Abstract
Chronic postnatal hyperoxia attenuates the hypoxic ventilatory response (HVR) of rats. To determine whether the ability to detect deficits in the HVR depends on the degree of hypoxia, we assessed the HVR at several levels of hypoxia in adult rats reared in 60% O2 for the first two postnatal weeks. Hyperoxia-treated rats exhibited smaller increases in ventilation than control rats at 12% O2 (30±8 vs. 53±4% baseline, mean±SEM; P=0.02) but not at 10% O2 (83±11 vs. 96±14% baseline; P=0.47). Interestingly, 10% O2 was used as the test gas in the only study to assess HVR in mice exposed to developmental hyperoxia, and that study reported normal HVR (Dauger et al., Chest 123:530-8, 2003). Therefore, we assessed the HVR at 12.5% O2 in adult mice reared in 60% O2 for the first two postnatal weeks. Hyperoxia-treated mice exhibited smaller increases in ventilation (28±7 vs. 58±8% baseline; P<0.01) and smaller carotid bodies than control mice. We conclude that hyperoxia impairs the HVR in both rats and mice, but this effect is most evident at moderate levels of hypoxia.
Keywords: control of breathing, respiration, developmental plasticity, carotid body, mouse
1. Introduction
Chronic exposure to hyperoxia (30-60% O2) during the postnatal period attenuates the hypoxic ventilatory response (HVR) exhibited by adult rats (Ling et al., 1996; Bavis & Mitchell, 2008). This plasticity is specific to development: equivalent hyperoxic exposures beginning after the second postnatal week do not appear to have lasting effects on respiratory control (Bavis et al., 2002). Persistent changes in the HVR reflect abnormal development of the carotid body and its afferent neurons. Specifically, rats reared in hyperoxia exhibit carotid body hypoplasia and have fewer chemoafferent axons in the carotid sinus nerve (CSN) (Erickson et al., 1998; Prieto-Lloret et al., 2004). As a result, whole-nerve CSN responses to hypoxia are greatly reduced throughout life (Fuller et al., 2002). Similar effects of developmental hyperoxia have been noted in cats (Hanson et al., 1989), quail (Simons & Bavis, 2007), and zebrafish (Vulesevic & Perry, 2006), suggesting that chronic hyperoxia influences the development of peripheral O2 sensors across vertebrate taxa.
Despite repeated observations of this hyperoxia-induced developmental plasticity, there also have been studies with rats and mice that did not detect blunting of the adult HVR after chronic postnatal hyperoxia. For example, Prieto-Loret et al. (2004) studied adult rats (3.5-4.5 mo of age) that had been exposed to 60% O2 for the first postnatal month. Although these rats had smaller carotid bodies and, on average, diminished carotid body responses to hypoxia, their HVR (gauged by changes in respiratory frequency alone) were similar to those of age-matched controls. Likewise, Dauger et al. (2003) found that mice (45 or 100 d of age) reared in 65% O2 for the first postnatal month displayed normal HVR; no other studies have investigated the effects of chronic hyperoxia on respiratory control in mice. As pointed out by the authors of these studies, however, 10% O2 was used to assess the HVR in these experiments whereas other experiments have employed more moderate hypoxia (12-12.5% O2 or greater) to demonstrate blunted HVR in hyperoxia-treated rats (Dauger et al., 2003; Prieto-Lloret et al., 2004). One possibility, therefore, is that hyperoxia-treated rats and mice are capable of achieving increases in ventilation similar to untreated (control) animals but require a more severe hypoxic exposure to elicit this response. If this hypothesis is correct, one would predict that hyperoxia-induced blunting of the adult HVR would be more apparent when assessed at mild or moderate levels of hypoxia (i.e., in response to submaximal stimuli).
In the present study, we investigated whether the ability to detect deficits in the rat HVR varies depending on the degree of hypoxia. Specifically, we studied the HVR at three levels of hypoxia (10%, 12%, and 14% O2) in adult rats that had been reared in 60% O2 for the first two postnatal weeks. Based on this analysis, we subsequently revisited the question of whether developmental hyperoxia attenuates the HVR of adult mice. Since diminished carotid body size is a consistent finding in hyperoxia-treated rats (Erickson et al., 1998; Fuller et al., 2002; Prieto-Lloret et al., 2004), we also studied the effects the chronic postnatal hyperoxia on carotid body volume in rats and mice.
