Abstract
Angiogenin (ANG), a 14 kDa angiogenic ribonuclease, is upregulated in human prostate cancers, especially in hormone refractory diseases, and is the highest upregulated gene in Akt-driven prostate intraepithelial neoplasia (PIN) in mice. ANG has been shown to undergo nuclear translocation in both prostate cancer cells and cancer-associated endothelial cells where it binds to the promoter region of ribosomal DNA (rDNA) and stimulates ribosomal RNA (rRNA) transcription. ANG thus plays an essential role in prostate cancer progression by stimulating both cancer cell proliferation and tumor angiogenesis. A variety of ANG antagonists, including its antisense oligonucleotide, siRNA, soluble binding proteins, monoclonal antibody, enzymatic inhibitors, and nuclear translocation blockers, have all been shown to inhibit prostate cancer in various animal models. Accumulating evidence indicates that ANG is a molecular target for prostate cancer drug development.
Keywords: Angiogenin, angiogenesis, prostate cancer, androgen-independence, rRNA transcription
PROSTATE CANCER, ANDROGEN RECEPTOR, AND RIBOSOMAL RNA TRANSCRIPTION
Prostate cancer is the most commonly diagnosed cancer and the third leading cause of cancer death among men in the US [1]. Early stage prostate cancer requires androgen for growth and thus responds to androgen deprivation therapy. However, the disease progresses to an androgen-independent (hormone-refractory) state that is unresponsive to androgen ablation. Treatment of these hormone-refractory prostate cancer patients with chemotherapeutic agents is generally unsatisfactory, and recurrent prostate cancer has been considered incurable. However, recent studies with newer agents have shown encouraging results [2]. For the first time, docetaxel-based regimens have been shown to improve survival in patients with hormone-refractory prostate cancer in two phase III studies. This discovery changed the perceptions of chemotherapy for this disease [3].
The androgen receptor (AR) is a critical player in prostate cancer development and progression [4]. Androgen-dependent prostate cancers strictly depend on AR for growth and progression. Accumulating evidence shows that even a majority of androgen-independent prostate cancers continue to depend on AR signaling for survival and growth [5]. Given the exquisite dependence of prostate cancer cells on the androgen signaling axis [6], extensive effort has been undertaken to determine the mechanism(s) by which androgens induce prostate cancer cell proliferation and survival. Mechanistic investigation has revealed that AR acts as a master regulator of G1-S phase progression. It induces signals that promote CDK activity and inhibit RB function [7]. Both canonical and noncanonical AR-responsive elements have been identified, providing mechanistic insights of the transcription network that mediates AR-dependent prostate cancer growth [8]. However, an important question has not yet been answered, that is, how rRNA transcription is regulated by AR as there are no AR responsive elements found in the promoter region of rDNA located on chromosomes 21 and 22 [8]. A total of 400 copies of rDNA genes are located in clusters in chromosome 13, 14, 15, 21, and 22. The entire chromosomes 21 and 22 have been analyzed for AR binding sites [8]. rRNA transcription is essential for ribosome biogenesis, protein translation and therefore for cell growth and proliferation [9]. It has been well documented that androgens regulate the accumulation of rRNA during androgen-dependent cell growth [10, 11] and that androgen-stimulated rRNA synthesis is the mechanism by which androgens affect growth [12]. Recent advance on the mechanism and function of ANG indicate that this 14 kDa angiogenic ribonuclease mediates AR-dependent and -independent rRNA transcription in both androgen-dependent and -independent prostate cancers, playing an essential role in prostate cancer development and progression [13–15].
UP-REGULATION OF ANG IN PROSTATE CANCER
ANG was originally isolated from the conditioned medium of HT29 human colon adenocarcinoma cells based on its angiogenic activity [16]. ANG has been shown to play a role in tumor angiogenesis [17, 18], and its expression is up-regulated in many types of cancers [19], particularly in prostate cancer [13, 18, 20–22]. A comparison of ANG expression level by immunohistochemical (IHC) staining with the anti-ANG monoclonal antibody 26-2F in human prostate tissues from 23 prostate adenocarcinoma, 20 benign prostate hyperplasia (BPH), and 10 normal patients found that ANG is positively stained in all 23 prostate cancer and 20 BPH samples [13]. No ANG was detected in the cytoplasm and nucleus of the glandular epithelial cells in all 10 normal prostate tissue specimens. The sample size in this study was too small for statistical analysis, but increased nuclear staining of ANG was obvious in 17 of the 23 prostate cancer samples. In a large cohort (n=107) of radical prostatectomy specimens, IHC study found that ANG expression increased progressively as prostatic epithelial cells evolve from a benign to an invasive phenotype [20]. ANG staining was detected in 60% of the prostate cancer samples, whereas only 17% of the normal control samples were stained positive for ANG. These data indicate that although upregulation of ANG in prostate cancer is not 100%, it is clear that enhanced expression of ANG is correlated with the occurrence of the disease and with its progression. In another study, ANG protein content in the serum of patients with hormone refractory prostate cancer (40 patients); newly diagnosed, untreated prostate cancer (39 patients); and control patients with no evidence of prostate cancer (37 patients) was determined to be 436 ± 24, 392 ± 17, and 328 ± 20 ng/ml [21], respectively. There is a statistically significant difference in ANG levels between the controls and untreated, hormone-naive prostate cancer patients (P<0.01) and between controls and hormone refractory prostate cancer patients (P<0.001). There is also a trend toward higher levels of ANG in hormone refractory patients compared with untreated patients. It is known that circulating ANG in normal plasma is mainly produced by the liver [23] and can reach a concentration of 250–350 ng/ml [24, 25]. Therefore, if the elevated plasma ANG level in prostate cancer patients results from the up-regulation of prostatic expression of ANG, it reflects a dramatic increase in ANG expression in the prostate.
