Abstract
The WNT signaling pathway plays important roles in the self-renewal and differentiation of mesenchymal stem cells (MSCs). Little is known about WNT signaling in adipocyte differentiation of human MSCs. In this study, we tested the hypothesis that canonical and non-canonical WNTs differentially regulate in vitro adipocytogenesis in human MSCs. The expression of adipocyte gene PPARγ2, lipoprotein lipase, and adipsin increased during adipocytogenesis of hMSCs. Simultaneously, the expression of canonical WNT2, 10B, 13, and 14 decreased, whereas non-canonical WNT4 and 11 increased, and WNT5A was unchanged. A small molecule WNT mimetic, SB-216763, increased accumulation of β-catenin protein, inhibited induction of Wnt4 and 11 and inhibited adipocytogenesis. In contrast, knockdown of β-catenin with siRNA resulted in spontaneous adipocytogenesis. These findings support the view that canonical WNT signaling inhibits and non-canonical WNT signaling promotes adipocytogenesis in adult human marrow-derived mesenchymal stem cells.
Keywords: Wnt, human mesenchymal stem cells, adipocytogenesis, β-catenin, SB-216763, gene silencing
Introduction
Adult human mesenchymal stem cells (hMSCs), also known as marrow stromal cells, have the capacity to differentiate into adipocytes, osteoblasts, and chondrocytes [1-3]. WNT signaling regulates many processes during embryonic development and adult homeostasis [4], as well as in bone formation, remodeling, and repair [5, 6]. At least 19 types of Wnts, several families of secreted antagonists, and multiple receptors have been identified [7]. Two distinct Wnt signaling pathways have been described: the canonical pathway and the noncanonical pathway [4]. The canonical Wnt is not only a general stem cell growth factor but can also influence cell lineage decisions in certain stem cell types [8, 9]. Cell fate determination changes are often regulated by finely-tuned alterations in the canonical Wnt pathway [10]. In this regard, it has been demonstrated that canonical Wnt signaling is critically involved in activities of hMSC [11]. In previous studies, we [12-14] and others [16] showed evidence that Wnt/β-catenin signaling is involved in stimulation of chondrocytogenesis and inhibition of osteoblastogenesis and adipocytogenesis of human marrow stromal cells.
In humans, the proportions of fat, hematopoietic marrow and trabecular bone in the bone medullary cavity are affected by age or by osteoporosis [17]. In patients with unusually high bone mass, activation of LRP5, a WNT co-receptor, resulted in the inhibition of adipocyte differentiation in hMSCs [18]. Further, Wnt10b, a canonical Wnt, inhibits adipogenesis and stimulates osteoblastogenesis of murine 3T3-L1 preadipocytes [19]. Recently, the non-canonical Wnt4 and Wnt5a were shown to play crucial roles in murine adipogenesis as positive regulators [20]. Kang et al. reported that canonical Wnt signaling stimulates osteoblastogenesis of murine ST2 cells by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ (PPARγ) [21].
Although there is a growing body of information available about the mechanisms of adipocyte differentiation from studies with murine preadipocytes, little is known about the Wnt expression profile during human adipocytogenesis. We recently reported that there are age- and gender-dependent variations in constitutive expression of the WNT genes in hMSCs [22]. This study tests the hypothesis that canonical and non-canonical Wnts regulate adipocytogenesis in human cells. WNT genes were monitored during adipocytogenesis of hMSCs and the effects of modulating β-catenin with a small molecule agonist (SB-216763) or with targeted gene silencing were evaluated.
Materials and methods
Reagents
SB-216763 (Tocris Cookson Inc.) was dissolved in DMSO at 50 mM concentration and stored at −20°C. The stock solution was diluted with DMSO and the same volumes of DMSO were used as vehicle control.
Cell culture
Marrow was obtained as discarded material from patients undergoing total hip replacement [3, 22]. Samples from thirteen subjects, 6 women and 7 men, age from 36 to 85 years old, were included in this study. Not all specimens could be included in every experiment because of the surgical schedule and numbers of cells needed for each assay. Low density mononuclear cells were isolated by density centrifugation with Ficoll/Histopaque 1077 (Sigma, St Louis, MO, USA). Resident adipocytes were removed as a floating layer in the supernatant fraction. This procedure enriches for undifferentiated cells and includes a population of non-adherent hematopoietic cells as well as a fraction capable of adherence and differentiation into adipose and skeletal cells. Non-adherent cells were removed 24 hours after seeding. The adherent marrow stromal cells (MSCs) were expanded in phenol red-free α-MEM medium (Gibco BRL), 10% heat-inactivated fetal bovine serum (FBS-HI; Gibco BRL), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). Medium was replenished twice each week. The hMSCs were subcultured at a ratio of 1:5 when they attained approximately 80% confluence. Cells from passage 2 were used in these experiments.
A line of hMSCs, KM101 [15, 23] was used for some experiments. KM101 cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM; Gibco BRL) with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The medium was replenished twice each week until the cells reached near-confluence.
