion of cross-presenting CD4(−)/CD8(+) dendritic cells define a protective phenotype in the mouse model of coxsackievirus myocarditis. J Virol. 2008;82(16):8149–8160. doi: 10.1128/JVI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman NM, Kim KS, Drescher KM, Oka K, Tracy S. 5′ terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology. 2008;375(2):480–491. doi: 10.1016/j.virol.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KS, Tracy S, Tapprich W, et al. 5′-terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005;79(11):7024–7041. doi: 10.1128/JVI.79.11.7024-7041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldstone MBA. Anatomy of viral persistence. PLoS Pathogens. 2009;5(7):E1000523. doi: 10.1371/journal.ppat.1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wessely R, Klingel K, Santana LF, et al. Transgenic expression of replication-restricted enteroviral genomes in heart muscle induces defective excitation-contraction coupling and dilated cardiomyopathy. J Clin Invest. 1998;102(7):1444–1453. doi: 10.1172/JCI1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallewaard NL, Zhang L, Chen J-W, Guttenberg M, Sanchez MD, Bergelson JM. Tissue-specific deletion of the coxsackievirus and adenovirus receptor protects mice from virus-induced pancreatitis and myocarditis. Cell Host Microbe. 2009;6(1):91–98. doi: 10.1016/j.chom.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Shi Y, Chen C, Lisewski U, et al. Cardiac deletion of the coxsackievirus-adenovirus receptor abolishes coxsackievirus B3 infection and prevents myocarditis in vivo. J Am Coll Cardiol. 2009;53(14):1219–1226. doi: 10.1016/j.jacc.2008.10.064. Demonstrates a crucial role of virus infection of the cardiomyocyte in the pathogenesis of viral myocarditis. [DOI] [PubMed] [Google Scholar]
- 28▪.Yajima T, Yasukawa H, Jeon ES, et al. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation. 2006;114(22):2364–2373. doi: 10.1161/CIRCULATIONAHA.106.642454. Demonstrates the presence of significant antiviral mechanisms within the cardiomyocyte. [DOI] [PubMed] [Google Scholar]
- 29.Yasukawa H, Yajima T, Duplain H, et al. The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J Clin Invest. 2003;111(4):469–478. doi: 10.1172/JCI16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yajima T, Knowlton KU. Viral myocarditis from the perspective of the virus. Circulation. 2009;119(19):2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- 31.Leipner C, Grun K, Schneider I, Gluck B, Sigusch HH, Stelzner A. Coxsackievirus B3-induced myocarditis. differences in the immune response of C57BL/6 and Balb/c mice. Med Microbiol Immunol (Berl) 2004;193(2–3):141–147. doi: 10.1007/s00430-003-0199-5. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J, Liu Z, Lim T, et al. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ Res. 2009;104(5):628–638. doi: 10.1161/CIRCRESAHA.108.192179. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura A. Regulation of cytokine signaling by the SOCS and spred family proteins. Keio J Med. 2009;58(2):73–83. doi: 10.2302/kjm.58.73. [DOI] [PubMed] [Google Scholar]
- 34.Chelbialix M, Wietzerbin J. Interferon, a growing cytokine family: 50 years of interferon research. Biochimie. 2007;89(6–7):713–718. doi: 10.1016/j.biochi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YX, da Cunha V, Vincelette J, et al. Antiviral and myocyte protective effects of murine interferon-β and -α2 in coxsackievirus B3-induced myocarditis and epicarditis in Balb/c mice. Am J Physiol Heart Circ Physiol. 2007;293(1):H69–H76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- 37.Schmidtke M, Gluck B, Merkle I, Hofmann P, Stelzner A, Gemsa D. Cytokine profiles in heart, spleen, and thymus during the acute stage of experimental coxsackievirus B3-induced chronic myocarditis. J Med Virol. 2000;61(4):518–526. [PubMed] [Google Scholar]
- 38.Wessely R, Klingel K, Knowlton KU, Kandolf R. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation. 2001;103(5):756–761. doi: 10.1161/01.cir.103.5.756. [DOI] [PubMed] [Google Scholar]
- 39.Deonarain R, Cerullo D, Fuse K, Liu PP, Fish EN. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation. 2004;110(23):3540–3543. doi: 10.1161/01.CIR.0000136824.73458.20. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 41.Yokota S-I, Okabayashi T, Fujii N. The battle between virus and host: modulation of toll-like receptor signaling pathways by virus infection. Mediators Inflamm. 2010;2010:184328. doi: 10.1155/2010/184328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Negishi H, Osawa T, Ogami K, et al. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci USA. 2008;105(51):20446–20451. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richer MJ, Lavallee DJ, Shanina I, Horwitz MS. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS ONE. 2009;4(1):E4127. doi: 10.1371/journal.pone.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinzierl AO, Szalay G, Wolburg H, et al. Effective chemokine secretion by dendritic cells and expansion of cross-presenting CD4 −/CD8+ dendritic cells define a protective phenotype in the mouse model of coxsackievirus myocarditis. J Virol. 2008;82(16):8149–8160. doi: 10.1128/JVI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardarson HS, Baker JS, Yang Z, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292(1):H251–H258. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 46.Heil F. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 47.Triantafilou K, Orthopoulos G, Vakakis E, et al. Human cardiac inflammatory responses triggered by Coxsackie B viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell Microbiol. 2005;7(8):1117–1126. doi: 10.1111/j.1462-5822.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 48.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27(7):1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315(5817):1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 50.Fairweather D, Yusung S, Frisancho S, et al. IL-12 receptor β1 and Toll-like receptor 4 increase IL-1 β- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170(9):4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 51.Fuse K, Chan G, Liu Y, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112(15):2276–2285. doi: 10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