2. Methods
All experimental procedures were approved by the Animal Care and Use Committee at Bates College.
2.1 Experimental animals
Experiments were conducted on Sprague-Dawley rats (SAS SD; Charles River Laboratories, Wilmington, MA USA) and C57BL/6 mice (Charles River Laboratories) reared in 21% O2 (“Control”) or 60% O2 (“Hyperoxia”) for the first two postnatal weeks; experimental animals were derived from 4 rat litters (2 Control, 2 Hyperoxia) and 11 mouse litters (6 Control, 5 Hyperoxia). Pregnant rats and mice (housed singly in standard cages) were placed into large (~275 l) clear acrylic chambers 1-2 days prior to parturition. Chambers were flushed with gases at sufficient flow rates to maintain target gas levels (21% O2 or 60% O2; CO2<0.4%). Chambers were opened briefly (<15 min) to clean cages every few days as needed. After the first 14 postnatal days, rats were housed in room air in standard animal facilities until studied as adults (rats: 2-3 mo of age; mice: 3.5-4.5 mo of age). Animals were maintained in a 12:12 light cycle with food and water ad libitum throughout the study.
2.2 Surgical preparation
At least one week prior to ventilation measurements, temperature transponders (E-mitter G2; Mini-Mitter, Bend, OR USA) were surgically implanted into the abdominal cavity. Anesthesia was induced with isoflurane in a closed box and subsequently maintained via nose cone (~2.5% isoflurane, balance O2); adequacy of anesthesia was assessed by lack of a withdrawal response to toe pinch. Transponders were placed into the abdominal cavity through a ventral, midline incision. Carprofen (5 mg kg-1, s.c.; Pfizer Animal Health, Exton, PA USA) was administered immediately after surgery as an analgesic.
2.3 Ventilation measurements
Ventilation was measured for 21 rats (Control: 5 ♂, 4 ♀; Hyperoxia: 9 ♂, 3 ♀) and 31 mice (Control: 12 ♂, 3 ♀; Hyperoxia: 10 ♂, 6 ♀). Measurements were made on conscious, unrestrained animals via whole-body barometric plethysmography; all measurements were made during the light portion of the light-dark cycle. Individuals were placed into a clear acrylic chamber (rat: 14 cm i.d., ~1.7 l; mouse: 9.5 cm i.d., ~0.5 l). Air was forced through the chamber using a gas mixing mass flow controller (MFC-4; Sable Systems, Las Vegas, NV USA) and valves (series 840; Sierra Instruments, Monterey, CA USA); flow rates (STPD) were 2.0 l min-1 for rats and 0.6 l min-1 for mice. Air was warmed and humidified before entering the chamber and the chamber floor was covered with water beneath the animal. When sealed, respiratory-related pressure fluctuations within the chamber were measured with a differential pressure transducer (DP45; Validyne Engineering, Northridge, CA USA); the system was pre-calibrated by repeated 0.3 ml (rat) or 0.1 ml (mouse) air injections. Pressure fluctuations and chamber temperature (T-type thermocouple) were recorded to computer (Chart 5.2, ADInstruments, Colorado Springs, CO USA). Body temperature (Tb) was continuously recorded by telemetry (VitalView 4.1, Mini-Mitter, Bend, OR USA). These measurements were used to calculate tidal volume (ml (BTPS); Drorbaugh & Fenn, 1955), respiratory frequency (breaths min-1), and their product, minute ventilation (ml min-1 (BTPS)); volumes were then normalized to body weight.