For some experiments, medium was replaced with fresh medium supplemented with 1% FBS-HI with or without SB-216763. After 6 hours, cells were harvested for Western immunoblotting. After longer intervals, cells were harvested for gene expression analysis or enumeration of adipocytes.
Conditions for adipocytogenic differentiation
For adipocyte differentiation, upon confluence of hMSCs in 100-mm dishes or 12-well tissue culture plates, medium was changed to α-MEM, 1% FBS-HI with supplements (1 μM dexamethasone, 0.5 mM 1-methyl-3-isobutylxanthine, and 10 μg/ml insulin), 100 U/mL penicillin, and 100 μg/mL streptomycin, referred to as adipocytogenic medium, as previously described [12, 24]. Eighteen days after treatment, lipid accumulation in adipocytes was visualized by staining with oil red-O as follows: cells were fixed in 10% formalin for 1 hour and stained for lipid with 0.3% oil red-O for 15 minutes. After rinsing thrice with ddH2O, the red-staining cells in six random areas of 1-mm2 were enumerated for each well and presented as an average ± standard deviation for 3-6 replicate wells.
RNA isolation and RT–PCR
Total RNA was isolated with TRIZOL reagent (Invitrogen) at intervals following transfer to adipocytogenic medium. Two μg of total RNA was reverse transcribed into cDNA with M-MLV (Promega) following the manufacturer’s instructions. Concentrations of cDNA and amplification conditions were optimized to reflect the exponential phase of amplification. One-twentieth of the cDNA was used in each 50 μL PCR reaction (30–40 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min) as described [12, 13, 24]. The gene-specific human primers were: PPARγ2 [25]: forward: 5′-ATTCTCCTATTGACCCAGAAAGCG-3′, reverse: 5′-AGCTTTATCTCCACAGACACGACATT-3′, lipoprotein lipase (LPL) [24]: forward: 5′-GAGATTTCTCTGTATGGCACC-3′, reverse: 5′-CTGCAAATGAGACACTTTCTC-3′, adipsin [26]: forward: 5′-CAAGCAACAAAGTCCCGAGC-3′ reverse: 5′-CCTGCGTTCAAGTCATCCTC-3′, and GAPDH: forward: 5′-GGGCTGCTTTTAACTCTGGT-3′, reverse: 5′-TGGCAGGTTTTTCTAGACGG-3′. The gene-specific primers for human WNTs had been designed for previous studies [22, 27]. PCR products were separated by 2% agarose gel electrophoresis and expression levels were measured by semi-quantitative RT-PCR. Images of bands were captured with KODAK Gel Logic 200 Imaging System and measured by KODAK Molecular Imaging Software (KODAK, Molecular Imaging Systems, New Haven, CT, USA). Quantitative data were expressed by normalizing the densitometric units to GAPDH (internal control).
Western immunoblotting
After 6 hours treatment with SB-216763 or DMSO control, human marrow stromal cells were harvested with lysis buffer containing 150 mM NaCl, 3 mM NaHCO3, 0.1% Triton x-100 and a mixture of protease inhibitors (Roche Diagnostics, CA) as previously described [12]. Cells were scraped from dishes and were homogenized in lysis buffer with a Kontes’ Pellet Pestle. Insoluble cellular materials were removed by centrifugation at 16,000 g. Protein concentration was determined with the BCA system (Pierce, Rockford, IL, USA). Proteins were resolved by electrophoresis on 4-12% SDS-PAGE (NuPAGE Bis-Tris gel; Invitrogen) and were transferred onto polyvinylidene fluoride membranes (Amersham Biosciences). The membranes were blocked with 5% nonfat milk in PBS buffer containing 0.1% Tween-20 (PBSMT) for 2–3 h at room temperature and incubated with primary antibodies overnight at 4°C: anti-β-catenin (E5, Santa Cruz Biotechnology) and anti-β-actin (Sigma). After removal of unbound primary antibodies by three 10-minute washes with PBS buffer containing 0.1% Tween-20 (PBST), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature and washed thrice for 10 minutes with PBST. The second antibody anti-mouse IgG-HRP was from Amersham, and anti-rabbit IgG-HRP was from Santa Cruz Biotechnology. The antibody-associated protein bands were revealed with the ECL-plus Western immunoblot system (Amersham Biosciences).
Transient transfection of β-catenin siRNA
Transient transfection of β-catenin siRNA (Stealth RNAi duplex siRNA, Invitrogen) or control siRNA (SiRNA-A, Santa Cruz Biothech., Inc.) into hMSCs was performed by electroporation with the Human MSC Nucleofector Kit (Lonza) according to the manufacturer’s instruction and as described [13]. In brief, hMSCs were harvested by trypsinization, and resuspended at one million cells in 100 μL of nucleofector solution for human MSCs with 100 pmole of β-catenin siRNA or control siRNA. Electroporation was performed in a Nucleofector™ II with program U-23 provided by Lonza/Amaxa Biosystems. Immediately after electroporation, the cells were transferred to 35- or 60-mm dishes in MEM-α with 10% FBS-HI. After confluence, cells in 60-mm dishes were prepared for Western immunoblot. Cells in 35-mm dishes were cultured for 14 days in expansion medium.
