Abstract
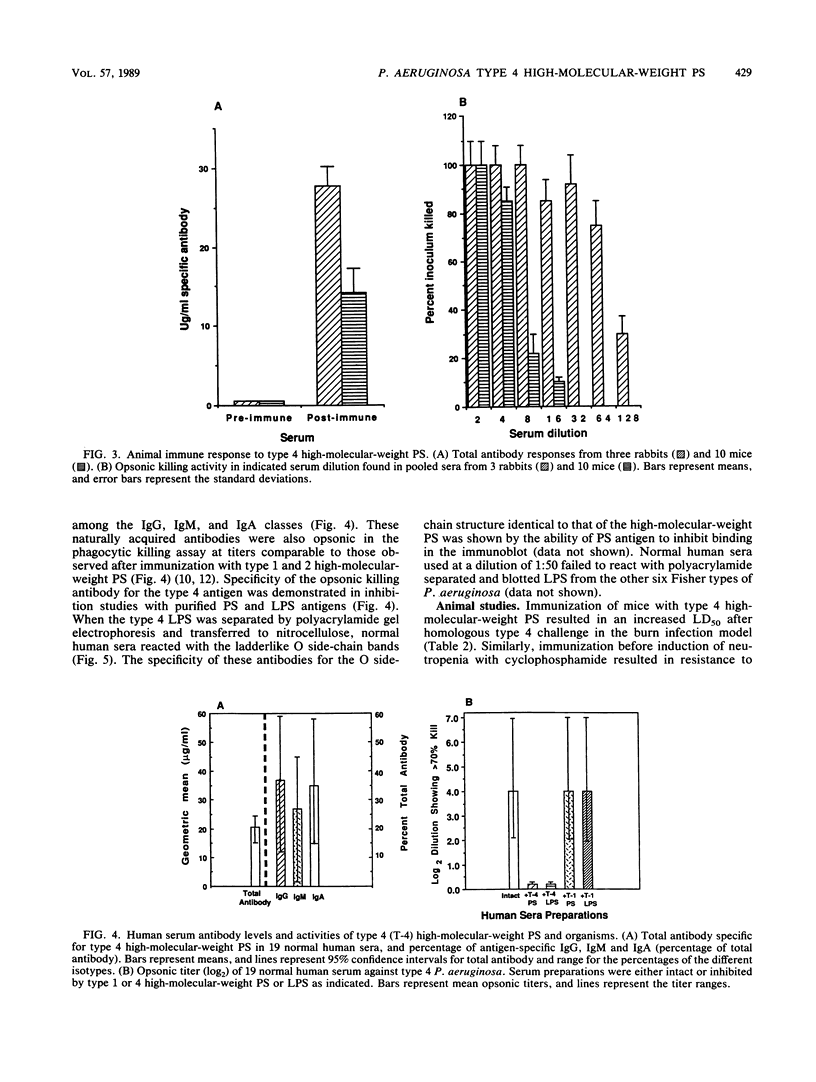
A high-molecular-weight, immunogenic form of the lipopolysaccharide O side chain of Pseudomonas aeruginosa Fisher immunotype 4 (type 4, International Antigenic Typing System 1, Lanyi O:6) was isolated and characterized. Analysis by nuclear magnetic resonance spectroscopy confirmed the structural similarity of this high-molecular-weight polysaccharide and the type 4 O side chain. The polysaccharide was immunogenic in rabbits and mice, eliciting opsonophagocytic killing antibodies. Immunization with the polysaccharide produced significant protection against homologous challenge in both burned and granulocytopenic mice. Naturally acquired opsonic killing antibodies to type 4 polysaccharide were present in sera from unimmunized normal adults at levels comparable to postimmunization levels achieved after immunization with other type-specific polysaccharides. The specificity of the naturally occurring antibodies for the O side chain was documented by immunoblot analysis and inhibition studies. Naturally occurring polysaccharide-specific antibodies were comparable in their protective activity against live challenge in neutropenic animals to immunization-induced murine antibodies with similar specificity. These data suggest that naturally occurring serum antibody to P. aeruginosa type 4 lipopolysaccharide O side chains in most adults is not distinguishable in quantity or quality from immunization-induced antibodies in mice; evaluation of type 4-specific vaccines in humans may be complicated by this finding.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cadieux J. E., Kuzio J., Milazzo F. H., Kropinski A. M. Spontaneous release of lipopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1983 Aug;155(2):817–825. doi: 10.1128/jb.155.2.817-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Cross A. S., Wegmann A., Germanier R., Sadoff J. C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987 Jul;80(1):51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Passive protection against Pseudomonas aeruginosa infection in an experimental leukopenic mouse model. Infect Immun. 1983 May;40(2):659–664. doi: 10.1128/iai.40.2.659-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev B. A., Kocharova N. A., Knirel Y. A., Shashkov A. S., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic Antigens of Pseudomonas aeruginosa. The structure of the polysaccharide chain of Ps.aeruginosa O:6 (Lanyi) lipopolysaccharide. Eur J Biochem. 1982 Jun 15;125(1):229–237. doi: 10.1111/j.1432-1033.1982.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Knirel YuA, Vinogradov E. V., Kocharova N. A., Paramonov N. A., Kochetkov N. K., Dmitriev B. A., Stanislavsky E. S., Lányi B. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa (a review). Acta Microbiol Hung. 1988;35(1):3–24. [PubMed] [Google Scholar]
- Käyhty H., Jousimies-Somer H., Peltola H., Mäketä P. H. Antibody response to capsular polysaccharides of groups A and C neisseria meningitidis and Haemophilus influenzae type b during bacteremic disease. J Infect Dis. 1981 Jan;143(1):32–41. doi: 10.1093/infdis/143.1.32. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Bennett S. E. Structural analysis and immunogenicity of Pseudomonas aeruginosa immunotype 2 high molecular weight polysaccharide. J Clin Invest. 1986 Feb;77(2):491–495. doi: 10.1172/JCI112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Cohen M., Jennings H. Further purification and characterization of high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1983 Dec;42(3):936–941. doi: 10.1128/iai.42.3.936-941.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Cross-protection by Pseudomonas aeruginosa polysaccharides. Infect Immun. 1982 Dec;38(3):1117–1122. doi: 10.1128/iai.38.3.1117-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Pollack M., Cohen M. Immunochemical characterization of high-molecular-weight polysaccharide from Fisher immunotype 3 Pseudomonas aeruginosa. Infect Immun. 1984 Aug;45(2):309–313. doi: 10.1128/iai.45.2.309-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Safety and immunogenicity of high molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J Clin Invest. 1982 Feb;69(2):303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosa immunotype 2. Infect Immun. 1981 Nov;34(2):461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Thomas D. M. Characterization of the human immune response to a polysaccharide vaccine from Pseudomonas aeruginosa. J Infect Dis. 1983 Aug;148(2):206–213. doi: 10.1093/infdis/148.2.206. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Thomas D. M. Lipopolysaccharide and high-molecular-weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982 Feb;145(2):217–223. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- Pollack M. Antibody activity against Pseudomonas aeruginosa in immune globulins prepared for intravenous use in humans. J Infect Dis. 1983 Jun;147(6):1090–1098. doi: 10.1093/infdis/147.6.1090. [DOI] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Stoll B. J., Pollack M., Young L. S., Koles N., Gascon R., Pier G. B. Functionally active monoclonal antibody that recognizes an epitope on the O side chain of Pseudomonas aeruginosa immunotype-1 lipopolysaccharide. Infect Immun. 1986 Sep;53(3):656–662. doi: 10.1128/iai.53.3.656-662.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]