On the day of the experiment, individuals were weighed and placed into the plethysmograph chamber. Individuals were initially exposed to 21% O2 (balance N2) for at least one hour. When the animal was resting quietly, the chamber was briefly sealed (~1 min) to record respiratory-related pressure fluctuations. Our objective was to obtain 45-60 s of data free from movement artifacts and sighs, and measurements were repeated if necessary. After measurements were made at 21% O2, rats were exposed to three levels of hypoxia in random order: 14% O2, 12% O2, and 10% O2 (balance N2); ventilation was measured after 15 min in hypoxia, and inspired O2 was not returned to 21% O2 between hypoxic exposures. Mice were exposed to only one level of hypoxia (12.5% O2, balance N2) and ventilation was measured after 15 min. Barometric pressure was recorded during each ventilation measurement and averaged 754±6 mmHg (mean±SD).
2.4 Carotid body volume measurements
Carotid body volume was estimated for adult male rats (n= 5 Control, 4 Hyperoxia) and mice (n= 5 Control, 5 Hyperoxia) at least one week after the ventilation measurements were completed. Rats were euthanized with 100% CO2 and transcardially perfused with ice cold 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS; pH 7.4). Mice were euthanized with 100% CO2 and decapitated (i.e., no perfusion step). For both species, carotid bifurcations were dissected out en bloc and post-fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.4 for 1 h) and cryoprotected in sucrose (1 d in 20% sucrose (rat) or 30% sucrose (mouse) in PBS). Extraneous tissue was dissected away and carotid bifurcations were then embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA, USA) on dry ice and stored at -80°C. Samples were later sectioned (12 μm (rat) or 9 μm (mouse)) with a cryostatic microtome onto Fisherbrand Superfrost Plus slides.
For rats, all slides were processed for morphological analysis using hematoxylin and eosin (H&E) staining. For mice, however, preliminary analysis of H&E stained slides revealed that the carotid body had a much more irregular shape than in rats with poorly defined borders. Accordingly, immunohistochemical labeling for tyrosine hydroxylase (marker for glomus cells) and vimentin (marker for sustentacular cells) was used to facilitate visualization of the carotid body. Every ninth section was stained with H&E to identify the range of sections containing the carotid body. The remaining sections were then incubated for 1 h with 10% Normal Donkey Serum (NDS) (Jackson ImmunoResearch, West Grove, PA, USA) and 0.3% Triton X-100 in PBS to block non-specific binding sites and permeabilize cell membranes. Sections were sequentially incubated overnight at 4°C with primary antibodies (diluted in 1.5% NDS in PBS + 0.05% Tween-20) for tyrosine hydroxylase (sc-7848, 1:50) and vimentin (sc-5565, 1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following each primary antibody incubation, sections were incubated for 1 h with their corresponding secondary antibody diluted 1:100 in 3% NDS in PBS and conjugated to either TRITC (for TH labeling; sc-2094) or FITC (for vimentin labeling; sc-2090) (Santa Cruz Biotechnology). Slides were coverslipped with Vectashield HardSet mounting medium + DAPI (Vector Labs, Burlingame, CA, USA).
Serial sections containing the carotid body were imaged with Nikon’s NIS Elements software using a Nikon Eclipse 80i microscope with a 2 megapixel camera. The carotid body was outlined and the area calculated using Image J software (National Institutes of Health, Bethesda, MD, USA). To estimate the area for missing sections (i.e., H&E stained sections for mouse carotid bodies), the areas of the two adjacent sections were averaged. Carotid body volume was then estimated from the area of the carotid body on each section, section thickness, and the total number of sections containing the carotid body: Σ[carotid body area in each section (μm2) × section thickness (μm)].
2.5 Data analysis
Raw values for respiratory frequency, tidal volume, and minute ventilation were compared between treatment groups using two-way repeated measures ANOVA (factor 1: treatment group, factor 2: inspired O2 (FiO2)) and, where appropriate, Tukey post hoc tests. Baseline ventilation, normalized HVR (i.e., increase in minute ventilation as a percentage of baseline), and carotid body volumes were compared between treatment groups using independent samples t-tests. Sex was not included in the statistical models given the relatively small sample sizes (particularly for females); however, chronic postnatal hyperoxia has similar effects on the respiratory control of male and female rats (Bavis et al., 2007), and no obvious sex differences were noted for the HVR of rats or mice in the present study. All statistical tests were run using SigmaStat 3.11 (SPSS, Chicago, IL), and P<0.05 was considered significant. Unless otherwise noted, values are reported as mean±SEM.