Statistical analyses
All experiments were performed three times, with 3 to 6 replicate wells per treatment. Data are presented as means ± standard error (SE). Datum that was more than ± 5 × SD from the mean of the rest of the samples was excluded as an outlier. Quantitative data were analyzed with either the non-parametric Mann-Whitney test or unpaired Student’s two-tailed t-test for independent samples. A value of p< 0.05 was considered significant.
Results
Expression of signature genes during adipocyte differentiation of hMSCs
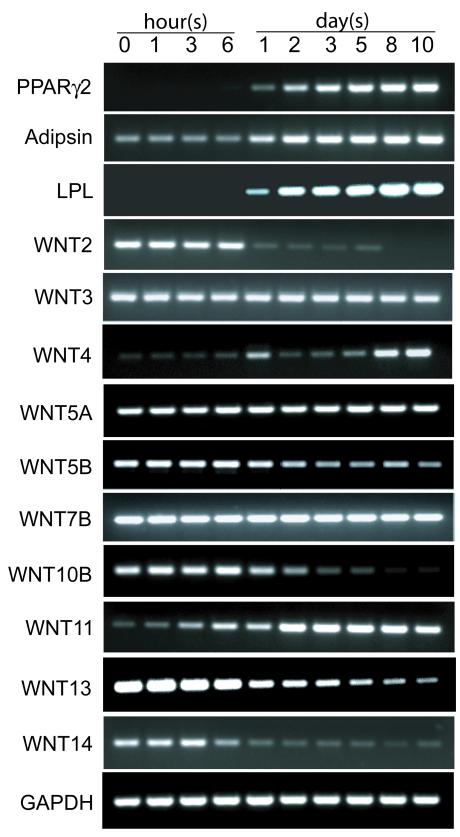
Human MSCs were cultivated in α-MEM with 1% FBS-HI and adipocytogenic supplements. Adipocyte signature genes, PPARγ2, LPL, and adipsin were examined at intervals with RT-PCR. Time course analysis indicated that expression of PPARγ2 and LPL was undetectable during the first 6 hour period in adipocytogenic medium and became detectable at 1 day (Figure 1). The expression of PPARγ2, LPL, and adipsin increased with time thereafter.
Figure 1.
Modulation of expression of adipocyte marker genes (PPARγ2, adipsin, and lipoprotein lipase, LPL) and WNT genes in representative hMSCs (60-year-old woman) at intervals after transfer to adipocytogenic medium. Agarose gel electrophoretograms show effects of time on RT-PCR products.
Expression of WNT genes during adipocyte differentiation of hMSCs
The expression of WNT genes was determined with RT-PCR in hMSCs undergoing adipocytogenesis at intervals to 10 days (Figure 1). The earliest change after transfer to adipocytogenic medium was an increase in non-canonical WNT11. There was a later upregulation of WNT4. In contrast, there were decreases in the expression of canonical WNT genes, WNT2, 10B, 13, and 14. The expression levels of WNT3, 5A, and WNT7B were unchanged during the 10 day experimental period. Compared with dramatic reductions in expression of WNT2, 10B, 13, and 14, there was a smaller and later decrease in expression of WNT5B. The expression of WNT10B was inversely correlated with PPARγ2 expression (r=−0.938, P= 0.018). The expression level of WNT3A was below detection through the evaluation period. WNT6 was expressed at levels too low for assessing variations. The expression of WNT16B in hMSCs appeared bimodal, with an increase from 0 to 24 hours, and decrease thereafter in adipocytogenic medium (Data not shown).
SB-216763 mimics WNT signaling pathway by accumulation of β-catenin in hMSCs
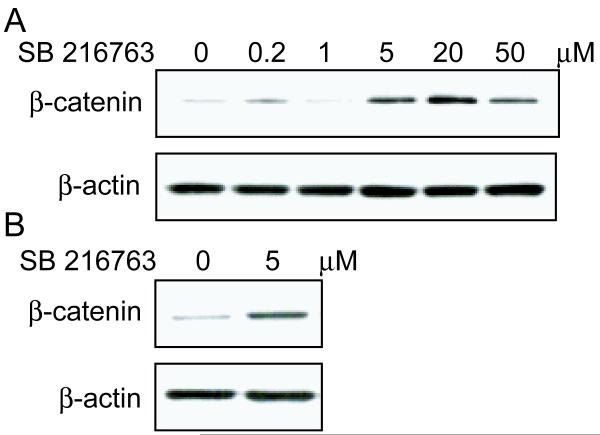
The line of KM101 human marrow stromal cells and hMSCs were analyzed for accumulation of β-catenin, a key member of the canonical Wnt signaling pathway, in the absence and presence of SB-216763, a small molecule WNT mimic. As shown in a representative result from two independent experiments, six hours of treatment with SB-216763 increased β-catenin in KM101 cells at concentrations at or greater than 5 μM (Figure 2A). Similarly, 5 μM SB-216763 increased cellular β-catenin in hMSCs (Figure 2B); that dose was used for subsequent experiments.