- 52.Riad A, Westermann D, Zietsch C, et al. TRIF is a critical survival factor in viral cardiomyopathy. J Immunol. 2011;186(4):2561–2570. doi: 10.4049/jimmunol.1002029. [DOI] [PubMed] [Google Scholar]
- 53.Ukimura A, Deguchi H, Kitaura Y, et al. Intracellular viral localization in murine coxsackievirus-B3 myocarditis – ultrastructural study by electron microscopic in situ hybridization. Am J Pathol. 1997;150(6):2061–2074. [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneyama M, Kikuchi M, Matsumoto K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175(5):2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 55▪.Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. Discovery of cytosolic RNA virus sensing mechanism through RIG-I. [DOI] [PubMed] [Google Scholar]
- 56.Satoh T, Kato H, Kumagai Y, et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc Natl Acad Sci USA. 2010;107(4):1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10(10):1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪.Chiu YH, MacMillan JB, Chen ZJJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–591. doi: 10.1016/j.cell.2009.06.015. Discovery of cytosolic DNA virus sensing mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 60.Kato H, Sato S, Yoneyama M, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23(1):19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 62.Kato H, Takeuchi O, Mikamo-Satoh E, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205(7):1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hornung V, Ellegast J, Kim S, et al. 5′-triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 64.Pichlmair A, Schulz O, Tan CP, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 65.Gitlin L, Barchet W, Gilfillan S, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidyl ic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103(22):8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loo YM, Fornek J, Crochet N, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82(1):335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahasi K, Kumeta H, Tsuduki N, et al. Solution structures of cytosolic RNA sensor MDA5 and LGP2 C-terminal domains: identification of the RNA recognition loop in rig-I-like receptors. J Biol Chem. 2009;284(26):17465–17474. doi: 10.1074/jbc.M109.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahasi K, Yoneyama M, Nishihori T, et al. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29(4):428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 69.Kawai T, Takahashi K, Sato S, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6(10):981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 70.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437(7062):1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 71▪.Seth RB, Sun L, Ea C-K, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. First molecular evidence that links mitochondria to innate antiviral mechanisms. [DOI] [PubMed] [Google Scholar]
- 72.Xu L-G, Wang Y-Y, Han K-J, Li L-Y, Zhai Z, Shu H-B. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell. 2005;19(6):727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 73.Dixit E, Boulant S, Zhang Y, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141(4):668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29(2):178–181. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Michallet M-C, Meylan E, Ermolaeva MA, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28(5):651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Kumar H, Kawai T, Kato H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203(7):1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Q, Sun L, Liu H-H, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Hühn MH, McCartney SA, Lind K, Svedin E, Colonna M, Flodström-Tullberg M. Melanoma differentiation-associated protein-5 (MDA-5) limits early viral replication but is not essential for the induction of type 1 interferons after Coxsackievirus infection. Virology. 2010;401(1):42–48. doi: 10.1016/j.virol.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 79.Wang JP, Cerny A, Asher DR, Kurt-Jones EA, Bronson RT, Finberg RW. MDA5 and MAVS mediate Type I interferon responses to Coxsackie B virus. J Virol. 2009;84(1):254–260. doi: 10.1128/JVI.00631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE, Bevilacqua PC. 5′-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science. 2007;318(5855):1455–1458. doi: 10.1126/science.1147347. [DOI] [PubMed] [Google Scholar]
- 81.Gruez A, Selisko B, Roberts M, et al. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J Virol. 2008;82(19):9577–9590. doi: 10.1128/JVI.00631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44(4):654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 87▪.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7(10):767–777. doi: 10.1038/nri2161. Comprehensive review of the role of autophagy in immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wileman T. Aggresomes and autophagy generate sites for virus replication. Science. 2006;312(5775):875–878. doi: 10.1126/science.1126766. [DOI] [PubMed] [Google Scholar]