3. Results
3.1 Rats
Ventilation was measured in adult rats at four levels of inspired O2 (FiO2): 21, 14, 12, and 10% (Fig. 1). As expected, minute ventilation increased significantly as FiO2 decreased for both Hyperoxia and Control rats (main effect for FiO2, P<0.001) (Fig. 1C). Overall, minute ventilation was significantly lower in Hyperoxia rats than in Control rats (main effect for treatment, P<0.01). Closer inspection of Fig. 1C suggests that this effect is driven by lower minute ventilation during hypoxia (particularly at 12-14% O2). Although ANOVA did not detect a significant treatment × FiO2 interaction for minute ventilation (P=0.14), baseline (21% O2) minute ventilation was nearly identical in Hyperoxia and Control rats (51.5±2.7 vs. 53.2±1.5 ml min-1 (100g)-1, respectively; P=0.61, t-test). Moreover, when the HVR was expressed as the percentage increase from baseline (i.e., 21% O2), the HVR was significantly lower in Hyperoxia rats than in Control rats at 12% O2 (29.6±7.6 vs. 53.2±4.4%, respectively; P=0.02), but not at 14% O2 (8.9 ±7.6 vs. 28.7 ±11.5%; P=0.15) or 10% O2 (83.1±10.7 vs. 95.9±14.2%; P=0.47).
Fig. 1.
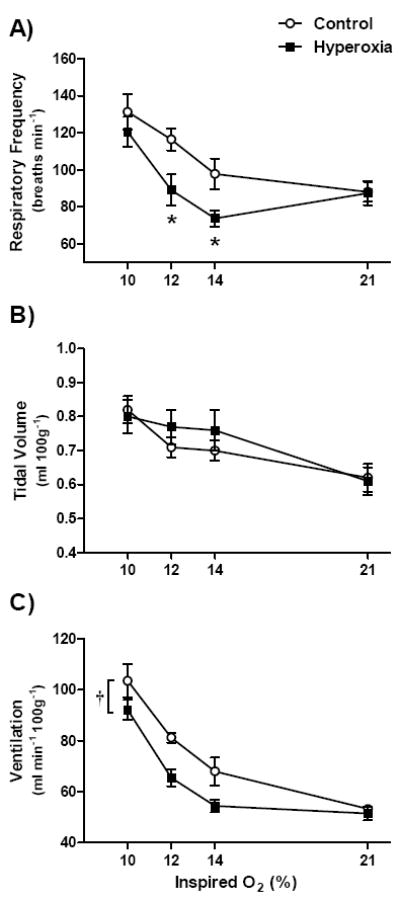
(A) Respiratory frequency, (b) tidal volume, and (C) minute ventilation for 2-3 mo old rats breathing 21%, 14%, 12%, and 10% O2. Rats were reared in 21% O2 (Control; n=9) or 60% O2 (Hyperoxia; n=12) for the first two postnatal weeks. Values are mean±SEM. Data were analyzed by two-way ANOVA. Where the treatment × FiO2 interaction was significant (panel A), * denotes P<0.05 vs. Control at the same FiO2. In panel C, † denotes P<0.05 for the main effect for hyperoxia treatment.
As in previous studies (Ling et al., 1996; Bavis et al., 2007), the blunted HVR of Hyperoxia rats was primarily explained by a smaller increase in respiratory frequency (Fig. 1A). Importantly, there was a significant interaction between treatment group and FiO2 (P=0.01) for respiratory frequency. Post hoc tests revealed that respiratory frequency was significantly lower in Hyperoxia rats than in Control rats while breathing 14% O2 (P=0.03) or 12% O2 (P=0.01) but not while breathing 21% O2 (P=0.95) or 10% O2 (P=0.31); thus, as for minute ventilation, differences between treatment groups were most apparent at moderate levels of hypoxia. Although tidal volume increased in hypoxia (main effect for FiO2, P<0.001), this response was not affected by postnatal hyperoxia (treatment, P=0.70; treatment × FiO2, P=0.12) (Fig. 1B).