Figure 2.
Effects of SB-216763 on β-catenin levels in hMSCs. (A) There was a dose-dependent effect of SB-216763 on β-catenin in KM101 cells. (B) SB-216763 (5 μM) increased β-catenin in hMSCs (61-year-old man). Protein was extracted from cells after 6 hours incubation with or without SB-216763 and subjected to immunoblot for β-catenin and β-actin.
SB-216763 blocked induction of adipocyte genes in hMSCs
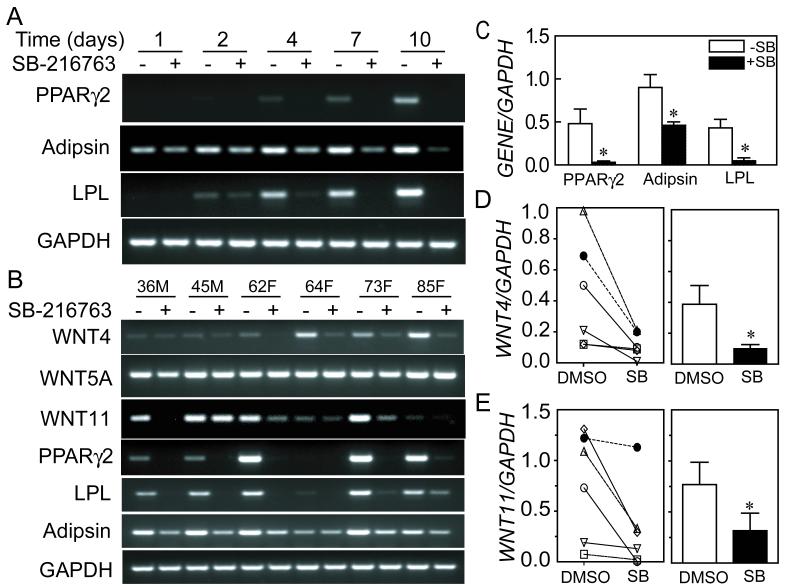
The effects of 5μM SB-216763 on induction of adipocyte gene expression in hMSCs were determined at intervals during culture in adipocytogenic medium (Figure 3A). There was a time-dependent increase in expression of PPARγ2, LPL, and adipsin in the absence of SB-216763, similar to the findings shown with another sample in Figure 1. In cells treated with 5 μM SB-216763, however, the expression of PPARγ2 was not detected at any time during the 10-day experiment. The expressions of LPL and adipsin were reduced or eliminated by 5 μM SB-216763. Reproducibility of the effect of SB-216763 was assessed with hMSCs from a series of 6 subjects after 7 days in adipocytogenic medium (Figure 3B, 36-85 years old, 4 women and 2 men). In these hMSCs, SB-216763 significantly inhibited expression of PPARγ2 (6% of control, p=0.044), adipsin (51%, p=0.018), and LPL (11%, p=0.013) (paired t-test) (Figure 3 C).
Figure 3.
Effects of SB-216763 on adipocyte differentiation and expression of WNT4 and WNT11 in hMSCs. (A) Expression of adipocyte signature genes in hMSCs was inhibited by SB-216763 after transfer to adipocytogenic medium. Agarose gel electrophoretogram shows PCR products for hMSCs (36-year-old man) after indicated days in adipocytogenic medium in the absence or presence of 5 μM SB-216763 (B) Expression of WNT4, WNT11 and adipocyte signature genes was inhibited by 5 μM SB-216763 in hMSCs from 6 subjects, evaluated 7 days after transfer to adipocytogenic medium. There was little or no effect on WNT5A. Each pair of lanes indicates the age in years and the gender (M, male; F, female) of the subject from whom the cells were obtained. (C) Expression of adipocyte signature genes was significantly inhibited by 5 μM SB-216763. Data are shown for 6 samples as the mean and standard error (SE) for each gene (* p<0.05, paired t-test). (D) Expression of WNT4 relative to GAPDH is shown (left panel) for each specimen in DMSO vehicle control and 5 μM SB-216763, and (right panel) as the group means and standard error (*p=0.031, Wilcoxon matched-pairs signed-ranks test). (E) Expression of WNT11 relative to GAPDH is shown (left panel) for each specimen in DMSO vehicle control and 5 μM SB-216763, and (right panel) as the group means and standard error (*p=0.031, Wilcoxon matched-pairs signed-ranks test).