Consistently, mouse ANG is the most significantly up-regulated gene in AKT-induced PIN in the murine prostate-restricted AKT kinase transgenic (MPAKT) mice [21]. In these mice, expression of AKT in the ventral prostate results in activation of the p70S6K pathway and induction of PIN with the characteristics similar to that observed in PTEN+/− mice [26]. Upregulation of ANG in MPAKT mice is an early and lasting event. ANG protein levels are higher in the ventral prostate of MPAKT mice than in that of the WT littermates across the age ranging from 4 to 12 weeks [14], implicating a potential role of ANG both in initial cell proliferation and in cell survival in AKT-induced PIN. These findings also suggest that ANG may be a causative factor rather than a consequence of PIN formation.
KNOCKING-DOWN MOUSE ANG-1 PREVENTS PIN FORMATION
The role of ANG in Akt-driven PIN in MPAKT mice was first examined by lentivirus-based siRNA method [27]. A single intraprostate injection of lentivirus containing ANG-1-specific siRNA significantly prevented the formation of PIN in these mice. A nonspecific control shRNA had no effect, indicating that the observed inhibition was not due to an off-target effect of siRNA.
It is of note that the treatment of ANG-1 siRNA did not alter AKT transgene expression and activation. The phosphorylation status of AKT and its down-stream target S6RP was not changed, indicating that the signal transduction pathway of Akt-S6K-S6RP was not affected. The fact that ANG-1 siRNA inhibited PIN formation, despite continuous expression of AKT transgene and activation of its down-stream targets, demonstrated that proliferation, expansion, and maintenance of the intraluminal cells driven by AKT require the participation of ANG. Knocking-down ANG-1 completely abolished AKT-induced increase in rRNA transcription and restored the nucleolar organizer region (NOR) to the level of WT littermates, suggesting that rRNA transcription in AKT-induced PIN was mediated by ANG. These observations were consistent with the established role of ANG in rRNA transcription [13, 28], and suggested that upregulation of ANG stimulates transcription of rRNA that, together with the ribosomal proteins stimulated by the AKT-mTOR-S6K pathway, allows ribosome biogenesis to take place. In other word, both ANG-stimulated rRNA and mTOR-stimulated ribosomal proteins are required for PIN formation. Consistent with decreased rRNA transcription, knocking-down ANG-1 decreased cell size and Ki-67 positive cells.
ANG AS A MOLECULAR TARGET FOR PROSTATE CANCER DRUG DEVELOPMENT
ANG was originally identified as an angiogenic protein, so previous efforts have been focused on its angiogenic properties. These efforts have lead to the finding that ANG undergoes nuclear translocation in endothelial cells where it stimulates rRNA transcription [28, 29]. As rRNA transcription is critical for cell growth and proliferation, ANG-stimulated rRNA transcription has been demonstrated to be necessary for angiogenesis induced by other angiogenic factors including VEGF, bFGF, aFGF, and EGF [30]. Thus, ANG has been proposed as a permissive factor for angiogenesis in general and that ANG-induced rRNA transcription is a general requirement for angiogenesis regardless the angiogenic stimuli [30]. ANG inhibitors have been shown to inhibit angiogenesis induced not only by ANG but also by other angiogenic factors [30]. Because these angiogenic factors have been shown to play a role in prostate tumor angiogenesis [31–33], an essential role of ANG in prostate tumor angiogenesis can already be envisioned.
The essential role of ANG in mediating rRNA transcription in both endothelial cells and prostate cancer cells suggests that ANG is a molecular target for prostate cancer drug development. Both ANG and its receptor can be targeted for this purpose. Proof of concept has been established for targeting ANG itself as ANG-specific siRNA and antisense that inhibit ANG synthesis, and monoclonal antibody and binding proteins that neutralize secreted ANG proteins have all been shown to inhibit xenograft growth of human prostate cancer cells in athymic mice [13, 18, 22]. One caveat of this strategy is the relatively high circulating ANG protein (~250–350 ng/ml) in plasma [24, 25]. The majority of the circulating ANG is produced by the liver [23]. Moreover, with a seemingly fast turnover rate and a half-life of 2 h [34], a large quantity of ANG inhibitors would be needed to neutralize the circulating ANG.
The cell surface receptor of ANG has not yet been identified. Therefore, targeting ANG receptor and its signaling pathway is currently not feasible. However, blockage of nuclear translocation of ANG seems to be a promising approach to inhibit the function of ANG. The biological function of ANG is related to rRNA transcription [35], which requires ANG to be physically in the nucleus [28]. Nuclear translocation of ANG is essential for its biological function [36]. Targeting nuclear translocation of ANG would avoid potential problems caused by high plasma concentration of ANG. Another distinct advantage of targeting nuclear translocation of ANG would be that it might not have serious side effects since nuclear translocation of ANG occurs only in proliferating endothelial cells and in cancer cells [37].