Hyperoxia rats had smaller carotid bodies as adults than Control rats (9.2±1.4 vs. 32.9±1.9 × 106 μm3, respectively; P<0.001).
3.2 Mice
Since the effects of postnatal hyperoxia were more apparent in rats when the HVR was assessed at moderate levels of hypoxia (12-14% O2), we studied the HVR at 12.5% O2 for mice reared in hyperoxia (Fig. 2). There was a significant interaction between treatment and FiO2 for minute ventilation (P<0.01). Post hoc tests revealed that Hyperoxia rats had lower minute ventilation while breathing 12.5% O2 (P=0.02) but not while breathing 21% O2 (P=0.64) (Fig. 2C). Similarly, the HVR was significantly lower in Hyperoxia mice than in Control mice when expressed as the percentage increase from baseline (28.3±6.5 vs. 57.5±8.1%, respectively; P<0.01).
Fig. 2.
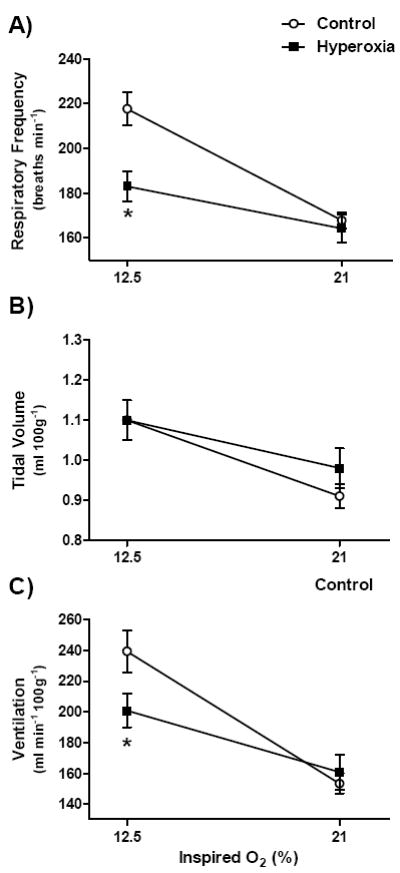
(A) Respiratory frequency, (b) tidal volume, and (C) minute ventilation for 3.5-4.5 mo old mice breathing 21% or 12.5% O2. Mice were reared in 21% O2 (Control; n=15) or 60% O2 (Hyperoxia; n=16) for the first two postnatal weeks. Values are mean±SEM. Data were analyzed by two-way ANOVA. Where the treatment × FiO2 interaction was significant (panels A and C), * denotes P<0.05 vs. Control at the same FiO2.
As in rats (Fig. 1), the blunted HVR was almost entirely explained by a smaller increase in respiratory frequency in Hyperoxia mice (treatment × FiO2, P<0.01) (Fig. 2A). Hyperoxia mice had lower respiratory frequency while breathing 12.5% O2 (P<0.001) but not while breathing 21% O2 (P=0.69) (Fig. 2A). Although tidal volume increased in hypoxia (main effect for FiO2, P<0.001), this response was not affected by postnatal hyperoxia (treatment, P=0.61; treatment × FiO2, P=0.18) (Fig. 2B).
Hyperoxia mice had smaller carotid bodies as adults than Control mice (1.3±0.2 vs. 4.8±0.8 × 106 μm3, respectively; P<0.01).
4. Discussion
We observed that chronic postnatal hyperoxia (2 weeks in 60% O2) caused long-lasting attenuation of the HVR in both rats and mice. When acutely exposed to 12-12.5% O2 as adults (i.e., after >1.5 mo recovery in room air), the HVR of hyperoxia-treated animals was approximately half that of age-matched controls; in both species, this blunting reflected a smaller increase in respiratory frequency. Although this developmental plasticity has been well characterized in rats (Ling et al., 1996; Bavis & Mitchell, 2008), this is the first report of a similar effect in mice. Importantly, as in rats (Erickson et al., 1998; Fuller et al., 2002; Prieto-Lloret et al., 2004), this plasticity appears to be mediated at least in part by abnormal development of the carotid body in mice. In both species, the carotid body of hyperoxia-treated animals was only about one-fourth the normal size; it is likely that postnatal hyperoxia elicited other morphological changes to the carotid chemoafferent pathway (e.g., degeneration of chemoafferent neurons; Erickson et al., 1998), but the present study did not investigate these potential effects. Interestingly, baseline ventilation was not affected by postnatal hyperoxia in either species despite these changes to the carotid body, suggesting a relatively small contribution of peripheral chemoreceptors to normoxic ventilatory drive in adults or, perhaps, compensatory plasticity / redundancy in this control system (e.g., Forster, 2003). Since mice respond to chronic postnatal hyperoxia in much the same way as rats, transgenic mouse models may prove useful to explore the molecular mechanisms underlying this important model of developmental plasticity.