SB-216763 inhibited WNT4and WNT11 induction in hMSCs
To determine whether activation of the canonical WNT signaling pathway alters expression of non-canonical WNT genes, we analyzed expression of WNT4, WNT5, and WNT11 at day 7 in adipocytogenic medium in the presence and absence of SB-216763. The expression of WNT4 was significantly reduced by SB-216763 (27% of DMSO vehicle control, p=0.031, Wilcoxon matched-pairs signed-ranks test, Figure 3B, D). WNT11 was reduced by SB-216763 to 40% of control (p=0.031) (Figure 3B, E). There were no significant effects of SB-216763 on expression of WNT5A in the series of 6 samples (Figure 3B).
SB-216763 inhibited adipocytogenesis in a dosage and duration-dependent way
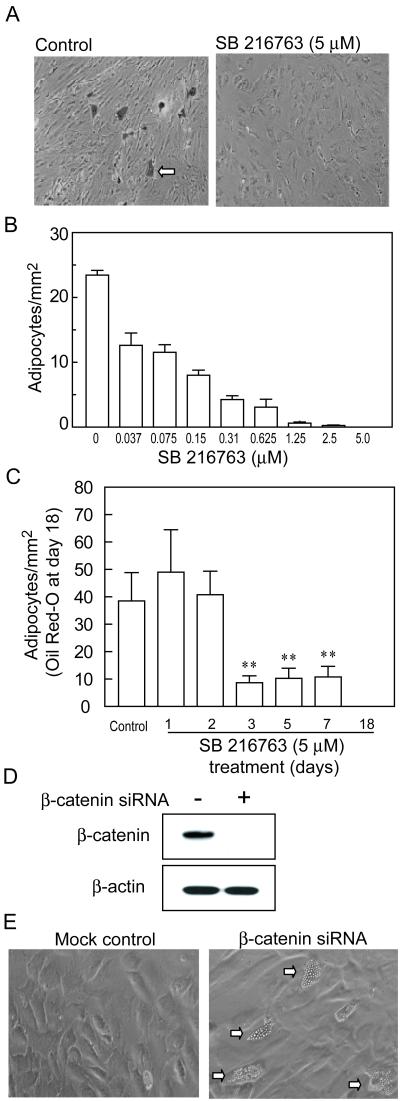
Human marrow stromal cells were used to determine the effects of different concentrations of SB-216763 on adipocyte differentiation (Figure 4). Generation of oil red-O-positive cells after 18 days of culture was inhibited significantly by 0.037μM SB-216763 (54.0% of control, p<0.0001) (Figure 4B). The number of adipocytes was decreased further with higher concentrations of SB-216763. At the concentration of 5 μM SB-216763, adipocyte differentiation was blocked completely (Figure 4A, B).
Figure 4.
Effects of SB-216763 and β-catenin siRNA on adipocytogenesis in hMSCs. (A) Photomicrographs of cultures of representative hMSCs (47-year-old man) without and with 5μM SB-216763 for 18 days show oil red-O-positive cells (arrow) in control, but not in treated cultures. (B) Cultures of hMSCs (47-year-old man) were treated with different concentrations of SB-216763 for 18 days. Bars indicate the numbers of oil red-O-positive cells per mm2, presented as mean ± S.D. of 4 replicate wells. (C) Cultures of hMSCs (60-year-old man) were treated with 5 μM SB-216763 for the first 1, 2, 3, 5, 7, days or the entire 18 days. Bars indicate the numbers of oil red-O-positive cells per mm2, enumerated at day 18, presented as mean ± S.D. of triplicate wells. Asterisks indicate significance (**p<0.01) of treatments compared with control (no exposure to SB-216763). (D) Western immunoblot shows that β-catenin protein in hMSCs was absent in cells transfected with β-catenin siRNA, but not control siRNA. (E) Phase-contrast photomicrograph shows that there was spontaneous generation of lipid-containing adipocytes (arrows) in control medium in hMSCs transfected with β-catenin siRNA, but not in control hMSCs.
Reproducibility of the inhibitory effect of 5 μM SB-216763 on adipocyte differentiation was assessed with hMSCs from 6 subjects (57-82 years old, 2 women and 4 men). There was a range in the numbers of adipocytes generated in cultures of hMSCs from different subjects (4.9 to 59.3 per mm2), without an apparent effect of age or gender. There were no oil-red-O-positive cells in cultures treated with 5μM SB-216763.
The duration of exposure to SB-216763 necessary to inhibit adipocyte differentiation was assessed (Figure 4C). The number of adipocytes generated 18 days after transfer to adipocytogenic medium was similar in controls and in hMSCs that were exposed to 5 μM SB-216763 for only the first 1 or 2 days. When exposure duration was between 3 and 7 days, the number of adipocytes was between 23 and 28% of controls. Continuous exposure to SB-216763 for the 18 days of the experiment resulted in complete inhibition of adipocytogenesis.