NEOMYCIN BLOCKS NUCLEAR TRANSLOCATION OF ANG AND INHIBITS ANG-MEDIATED ANGIOGENESIS
During the study of the mechanism by which ANG is translocated to the nucleus of endothelial cells, neomycin, a phospholipase C (PLC) inhibitor, was found to block nuclear translocation of ANG [38]. Neomycin is an aminoglycoside antibiotic that has also been reported as a PLC inhibitor. However, the PLC-inhibitory activity may not be involved in blocking nuclear translocation of ANG because another inhibitor of PLC, U-73122 and its inactive analog U-73343 [39, 40], only marginally block nuclear translocation of ANG. Subsequently, neomycin was found to inhibit ANG-induced endothelial cell proliferation and angiogenesis [38]. Other structurally related aminoglycosides including streptomycin, kanamycin, gentamicin, and paromomycin have a similar anti-bacterial spectrum but fail to inhibit nuclear translocation of ANG and therefore, are not anti-angiogenic [38]. The anti-angiogenic activity of neomycin is thus related to its capacity of blocking nuclear translocation of ANG but is not associated with its antibiotic properties.
NEOMYCIN INHIBITS XENOGRAFT GROWTH OF PC-3 CELL TUMOR IN ATHYMIC MICE
The anti-prostate cancer activity of neomycin has been examined in a xenograft nude mouse model [13]. Treatment with neomycin s.c. at a dose of 60 mg/kg of body weight prevented tumor establishment in 50% of the animals. In the other 50% of the animals that did develop tumors, the appearance of palpable tumors was significantly delayed. The average tumor weight was decreased by 77% as compared with the untreated animals. PCNA-positive cells and tumor angiogenesis were decreased by 60 and 81%, respectively, in neomycin-treated tumor tissues as compared with untreated controls. These results demonstrated that blocking nuclear translocation of ANG by neomycin effectively inhibits PC-3 cell tumor growth in mice, presumably through inhibition of both tumor cell proliferation and angiogenesis.
NEAMINE IS AN EQUALLY POTENT INHIBITOR OF ANG
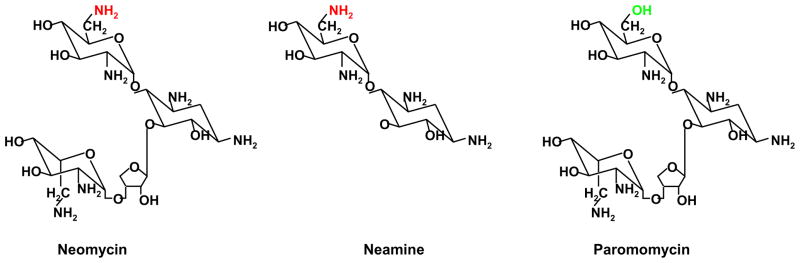
Although neomycin is approved by FDA as an antibiotic, it is also known to be nephro- and oto-toxic [41], which preclude its prolonged use as an anti-cancer agent. In an effort to search for less toxic analogues and derivatives, neamine [42], a nontoxic degradation product of neomycin, has been found to effectively inhibit nuclear translocation of ANG [43]. Fig. 1 shows the structure of neomycin and neamine. Paromomycin differs from neomycin only at the C-6 position of the D-glucopyranosyl ring (-OH instead of -NH2) but does not block nuclear translocation of ANG.
Fig. 1.
Structure of neomycin, neamine and paromomycin. The amino group in neomycin and neamine shown in red is essential for blocking nuclear translocation of ANG.
Neamine has also been shown to inhibit angiogenesis induced both by ANG and by bFGF and VEGF [30]. Moreover, it inhibits xenograft growth of HT-29 human colon adenocarcinoma and MDA-MB-435 human breast cancer cells in athymic mice [43]. Since the toxicity profile of neamine is similar to that of streptomycin and kanamycin, which is at least ~20-fold less toxic than neomycin [44, 45], neamine may serve as a lead compound for the development of prostate cancer therapeutic agents. Its capacity to prevent the establishment and to inhibit the growth of PC-3 human prostate cancer cells in mice, as well as to inhibit AKT-induced PIN has recently been established [15]. Subcutaneous treatment with neamine at 30 mg/kg, a nontoxic dose that is 42-fold lower than the reported LD50 of 1,250 mg/kg [41], prevented PC-3 tumor establishment in 50% of the athymic mice. In the animals that did develop tumors, their growth rate was decreased significantly (72.5% inhibition). This inhibition is accompanied by a blockade of nuclear translocation of ANG and a decrease in rRNA transcription, in PC-3 cell proliferation as well in angiogenesis [15]. Thus, neamine seems to block nuclear translocation of ANG thereby suppressing rRNA transcription, cell proliferation and angiogenesis, consistent with the previous report that ANG plays a dual role in prostate cancer progression by stimulating both angiogenesis and cancer cell proliferation [13]. They also concur with the reports that nuclear function of ANG is related to rRNA transcription [28] and that the neomycin family of aminoglycoside antibiotics blocks nuclear translocation of ANG [38].