It perhaps is not surprising that chronic postnatal hyperoxia blunts the HVR in both rats and mice since developmental hyperoxia has similar effects in other mammals (e.g., cats: Hanson et al., 1989) as well as in other vertebrate taxa (e.g., quail: Simons & Bavis, 2007; fish: Vulesevic & Perry, 2006). However, our findings do contrast with previous studies by Prieto-Lloret et al. (2004) and Dauger et al. (2003) that reported normal HVR for rats and mice, respectively, reared in 60-65% O2 for the first postnatal month. We propose that the HVR data for rats in the present study reconcile these apparently conflicting findings. When the HVR was assessed at three different levels of hypoxia, blunting was more apparent at moderate levels of hypoxia (12% O2, and to some extent 14% O2) compared to severe hypoxia (10% O2) (see Fig. 1, particularly panel A). Since Prieto-Lloret et al. (2004) and Dauger et al. (2003) used 10% O2 to assess the HVR in their studies, the data from all three studies are seemingly in agreement (i.e., no significant blunting at 10% O2); our data suggest that Prieto-Lloret et al. and Dauger et al. may have detected blunted HVR if they used hypoxic challenges with FiO2>10% O2. Importantly, Prieto-Loret et al. (2004) did report abnormal carotid body morphology and carotid body responses to hypoxia in their study, even though HVR appeared normal. Thus, our data may also help to explain their paradoxical results (i.e., the HVR likely was abnormal, as predicted from the carotid body data, but this effect was not discernible with severe hypoxia).
The present data suggest that the FiO2 used to assess HVR can explain why some studies detect blunted HVR after chronic postnatal hyperoxia while others do not, but this may not be the only explanation for differing conclusions. For example, rats and mice were reared in hyperoxia for the first postnatal month in the studies by Prieto-Loret et al. (2004) and Dauger et al. (2003) whereas the present study employed a two week hyperoxic exposure. In the seminal study by Ling et al. (1996), in which rats were exposed to 60% O2 for the first postnatal month, hyperoxia-treated rats exhibited lower arterial partial pressure of O2 (PaO2) during acute hypoxia compared to age-matched controls, suggesting gas exchange impairment. This difference in arterial hypoxemia at equivalent FiO2 tends to obscure differences in the HVR, and the blunted HVR of hyperoxia-treated rats is much more evident when the HVR is assessed at equivalent PaO2 (by varying FiO2) (see Fig. 2 in Ling et al., 1996). Hypoxic blood gases were not measured in the studies by Prieto-Lloret et al. (2004) or Dauger et al. (2003), but Dauger et al. reported lung injury and a trend toward lower normoxic PaO2 in their hyperoxia-treated mice. In the present study, we intentionally selected only a two week exposure to 60% O2 since we previously found that PaO2 in normoxia and hypoxia are normal in adult rats following this protocol (i.e., no evidence of impaired gas exchange) (Bavis et al., 2007).
Another difference between the present study and that of Dauger et al. (2003) is that Dauger et al. attempted to maintain isocapnia in their mice by raising FiCO2 to 3% during the hypoxic challenge; in the present study, the HVR was assessed under poikilocapnic conditions. However, it seems unlikely that this methodological difference explains the discrepancy between studies. Indeed, hyperoxia-treated rats (Ling et al., 1996; Prieto-Llore et al., 2004) and mice (Dauger et al., 2003) exhibit normal hypercapnic ventilatory responses as adults, suggesting that chronic postnatal hyperoxia does not cause any persistent change in CO2 sensitivity. Moreover, the phrenic nerve response to hypoxia is diminished in hyperoxia-treated rats despite maintaining strict isocapnia throughout the experimental protocol (Ling et al., 1997; Bavis et al., 2002; Fuller et al., 2002). Thus, after being reared in chronic postnatal hyperoxia, adult rats exhibit diminished respiratory responses to hypoxia in both isocapnic and poikilocapnic preparations.