Knockdown of β-catenin resulted in spontaneous adipocytogenesis in hMSCs
To further assess the role of β-catenin in adipocyte differentiation of hMSCs, we transfected β-catenin siRNA or control siRNA into hMSCs. Western immunoblot verified that β-catenin protein was absent in cells transfected with 100 pmole siRNA per million cells, but was present in cells transfected with control siRNA (Figure 4D). Knockdown of β-catenin with siRNA resulted in spontaneous adipocyte differentiation of hMSCs in basal medium (Figure 4E). After 14 days, there were 6.8 ± 1.5 adipocytes per mm2 in β-catenin siRNA hMSCs (n=4 dishes), compared with the control group (0.2 ± 0.5, n=5) (p=0.015, Mann-Whitney test). These data further support the conclusion that β-catenin inhibits differentiation of hMSCs into adipocytes.
Discussion
Adipocyte differentiation consists of a complex series of events in which cellular and extracellular factors interact to induce an undifferentiated marrow stromal cell or pre-adipocyte to develop into an adipocyte. PPARγ is a member of the nuclear hormone receptor superfamily and is expressed at high levels specifically in adipose tissue and is a central regulator of adipocyte gene expression and differentiation [28, 29]. Studies with animal cells established the Wnt/β-catenin signaling pathway as an important regulator of adipocyte differentiation [30, 31]. These studies with human marrow-derived mesenchymal stem cells show that the canonical WNT signaling pathway inhibits adipocyte differentiation in vitro. First, during adipocyte differentiation, canonical WNT2, 10B, 13, and 14 genes were down-regulated in hMSCs. Second, activation of Wnt/β-catenin signaling with highly selective inhibitor of GSK-3β, SB-216763, inhibited adipocytogenesis of hMSCs. Third, knockdown of endogenous β-catenin with siRNA resulted in spontaneous adipocyte differentiation. These lines of evidence indicate that canonical WNT/β-catenin pathway inhibits adipocytogenesis in human MSCs. While the expression of canonical WNT2, 10B, 13, and 14 was downregulated in hMSCs undergoing adipocyte differentiation, there was increased expression of WNT11 and 4. These results suggest that in human cells, canonical WNT genes may be inhibitors of adipocyte differentiation and non-canonical WNTs, in particular WNT4 and 11 may be enhancers of adipocyte differentiation. A previous analysis of constitutive expression of WNTs in human MSCs revealed an age-related decline in a number of canonical WNTs, but that WNT4 was unique in displaying an age-related increase in cells from men [22]. It is possible that WNT expression plays a role in age-related lineage restriction in bone cell progenitors. Adult human MSCs from discarded surgical tissue provide an opportunity to unravel the mechanisms of canonical and non-canonical Wnt interactions in adipocyte differentiation and effects of clinical factors, such as age, diabetes, and use of anti-diabetic drugs, on adipocyte differentiation.
These data with human MSCs are similar to some aspects of differentiation reported with murine preadipocyte 3T3-L1 cells; wnt10b was described as a potent inhibitor of murine adipocytogenesis [32], and wnt4 was described as a promoter of murine adipocytogenesis [20]. There is no retrievable literature on the role of Wnt11 in adipocytogenesis, but it was the Wnt that displayed the earliest change, an increase, and prior to detection of PPARγ2 upregulation. Whereas non-canonical wnt5a promotes murine adipocytogenesis [20], it appeared in this study to be unchanged during adipocytogenesis of hMSCs and upon treatment with SB-216763. Bilkovski et al. reported that non-canonical Wnt5a maintained osteoblast potential and inhibited adipocytogenic differentiation in hMSCs that were isolated from umbilical cord blood [48]. The difference in roles of Wnt5a in Bilkovski’s study and ours may be due to different biological behaviors in their neonatal cells and adult marrow-derived hMSCs used herein. For example, Jager et al. summarized the evidence that cord blood-derived MSCs have a niche-specific phenotype, are constitutively osteoblastic independent of the usual stimuli, and have greater osteoblast potential than do adult hMSCs [33]. It is likely that different Wnts may be involved in constitutive lineage potential in cord blood and adult marrow-derived hMSCs. The lack of an effect of SB-216763 on WNT5A while it downregulated WNT 4 and 11 also suggests that WNT5A is unlikely to be a key regulator of adipocytogenesis of adult human MSCs.
In studies with the murine preadipocyte cell line 3T3-L1, Wnt5b was reported to be upregulated during adipogenesis and overexpression of Wnt5b stimulated adipogenesis [34]. A recent study with 3T3-L1 cells indicated that Wnt5a was more abundantly expressed than Wnt5b, but both were down-regulated upon induction of adipogenic differentiation in this cell line [35]. Our present study with human cells shows that WNT5A was unchanged and WNT5B was decreased modestly in adipocyte differentiation, whereas wnt5a and 5b play stimulatory roles in mouse cells. Our results suggest that in human marrow stromal cells, WNT5B may be an inhibitor of adipocytogenesis, but the exact roles of WNT5B in human adipocytogenesis remain to be elucidated. Moreover, there may be differences in regulation of adipocytogenesis in human marrow-derived cells, compared with adipose tissue from normal subjects or from subjects with type 2 diabetes mellitus [36].