- 90.Kemball CC, Alirezaei M, Flynn CT, et al. Coxsackievirus infection induces autophagy-like vesicles and megaphagosomes in pancreatic acinar cells in vivo. J Virol. 2010;84(23):12110–12124. doi: 10.1128/JVI.01417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong J, Zhang J, Si X, et al. Autophagosome supports Coxsackievirus B3 replication in host cells. J Virol. 2008;82(18):9143–9153. doi: 10.1128/JVI.00641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson WT, Giddings TH, Taylor MP, et al. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3(5):861–871. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoon SY, Ha YE, Choi JE, et al. Coxsackievirus B4 uses autophagy for replication after calpain activation in rat primary neurons. J Virol. 2008;82(23):11976–11978. doi: 10.1128/JVI.01028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor MP, Kirkegaard K. Potential subversion of autophagosomal pathway by picornaviruses. Autophagy. 2008;4(3):286–289. doi: 10.4161/auto.5377. [DOI] [PubMed] [Google Scholar]
- 95.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gannagé M, Dormann D, Albrecht R, et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe. 2009;6(4):367–380. doi: 10.1016/j.chom.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kyei GB, Dinkins C, Davis AS, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186(2):255–268. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sir D, Chen W-l, Choi J, Wakita T, Yen TSB, Ou J-J. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48(4):1054–1061. doi: 10.1002/hep.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68(3):355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Jounai N, Takeshita F, Kobiyama K, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104(35):14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hacihanefioglu A, Tarkun P, Gonullu E. Acute severe cardiac failure in a myeloma patient due to proteasome inhibitor bortezomib. Int J Hematol. 2008;88(2):219–222. doi: 10.1007/s12185-008-0139-7. [DOI] [PubMed] [Google Scholar]
- 102.Orciuolo E, Buda G, Cecconi N, et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. 2007;138(3):396–397. doi: 10.1111/j.1365-2141.2007.06659.x. [DOI] [PubMed] [Google Scholar]
- 103.Wang XJ, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99(12):1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 104▪.Gao G, Luo HL. The ubiquitin-proteasome pathway in viral infections. Can J Physiol Pharmacol. 2006;84(1):5–14. doi: 10.1139/y05-144. Comprehensive review of the ubiquitin-proteasome pathway in viral infection. [DOI] [PubMed] [Google Scholar]
- 105.Si XN, Gao G, Wong J, Wang YH, Zhang JC, Luo HL. Ubiquitination is required for effective replication of Coxsackievirus B3. PLoS ONE. 2008;3(7):E2585. doi: 10.1371/journal.pone.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao G, Zhang JC, Si XN, et al. Proteasome inhibition attenuates coxsackievirus-induced myocardial damage in mice. Am J Physiol Heart Circ Physiol. 2008;295(1):H401–H408. doi: 10.1152/ajpheart.00292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao G, Wong J, Zhang J, et al. Proteasome activator REG enhances Coxsackieviral infection via facilitating p53 degradation. J Virol. 2010;84(21):11056–11066. doi: 10.1128/JVI.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jia Y, Song T, Wei C, et al. Negative regulation of MAVS-mediated innate immune response by PSMA7. J Immunol. 2009;183(7):4241–4248. doi: 10.4049/jimmunol.0901646. [DOI] [PubMed] [Google Scholar]
- 109.Chau DH, Yuan J, Zhang H, et al. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis. 2007;12(3):513–524. doi: 10.1007/s10495-006-0013-0. [DOI] [PubMed] [Google Scholar]
- 110.Joo CH, Hong HN, Kim EO, et al. Coxsackievirus B3 induces apoptosis in the early phase of murine myocarditis: a comparative analysis of cardiovirulent and noncardiovirulent strains. Intervirology. 2003;46(3):135–140. doi: 10.1159/000071453. [DOI] [PubMed] [Google Scholar]
- 111.Saraste A. Cardiomyocyte apoptosis in experimental coxsackievirus B3 myocarditis. Cardiovasc Pathol. 2003;12(5):255–262. doi: 10.1016/s1054-8807(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 112.Venteo L, Bourlet T, Renois F, et al. Enterovirus-related activation of the cardiomyocyte mitochondrial apoptotic pathway in patients with acute myocarditis. Eur Heart J. 2010;31(6):728–736. doi: 10.1093/eurheartj/ehp489. [DOI] [PubMed] [Google Scholar]
- 113▪.Clarke P, Tyler KL. Apoptosis in animal models of virus-induced disease. Nat Rev Microbiol. 2009;7(2):144–155. doi: 10.1038/nrmicro2071. Comprehensive review of the role of apoptosis in virus-induced disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeBiasi RL, Robinson BA, Sherry B, et al. Caspase inhibition protects against reovirus-induced myocardial injury in vitro and in vivo . J Virol. 2004;78(20):11040–11050. doi: 10.1128/JVI.78.20.11040-11050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiong D, Lee GH, Badorff C, et al. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med. 2002;8(8):872–877. doi: 10.1038/nm737. [DOI] [PubMed] [Google Scholar]
- 116.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mason JW, Oconnell JB, Herskowitz A, et al. A clinical-trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333(5):269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]