Aside from potentially explaining conflicting reports in the literature, it is interesting in itself that we did not detect differences in the HVR at 10% O2 between control and hyperoxia-treated rats. Strong respiratory challenges may be advantageous to discern treatment effects under some circumstances, perhaps accentuating differences between groups during submaximal stimuli (or when maximal responses differ). On the other hand, if an experimental treatment shifts the ventilatory response curve toward lower FiO2 without altering the maximal response, it is possible that the HVR of different treatment groups will converge at lower FiO2 (i.e., at or approaching the maximal response) and obscure important treatment effects. Importantly, in urethane-anesthetized, paralyzed, and vagotomized rats, Ling et al. (1997) noted that control animals reached their maximal phrenic response to isocapnic hypoxia at higher PaO2 (i.e., less hypoxic stimulus) than rats reared in hyperoxia. Rats are often distressed at FiO2 below 10% O2 (Aaron & Powell, 1993), so we did not study the HVR of rats in more severe hypoxia in the present study. It is possible, however, that the HVR of control and hyperoxia-treated rats were near maximal at 10% O2; indeed, respiratory frequency appears maximal at 10% O2 in rats acclimatized in chronic hypoxia (Aaron & Powell, 1993). In light of these observations, the present study suggests that caution should be used when employing a single FiO2 to assess the HVR, at least until a treatment effect is established; otherwise, important treatment effects may be overlooked.
Acknowledgments
This study was supported by grant number P20 RR-016463 from the National Center for Research Resources (NCRR), a component of National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron EA, Powell FL. Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J Appl Physiol. 1993;74:1635–1640. doi: 10.1152/jappl.1993.74.4.1635. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol. 2008;104:1220–1229. doi: 10.1152/japplphysiol.01086.2007. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Simons JC. Developmental hyperoxia attenuates the hypoxic ventilatory response in Japanese quail (Coturnix japonica) Respir Physiol Neurobiol. 2008;164:411–418. doi: 10.1016/j.resp.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol. 2002;92:1013–1018. doi: 10.1152/japplphysiol.00859.2001. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Russell KE, Simons JC, Otis JP. Hypoxic ventilatory responses in rats after hypercapnic hyperoxia and intermittent hyperoxia. Respir Physiol Neurobiol. 2007;155:193–202. doi: 10.1016/j.resp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol. 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Vidruk EH, Wang Z-Y, Olson EB, Jr, Bisgard GE, Mitchell GS. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol. 2002;538:947–955. doi: 10.1113/jphysiol.2001.012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Eden GJ, Nijhuis JG, Moore PJ. Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn. In: Lahiri S, Forster RE, Davies RO, Pack AI, editors. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. Oxford University Press; New York: 1989b. pp. 113–120. [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Phrenic responses to isocapnic hypoxia in adult rats following perinatal hyperoxia. Respir Physiol. 1997;109:107–116. doi: 10.1016/s0034-5687(97)00045-5. [DOI] [PubMed] [Google Scholar]
- Prieto-Lloret J, Caceres AI, Obeso A, Rocher A, Rigual R, Agapito MT, Bustamante R, Castañeda J, Perez-Garcia MT, López-López JR, González C. Ventilatory responses and carotid body function in adult rats perinatally exposed to hyperoxia. J Physiol. 2004;554:126–144. doi: 10.1113/jphysiol.2003.049445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulesevic B, Perry SF. Developmental plasticity of ventilatory control in zebrafish, Danio rerio. Respir Physiol Neurobiol. 2006;154:396–405. doi: 10.1016/j.resp.2006.01.001. [DOI] [PubMed] [Google Scholar]