Neamine also prevents and reverses AKT-induced PIN in MPAKT mice. Treatment of 4-week-old MPAKT mice with neamine at a daily i.p. dose of 10 mg/kg for 4 weeks decreased the percentage of PIN by 65%, again accompanied with a relocalization of mouse ANG from nucleus to cytoplasm [15]. Neamine treatment did not alter expression and phosphorylation of AKT transgene and its downstream effector S6P, demonstrating that nuclear ANG is not involved in the AKT signaling pathway. However, the level of 47S rRNA in the prostate epithelial cells decreased dramatically after neamine treatment, confirming the activity of nuclear ANG in rRNA transcription. Neamine treatment also decreased interluminal angiogenesis as well as luminal epithelial cell proliferation in these animals [15].
Treatment of 12-week-old MPAKT mice with fully established PIN with daily i.p. injection of neamine (10 mg/kg) for 4 weeks shrank the PIN lesion and restored normal luminal architectures of the ventral prostate in AKT over-expressing mice with a 100% response rate. The percentage of glands with PIN in the ventral prostate decreased substantially but AKT expression and phosphorylation were not affected. Again, rRNA transcription was inhibited by neamine. Moreover, TUNEL staining showed that neamine treatment induced apoptosis of the prostate luminal epithelial cells of MPAKT mice [15]. It is therefore clearly demonstrated that neamine blocked nuclear translocation of ANG thereby inhibiting rRNA transcription and inducing cell apoptosis, leading to a phenotypic reversal of established PIN, in spite of continuous AKT transgene expression and phosphorylation.
ANDROGEN-INDEPENDENT PROSTATE CANCER
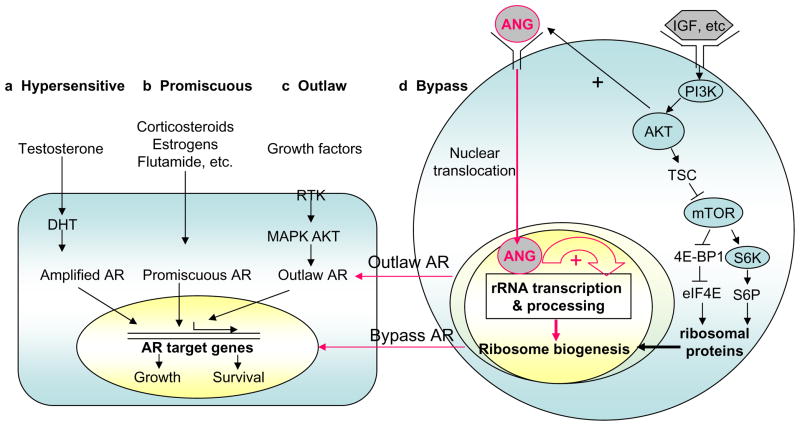
The main obstacle for prostate cancer treatment is the development of androgen-independent prostate cancer. Even after aggressive androgen ablation therapy such as surgical and chemical castration, cancer recurs in 15–30% of the patients after a few years as an advanced androgen-independent metastatic tumor. Various pathways have been proposed to be involved in the development of androgen-independent prostate cancer [6]. These include hypersensitive AR, promiscuous AR, outlaw AR, and bypass AR (Fig. 2).
Fig. 2.
Possible pathways to androgen independence and the involvement of ANG. Left panel, androgen-independent but AR-dependent pathways. (a) In the hypersensitive pathway, more AR is produced, or AR has enhanced sensitivity, or more testosterone is converted to the more potent DHT. (b) In the promiscuous pathway, the specificity of AR is broadened so that it can be activated by non-androgen molecules. (c) In the outlaw pathway, receptor tyrosine kinases (RTK) are activated and AR is phosphorylated by AKT or MAPK, producing a ligand-independent AR activation. Right panel, (d) Bypass pathway and the proposed role of ANG. IGF and other growth factors, or PTEN deficiency activate PI3K-AKT-mTOR pathway to enhance ribosomal protein production but it is unclear how rRNA is proportionally increased. ANG is known to be constitutively translocated to the nucleus of androgen-independent prostate cancer cells where it enhances rRNA transcription. Recent work has shown that upregulation of ANG and constitutive nuclear translocation in prostate cancer cells, especially in androgen-independent cells, result in an adequate supply of rRNA that is normally controlled by androgen-AR axis, thus enabling the cells to grow in the absence of androgens and AR.
Hypersensitive AR
The first mechanism is an increased sensitivity of AR to very low levels of androgens. This can be achieved by AR amplification, increased AR sensitivity, and increased local androgen levels. Approximately 30% of tumors that become androgen-independent after ablation therapy have an amplified AR gene, resulting in increased AR expression, whereas none of the primary tumors from the same patients before androgen ablation had an AR gene amplification [46]. The second hypersensitive pathway results from increased stability and enhanced nuclear localization of AR, which results in four orders of magnitude greater sensitivity of AR to androgen [47]. The third hypersensitive mechanism is by an increase in the local production of androgens to compensate for the overall decline in circulating testosterone. This is achieved by increased activity of 5α-reductase that converts testosterone to dihydrotestosterone (DHT), the more potent form of androgen with 5-fold higher affinity for AR. It has been reported that after androgen ablation therapy, serum testosterone levels decrease by 95%, but the concentration of DHT in the prostate tissue is reduced by only 60% [48].