The studies with SB-216763 indicate cross-talk between canonical and non-canonical regulators of adipocyte differentiation. Inhibition of adipocytogenesis with SB-216763 was accompanied by striking decreases in WNT4 and WNT11 expression. Research with murine cells also reveals crosstalk [37] and multiple mechanisms [38] regulating adipocytogenesis.
Wnts signaling occurs by activating membrane receptors of the Frizzled (Fz) family in a complex with members of the low-density-lipoprotein-related protein (LRP) family; the complex promotes the stability and nuclear localization of β-catenin by either degradation of Axin or inhibitory of GSK3β activity; thereafter, β-catenin activates transcription in conjunction with co-transcription factors Lefs/Tcfs [4, 39, 40]. Activating Wnt signaling pathway with other agents such as LiCl or GSK3 inhibitor CHIR 99021in 3T3-L1 pre-adipocytes was shown to block adipocyte differentiation of these cells [19, 32]. Studies from our lab [12] and from De Boer et al. [41] with human MSCs indicated that LiCl inhibited adipocyte differentiation. LiCl is not only an inhibitor of GSK-3β [19, 32]; LiCl enhances phospholipase C activity [42] and inhibits casein kinase-2 and MAPK activated protein kinase-2 [43]. Coghlan et al. developed SB-216763, a highly selective, cell permeable small molecule inhibitor of GSK-3β, which does not inhibit the activity of the kinases required for insulin signaling and cell survival [44]. SB-216763 has been used to manipulate β-catenin signaling [44-47]. The Western immunoblot results presented here show that treatment with SB-216763 resulted in the accumulation of β-catenin in both a line of hMSCs, KM101, and in freshly isolated human MSCs. This study shows that activation of WNT signaling by SB-216763 inhibited the induction of PPARγ2 and adipocyte differentiation in hMSCs. The maximum effective concentration of SB-216763 is known to depend on the cell type [43]. This study shows that hMSCs were sensitive to 0.037 μM SB-216763 and that 5 μM blocked adipocytogenesis, in a reproducible manner with hMSCs representing different ages and both genders.
Targeted knock-down of β-catenin in hMSCs with the gene silencing approach resulted in spontaneous generation of lipid-filled adipocytes. This observation adds evidence to the hypothesis that canonical Wnt signaling is important for constitutive suppression of adipocytogenesis.
In summary, these results suggest that in adult marrow-derived human mesenchymal stem cells, canonical WNTs, specifically WNT2, 10B, 13, and 14, may all be constitutive inhibitors of adipocyte differentiation and that non-canonical WNTs, in particular WNT11 and 4, may be enhancers that are upregulated during adipocyte differentiation. Activation of Wnt/β-catenin signaling with highly selective inhibitor of GSK-3β, SB-216763, inhibited adipocytogenesis of hMSCs and knockdown of β-catenin with siRNA stimulated adipocyte differentiation in hMSCs. Further, these studies indicate cross-talk between canonical and non-canonical regulators of adipocyte differentiation. These findings support the view that canonical and non-canonical WNT signaling pathways regulate adipocytogenesis in human marrow-derived mesenchymal stem cells.
Acknowledgements
This study was presented in part at the 30th ASBMR annual meeting, 2008, in Montreal, QC, Canada and at the topical meeting of the ASBMR: New Frontiers in Skeletal Research: Bone, Fat and Brain Connections, 2009, in Bethesda, MD, USA. The authors greatly appreciate Behnam Eslami, DDS, DMSc, and Sara Anderson for assistance. L.S. was supported by The China Scholarship Council (CSC). This work is based on a thesis by L.S. for the M.D. degree at Zhongshan Hospital, Fudan University, Shanghai, China. The project was supported by grants from NIH R01 AG 025015 and R01 AG 028114 (to J.G.) and by a grant from the American Federation for Aging Research (A09052 to S.Z.). The discarded marrow was obtained and studied with approval and annual review from the Partners Human Research Committee.