Promiscuous AR
Promiscuous AR results from point mutations, which decrease the specificity of ligand binding and allows inappropriate activation by various non-androgen steroids and androgen antagonists [49]. Somatic mutations of AR have been reported from a subset of hormone naive prostate cancers and more frequently from androgen-independent tumors [50]. In cells with gain-of-function AR mutations, the androgen signal is maintained by broadening the number of ligands that can bind to and activate the receptor. Normally, AR is specifically activated by testosterone and DHT, but mutations in the ligand-binding domain widen this specificity. As a result, malignant cells can continue to proliferate and avoid apoptosis by using other circulating steroid hormones as substitutes when the androgen level is low. The T877A mutation, which is found in 25% [51] to 31% [52] of metastatic prostate cancers, enables progestins and estrogens to bind and act as agonists [53]. Moreover, this mutation changes the AR response to anti-androgen flutamide from an antagonist to an agonist. The L701H mutation enhances the binding of AR to other adrenal corticosteroids, particularly the glucocorticoids cortisol and cortisone. The T877A and L701H double mutation has a synergistic effect by increasing the affinity of AR for glucocorticoids by 300% more than the L701H mutation alone [54]. Apart from AR mutation, co-regulator alterations can be another mechanism by which prostate cancer progresses to androgen independence [55].
Outlaw AR
AR can become an “outlaw” receptor that is activated by stimuli other than exogenous steroid ligands. Certain growth factors such as IGF, KGF and EGF can activate AR to induce AR target genes in the absence of androgen [56]. For example, IGF induces a 5-fold increase in PSA secretion in LNCaP cells [56]. However, the AR antagonist casodex completely blocks activation of the AR by IGF, KGF, and EGF, indicating that the AR ligand-binding domain is necessary. This demonstrated a pathway of ligand-independent but receptor-dependent mechanism of AR-regulated genes. AR-dependent gene expression in prostate cancer cells has been observed following activation of various signaling pathways such as protein kinase A [57, 58], MAPK [59, 60], and PI-3K/Akt [61, 62]. In these situations, the AR remains functional under androgen-depleted conditions.
Bypass AR
Another pathway to androgen independence is to bypass AR completely so that AR becomes dispensable. This hypothesis is supported by the finding that in recurrent prostate cancer patients, AR positive and AR negative cancer cells coexist [63]. An effective bypass of the androgen signaling cascade would facilitate proliferation and inhibit apoptosis in the absence of androgens and AR. The BCL2 gene is one of the bypass candidates that can block apoptosis. Normally BCL2 is not expressed in the secretory epithelial cells of the prostate [64], but is frequently expressed in PIN, as well as in androgen-independent prostate cancer [65]. Blocking BCL2 with an antisense oligo delayed the emergence of androgen-independent prostate cancer in an LNCaP xenografic model [66]. Upregulation of BCL2 could bypass the signal for apoptosis that is normally generated by androgen ablation. This is supported by reports that many cases of androgen-independent prostate cancer over-express BCL2 [65, 67]. Peptide growth factors have also been proposed as potential mediators for prostate cancer cells to bypass AR [68]. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) has been shown to alter the dependence of LNCaP on the androgen-AR axis for survival and proliferation. HB-EGF promotes a more aggressive phenotype in vivo and exerts effects that bypass both androgen- and AR-dependent signaling [69].
ANG-STIMULATED rRNA TRANSCRIPTION IN PROSTATE CANCER
Sustained cell growth requires the production of new ribosomes. The rate-limiting step in this process is the transcription of rRNA. Androgens have been shown to regulate the accumulation of rRNA during androgen-dependent cell growth in normal development and in pathological states of the prostate such as benign prostatic hyperplasia, PIN, and prostate cancers [10–12]. Therefore, a link exists between androgen stimulation and rRNA transcription in androgen-responsive cells. It is thus reasonable to assume that an androgen-independent pathway of rRNA transcription has to be functional in the growth of androgen-independent prostate cancer. ANG-stimulated rRNA transcription seems to be one such pathway in androgen-independent prostate cancer.
The function of ANG in stimulating rRNA transcription and the role of ANG in promoting the progression of androgen-independent prostate cancer is interwoven with the function of the kinase AKT and the tumor suppressor PTEN Inactivating somatic mutation of PTEN or loss of the PTEN protein are common in prostate cancer cell lines and in primary and metastatic tumor specimens [70–72]. Mutation of PTEN leads to deregulated PI3K signaling, resulting in constitutive activation of downstream targets including the AKT kinase family. AKT kinase activity is frequently elevated in prostate cancers [73]. AKT is activated through phosphorylation on Ser-473 and Thr-308. Activated AKT promotes both cell growth and cell survival through the mTOR pathway.
mTOR plays an important role in PI3K- and AKT-dependent oncogenesis, especially in the pathogenesis of prostate cancer [21, 74]. Transformation by PI3K or AKT directly correlates with activation of mTOR and its downstream target S6K [75]. S6 phosphorylation has been associated with translation of a specific class of mRNA termed TOP (a terminal oligopyrimidine track in the 5′ untranslated region) mRNA [76]. This class of mRNAs includes ribosomal proteins, elongation factors 1A1 and 1A2, and several other proteins involved in ribosome biogenesis or in translation control [77]. Thus, AKT activation will enhance ribosomal protein production. However, it is unknown how transcription of rRNA, which needs to be incorporated in an equimolar ratio, is proportionally elevated. ANG-stimulated rRNA transcription in prostate cancer cells thus fulfills this growth requirement. Fig. 2 summarizes the proposed action of ANG in promoting androgen independence through the Outlaw AR and Bypass AR pathways.
RATIONALE OF NEAMINE AS A LEAD AGENT FOR FURTHER DEVELOPMENT
In efforts to understand the mechanism by which ANG is translocated to the nucleus of endothelial cells, neomycin was discovered to block nuclear translocation of ANG and to inhibit ANG-induced cell proliferation and angiogenesis [38]. Moreover, neomycin has been shown to inhibit xenograft growth of PC-3 cells in athymic mice [13] and AKT-driven PIN in MPAKT mice [14]. Neomycin is an aminoglycoside antibiotic isolated originally from Streptomyces fradiae [78]. Similar to other aminoglycosides, neomycin has high activity against Gram-negative bacteria, and has partial activity against Gram-positive bacteria. However, neomycin is nephro- and oto-toxic to humans and its clinical use has been restricted to topical preparation and oral administration as a preventive measure for hepatic encephalopathy and hypercholesterolemia by killing bacteria in the small intestinal tract and keeping ammonia levels low [41]. The nephro-toxicity of neomycin is associated with selective accumulation in the kidney where the cortical levels may reach as high as 20 times those of circulating levels in serum. The mechanism underlying selective renal accumulation has been shown to be tubular re-absorption, extraction from the circulation at the basolateral surface, as well as brush border uptake [44]. The antibiotic activity and the renal toxicity of neomycin seem to be separable from its capacity to inhibit nuclear translocation of ANG. This led the search for less toxic derivatives and analogues of neomycin and led to the finding that neamine [42], a virtually nontoxic derivative of neomycin, has comparable activity in blocking nuclear translocation of ANG [43]. Neamine is equally effective in inhibiting angiogenesis induced by ANG as well as by other angiogenic factors [30].
Neamine is a degradation product of neomycin although there is some evidence that it is also produced in small amounts by Streptomyces fradiae [42]. Cell and organ culture experiments have shown that the nephro- and oto-toxicity of neamine is ~5 and 6%, respectively, of that of neomycin [44, 45]. Thus, the toxicity of neamine is similar to that of streptomycin, an antibiotic that is currently in clinical use. Neamine is also less neuromuscularly toxic than neomycin. The acute LD50 (subcutaneous) in mice for neamine, neomycin, and streptomycin is 1,250, 220, and 600 mg/kg, respectively [41]. The recommended dosage for intramuscular injection of streptomycin in humans is 25–30 mg/kg twice weekly [79]. Since neamine appears to be less toxic than streptomycin, the dose in the above study (30 mg/kg s.c., and 10 mg/kg i.p.) was tolerated well. Indeed no acute or chronic adverse side effects were reported in these experiments [15].
Neamine is effective in inhibiting prostate cancer growth in both the xenograft and spontaneous mouse models. With the xenograft animal model, neamine prevented the establishment of PC-3 cell tumors in 50% of the animals with an overall inhibition of 72.5% in the growth rate. Histology and IHC evaluation demonstrated that neamine inhibited both angiogenesis and cancer cell proliferation. These results closely resemble those observed with neomycin, confirming the dual role of ANG and suggesting a similar mechanism of inhibition mediated by neamine and neomycin. Indeed, neamine treatment blocked nuclear translocation of ANG and suppressed rRNA transcription in cancer cells.
Neamine is effective in preventing AKT-induced PIN in MPAKT mice, providing a strong rationale for its further development as an anti-prostate cancer agent. AKT kinase activity is frequently elevated in prostate cancers [73]. The findings that neamine treatment decreases rRNA transcription in the luminal epithelial cells and that it both prevents and reverses AKT-induced PIN indicate that ANG is important not only for cell proliferation but also for cell survival. Given the nontoxic nature of neamine and its potent activity against ANG-mediated rRNA transcription that is essential for prostate cancer progression, neamine is a promising lead agent for further development.
Acknowledgments
This work has been funded by NIH grant R01CA105241 and Department of Defense grant W81XWH-06-1-0031.