Footnotes
There are no conflicts associated with this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- [2].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [3].Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-Related Intrinsic Changes in Human Bone Marrow-Derived Mesenchymal Stem Cells and Their Differentiation to Osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Issack PS, Helfet DL, Lane JM. Role of wnt signaling in bone remodeling and repair. HSS J. 2008;4:66–70. doi: 10.1007/s11420-007-9072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Piters E, Boudin E, Van Hul W. Wnt signaling: a win for bone. Arch. Biochem. Biophys. 2008;473:112–116. doi: 10.1016/j.abb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- [7].Tamura M, Nemoto E, Sato MM, Nakashima A, Shimauchi H. Role of the Wnt signaling pathway in bone and tooth. Front. Biosci. 2010;2:1405–1413. doi: 10.2741/e201. [DOI] [PubMed] [Google Scholar]
- [8].Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- [9].Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- [10].Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neth P, Ciccarella M, Egea V, et al. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- [12].Zhou S, Eid K, Glowacki J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J. Bone Miner. Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- [13].Zhou S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J. Cell. Biochem. 2011;112:1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shen L, Zhou S, Glowacki J. The effects of WNT activator SB-216763 on chondrocytogenesis of human bone marrow stromal cells. Chinese Journal of Experimental Surgery. 2010;27:116–119. [Google Scholar]
- [15].Harigaya K, Handa H. Gene expression of functional clonal cell lines from bone marrow stroma. Proc. Natl. Acad. Sci. USA. 1985;83:3477–3480. doi: 10.1073/pnas.82.10.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu G, Vijayakumar S, Grumolato L, et al. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J. Cell Biol. 2009;185:67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Justesen J, Stenderup K, Ebbesen EN, et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- [18].Qiu W, Andersen TE, Bollerslev J, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J. Bone Miner. Res. 2007;22:1720–1731. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- [19].Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- [20].Nishizuka M, Koyanagi A, Osada S, et al. Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett. 2008;582:3201–3205. doi: 10.1016/j.febslet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- [21].Kang S, Bennett CN, Gerin I, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- [22].Shen L, Zhou S, Glowacki J. Effects of age and gender on WNT gene expression in human bone marrow stromal cells. J. Cell Biochem. 2009;106:337–343. doi: 10.1002/jcb.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kinjo K, Miyakawa Y, Uchida H, et al. All-trans retinoic acid directly up-regulates thrombopoietin transcription in human bone marrow stromal cells. Exp. Hematol. 2004;32:45–51. doi: 10.1016/j.exphem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- [24].Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-β/Smad3 signaling. J. Biol. Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schiller PC, D’Ippolito G, Brambilla R, Roos BA, Howard GA. Inhibition of gap-junctional communication induces the trans-differentiation of osteoblasts to an adipocytic phenotype in vitro. J. Biol. Chem. 2001;276:14133–14138. doi: 10.1074/jbc.M011055200. [DOI] [PubMed] [Google Scholar]
- [26].Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O’Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes. 1998;47:1384–1391. doi: 10.2337/diabetes.47.9.1384. [DOI] [PubMed] [Google Scholar]
- [27].Yates KE. Demineralized bone alters expression of Wnt network components during chondroinduction of post-natal fibroblasts. Osteoarthritis Cartilage. 2004;12:497–505. doi: 10.1016/j.joca.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [28].Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr. Opin. Genet. Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- [29].MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- [30].Prestwich TC, Macdougald OA. Wnt/β-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007;19:612–617. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shang YC, Zhang C, Wang SH, et al. Activated β-catenin induces myogenesis and inhibits adipogenesis in BM-derived mesenchymal stromal cells. Cytotherapy. 2007;9:667–681. doi: 10.1080/14653240701508437. [DOI] [PubMed] [Google Scholar]
- [32].Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- [33].Jäger M, Zilkens C, Bittersohl B, Krauspe R. Cord blood--an alternative source for bone regeneration. Stem Cell Rev. 2009;5:266–277. doi: 10.1007/s12015-009-9083-z. [DOI] [PubMed] [Google Scholar]
- [34].Kanazawa A, Tsukada S, Kamiyama M, et al. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005;330:505–510. doi: 10.1016/j.bbrc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [35].van Tienen FHJ, Laeremans H, van der Kallen CJH, et al. Wnt5b stimulates adipogenesis by activating PPARγ, and inhibiting the β-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem. Biophys. Res. Commun. 2009;387:207–211. doi: 10.1016/j.bbrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [36].Kanazawa A, Tsukada S, Sekine A, et al. Association of the gene encoding winglesstype mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am. J. Hum. Genet. 2004;75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Takada I, Kouzmenko AP, Kato S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- [38].Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J. Biol. Chem. 2005;280:24004–24010. doi: 10.1074/jbc.M501080200. [DOI] [PubMed] [Google Scholar]
- [39].Moon RT, Bowerman B, Boutros M, et al. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- [40].Nusse R. Wnt signaling in disease and in development. Cell Research. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- [41].De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- [42].Chretien L, Laporte SA, Escher E, et al. Use of LiCl in phospholipase C assays masks the impaired functionality of a mutant angiotensin II receptor. Cell. Signal. 1997;9:379–382. doi: 10.1016/s0898-6568(97)00032-6. [DOI] [PubMed] [Google Scholar]
- [43].Davies SP, Reddy H, Caivano M, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Coghlan MP, Culbert AA, Cross DA, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- [45].Coghlan MP, Culbert AA, Cross DA, L. S, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- [46].Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Culbert AA, Brown MJ, Frame S, Hagen T, Cross DA, Bax B, Reith AD. GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces Tau dephosphorylation and β-catenin stabilisation without elevation of glycogen synthase activity. FEBS Lett. 2001;507:288–294. doi: 10.1016/s0014-5793(01)02990-8. [DOI] [PubMed] [Google Scholar]
- [48].Bilkovski R, Schulte DM, Oberhauser F, et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J. Biol. Chem. 2010;285:6170–6178. doi: 10.1074/jbc.M109.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]