Abbreviations used
- ANG
angiogenin
- AR
androgen receptor
- bFGF
basic fibroblast growth factor
- BPH
benign prostate hyperplasia
- DHT
dihydrotestosterone
- IGF
insulin-like growth factor
- IHC
immunohistochemistry
- MPAKT
murine prostate-restricted AKT kinase transgenic
- PCNA
proliferating cell nuclear antigen
- PIN
prostate intraepithelial neoplasia
- PLC
phospholipase C
- rDNA
ribosomal DNA
- rRNA
ribosomal RNA
- VEGF
vascular endothelial growth factor
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Tan WW. Novel agents and targets in managing patients with metastatic prostate cancer. Cancer Control. 2006;13:194–8. doi: 10.1177/107327480601300306. [DOI] [PubMed] [Google Scholar]
- 3.Sinibaldi VJ. Docetaxel treatment in the elderly patient with hormone refractory prostate cancer. Clin Interv Aging. 2007;2:555–60. doi: 10.2147/cia.s1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taplin ME. Androgen receptor: role and novel therapeutic prospects in prostate cancer. Expert Rev Anticancer Ther. 2008;8:1495–508. doi: 10.1586/14737140.8.9.1495. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–8. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 7.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Li W, Liu XS, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–92. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenwald IB. Deregulation of protein synthesis as a mechanism of neoplastic transformation. Bioessays. 1996;18:243–50. doi: 10.1002/bies.950180312. [DOI] [PubMed] [Google Scholar]
- 10.Mainwaring WI, Wilce PA. The control of the form and function of the ribosomes in androgen-dependent tissues by testosterone. Biochemical Journal. 1973;134:795–805. doi: 10.1042/bj1340795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mainwaring WI, Derry NS. Enhanced transcription of rRNA genes by purified androgen receptor complexes in vitro. Journal of Steroid Biochemistry. 1983;19:101–8. [PubMed] [Google Scholar]
- 12.Kabler RL, Srinivasan A, Taylor LJ, Mowad J, Rothblum LI, Cavanaugh AH. Androgen regulation of ribosomal RNA synthesis in LNCaP cells and rat prostate. Journal of Steroid Biochemistry & Molecular Biology. 1996;59:431–9. doi: 10.1016/s0960-0760(96)00126-4. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci USA. 2006;103:14519–24. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibaragi S, Yoshioka N, Kishikawa H, et al. Angiogenin-stimulated Ribosomal RNA Transcription Is Essential for Initiation and Survival of AKT-induced Prostate Intraepithelial Neoplasia. Mol Cancer Res. 2009;7:415–24. doi: 10.1158/1541-7786.MCR-08-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibaragi S, Yoshioka N, Li S, et al. Neamine inhibits prostate cancer growth by suppressing angiogenin-mediated ribosomal RNA transcription. Clin Cancer Res. 2009;15:1981–8. doi: 10.1158/1078-0432.CCR-08-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fett JW, Strydom DJ, Lobb RR, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–6. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- 17.Olson KA, Fett JW, French TC, Key ME, Vallee BL. Angiogenin antagonists prevent tumor growth in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:442–6. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson KA, Byers HR, Key ME, Fett JW. Prevention of human prostate tumor metastasis in athymic mice by antisense targeting of human angiogenin. Clin Cancer Res. 2001;7:3598–605. [PubMed] [Google Scholar]
- 19.Tello-Montoliu A, Patel JV, Lip GY. Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost. 2006;4:1864–74. doi: 10.1111/j.1538-7836.2006.01995.x. [DOI] [PubMed] [Google Scholar]
- 20.Katona TM, Neubauer BL, Iversen PW, Zhang S, Baldridge LA, Cheng L. Elevated expression of angiogenin in prostate cancer and its precursors. Clin Cancer Res. 2005;11:8358–63. doi: 10.1158/1078-0432.CCR-05-0962. [DOI] [PubMed] [Google Scholar]
- 21.Majumder PK, Yeh JJ, George DJ, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100:7841–6. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson KA, Byers HR, Key ME, Fett JW. Inhibition of prostate carcinoma establishment and metastatic growth in mice by an antiangiogenin monoclonal antibody. Int J Cancer. 2002;98:923–9. doi: 10.1002/ijc.10282. [DOI] [PubMed] [Google Scholar]
- 23.Weiner HL, Weiner LH, Swain JL. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science. 1987;237:280–2. doi: 10.1126/science.2440105. [DOI] [PubMed] [Google Scholar]
- 24.Shimoyama S, Gansauge F, Gansauge S, Negri G, Oohara T, Beger HG. Increased angiogenin expression in pancreatic cancer is related to cancer aggressiveness. Cancer Research. 1996;56:2703–6. [PubMed] [Google Scholar]
- 25.Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. Increased angiogenin expression in the tumor tissue and serum of urothelial carcinoma patients is related to disease progression and recurrence. Cancer. 1999;86:316–24. [PubMed] [Google Scholar]
- 26.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 27.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–6. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 28.Xu ZP, Tsuji T, Riordan JF, Hu GF. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochemistry. 2003;42:121–8. doi: 10.1021/bi020465x. [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Xu C, Riordan JF. Human angiogenin is rapidly translocated to the nucleus of human umbilical vein endothelial cells and binds to DNA. J Cell Biochem. 2000;76:452–62. doi: 10.1002/(sici)1097-4644(20000301)76:3<452::aid-jcb12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–56. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- 31.Campbell SC. Advances in angiogenesis research: relevance to urological oncology. J Urol. 1997;158:1663–74. doi: 10.1016/s0022-5347(01)64090-4. [DOI] [PubMed] [Google Scholar]
- 32.Russell PJ, Bennett S, Stricker P. Growth factor involvement in progression of prostate cancer. Clin Chem. 1998;44:705–23. [PubMed] [Google Scholar]
- 33.Ferrer FA, Miller LJ, Andrawis RI, et al. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells [see comments] Journal of Urology. 1997;157:2329–33. [PubMed] [Google Scholar]
- 34.Hatzi E, Bassaglia Y, Badet J. Internalization and processing of human angiogenin by cultured aortic smooth muscle cells [In Process Citation] Biochem Biophys Res Commun. 2000;267:719–25. doi: 10.1006/bbrc.1999.2015. [DOI] [PubMed] [Google Scholar]
- 35.Xu ZP, Tsuji T, Riordan JF, Hu GF. The nuclear function of angiogenin in endothelial cells is related to rRNA production. Biochem Biophys Res Commun. 2002;294:287–92. doi: 10.1016/S0006-291X(02)00479-5. [DOI] [PubMed] [Google Scholar]
- 36.Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1677–81. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuji TYS, Kishimoto K, et al. Angiogenin is translocated to the nucleus of HeLa cells and is involved in rRNA transcription and cell proliferation. Cancer Research. 2005;65:1352–60. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- 38.Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9791–5. doi: 10.1073/pnas.95.17.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somjen D, Kohen F, Lieberherr M. Nongenomic effects of an anti-idiotypic antibody as an estrogen mimetic in female human and rat osteoblasts. J Cell Biochem. 1997;65:53–66. doi: 10.1002/(sici)1097-4644(199704)65:1<53::aid-jcb6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 40.Hildebrandt JP, Plant TD, Meves H. The effects of bradykinin on K+ currents in NG108-15 cells treated with U73122, a phospholipase C inhibitor, or neomycin. Br J Pharmacol. 1997;120:841–50. doi: 10.1038/sj.bjp.0700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glasby JS. Encyclopedia of antibiotics. New York, NY: John Wiley & Sons Ltd; 1993. pp. 363–7. [Google Scholar]
- 42.Leach BE, Teeters CM. Neamine, an antibacterial degradation product of neomycin. J Am Chem Soc. 1951;73:2794–7. [Google Scholar]
- 43.Hirukawa S, Olson KA, Tsuji T, Hu GF. Neamine inhibits xenografic human tumor growth and angiogenesis in athymic mice. Clin Cancer Res. 2005;11:8745–52. doi: 10.1158/1078-0432.CCR-05-1495. [DOI] [PubMed] [Google Scholar]
- 44.Williams PD, Bennett DB, Gleason CR, Hottendorf GH. Correlation between renal membrane binding and nephrotoxicity of aminoglycosides. Antimicrob Agents Chemother. 1987;31:570–4. doi: 10.1128/aac.31.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Au S, Weiner N, Schacht J. Membrane perturbation by aminoglycosides as a simple screen of their toxicity. Antimicrobial Agents & Chemotherapy. 1986;30:395–7. doi: 10.1128/aac.30.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 47.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–8. [PubMed] [Google Scholar]
- 48.Labrie F, Dupont A, Belanger A, et al. Treatment of prostate cancer with gonadotropin-releasing hormone agonists. Endocr Rev. 1986;7:67–74. doi: 10.1210/edrv-7-1-67. [DOI] [PubMed] [Google Scholar]
- 49.Buchanan G, Greenberg NM, Scher HI, Harris JM, Marshall VR, Tilley WD. Collocation of androgen receptor gene mutations in prostate cancer. Clin Cancer Res. 2001;7:1273–81. [PubMed] [Google Scholar]
- 50.Marcelli M, Ittmann M, Mariani S, et al. Androgen receptor mutations in prostate cancer. Cancer Res. 2000;60:944–9. [PubMed] [Google Scholar]
- 51.Gaddipati JP, McLeod DG, Heidenberg HB, et al. Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res. 1994;54:2861–4. [PubMed] [Google Scholar]
- 52.Taplin ME, Bubley GJ, Ko YJ, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–5. [PubMed] [Google Scholar]
- 53.Veldscholte J, Berrevoets CA, Ris-Stalpers C, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–9. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 55.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 56.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- 57.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–83. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 58.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–7. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 59.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–84. [PubMed] [Google Scholar]
- 60.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96:5458–63. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graff JR, Konicek BW, McNulty AM, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–5. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 62.Wen Y, Hu MC, Makino K, et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–5. [PubMed] [Google Scholar]
- 63.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 64.McDonnell TJ, Troncoso P, Brisbay SM, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–4. [PubMed] [Google Scholar]
- 65.Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 66.Gleave M, Tolcher A, Miyake H, et al. Progression to androgen independence is delayed by adjuvant treatment with antisense Bcl-2 oligodeoxynucleotides after castration in the LNCaP prostate tumor model. Clin Cancer Res. 1999;5:2891–8. [PubMed] [Google Scholar]
- 67.Furuya Y, Krajewski S, Epstein JI, Reed JC, Isaacs JT. Expression of bcl-2 and the progression of human and rodent prostatic cancers. Clin Cancer Res. 1996;2:389–98. [PubMed] [Google Scholar]
- 68.Culig Z, Hobisch A, Cronauer MV, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 69.Adam RM, Kim J, Lin J, et al. Heparin-binding epidermal growth factor-like growth factor stimulates androgen-independent prostate tumor growth and antagonizes androgen receptor function. Endocrinology. 2002;143:4599–608. doi: 10.1210/en.2002-220561. [DOI] [PubMed] [Google Scholar]
- 70.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 71.Trotman LC, Niki M, Dotan ZA, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sellers WR, Sawyers CA. The Somatic Genetics of Prostate Cancer. In: Kantoff PW, editor. Prostate Cancer Principles and Practice. Philadephia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 73.Sun M, Wang G, Paciga JE, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–7. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. Epub 2004 May 23. [DOI] [PubMed] [Google Scholar]
- 75.Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc Natl Acad Sci U S A. 2001;98:136–41. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. Embo J. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci U S A. 1994;91:11477–81. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waksman SA, Lechevalier HA. Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. Science. 1949;109:305–7. doi: 10.1126/science.109.2830.305. [DOI] [PubMed] [Google Scholar]
- 79.Wintrobe M, Thorn G, Adams R, et al. Harrison’s Principles of Internal Medicine. 6. New York: McGRAW-HILL; 1971. p. 749. [Google Scholar]