Abstract
Objective
To test the impact of participation in a peer-based intervention for symptom management for women living with HIV infection on selected outcome measures including, symptom intensity, medication adherence, viral control, and quality of life.
Design
Randomized clinical trial.
Methods
Participants were recruited using a convenient, consecutive sampling method. Those participants randomized to the experimental condition attended seven, peer-led sessions over seven weeks. Participants randomized to the control condition received a copy of HIV Symptom Management Strategies: A Manual for People Living with HIV/AIDS. Participants completed four surveys assessing change over time in the aforementioned outcome variables.
Results
Eighty-nine HIV-infected women followed over 14 weeks and there were no differences between the two groups on baseline demographic variables. Mixed-effects regression indicated no significant difference between groups across time in total symptom intensity score and medication adherence. There was a significant difference between groups across time for two of the nine quality of life scales – HIV Mastery (χ2 = 25.08; p < 0.005) and Disclosure Worries (χ2 = 24.67; p < 0.005).
Conclusions
In urban-dwelling women living with HIV/AIDS, results suggest that a peer-based symptom management intervention may not decrease symptom intensity or increase medication adherence. There is positive evidence that suggests that the intervention may increase some important aspects of quality of life. However, further research is warranted to elucidate the effect of peer-based interventions in achieving positive self-management outcomes.
Keywords: HIV, AIDS, peer, medication adherence, clinical trial
Introduction
Women, particularly vulnerable women, in the USA are increasingly infected with HIV (CDC, 2006; Hall et al., 2008). As a chronic disease, women living with HIV must learn to manage their symptoms and care. This occurs in the face of numerous challenges including stigma which can prevent women from seeking HIV testing and treatment (Gupta, 2002; Sandelowski, Lambe, & Barroso, 2004); lack of economic power (Gupta, 2002; Hunter, 2002); fear of violence (Garcia-Moreno & Watts, 2000; Maman et al., 2002); and the growing burden of care of infected family members (Ogden & Esim, 2003; Steinberg, Johnson, Schierhout,& Ndewa, 2002). These challenges can prevent women from managing their own, much-needed care. One important area of self-management is symptom management. The symptoms of HIV and its treatments are complex, painful, and distressing to the women who experience them (Johnson, Stallworth,& Neilands, 2003; Mannheimer et al., 2008; Sowell et al., 1997; van Servellen, Sarna,& Jablonski, 1998). These symptoms affect all aspects of a woman’s life, including her quality of life (Lorenz, Cunningham, Spritzer,& Hays, 2006; Lorenz, Shapiro, Asch, Bozzette,& Hays, 2001; Sousa, Holzemer, Bakken Henry,& Slaughter, 1999), her adherence to HIV therapy (Holzemer, Henry, Portillo,& Miramontes, 2000), and her treatment decisions (Portillo, Holzemer, & Chou, 2007; Siegel, Schrimshaw,& Dean, 1999).
Peer-based interventions have the potential to increase symptom management (Chou, Holzemer, Portillo,& Slaughter, 2004; Gifford, Laurent, Gonzales, Chesney, & Lorig, 1998; Lorig, Ritter, & Plant, 2005; Sowell et al., 1997; van Servellen et al., 1998) and enhance health equity in women with HIV/AIDS (HRSA, 2005). They have been found to improve access to health care services, provide support, improve self-efficacy and self-confidence, and facilitate involvement in self-care activities (Doull, O’Conner, Robins on, Wells,& Tugwell, 2004; Higgins, Thompson, Deeks, & Altman, 2003; van Rompay et al., 2008). Consequently, a peer intervention model may facilitate change in the symptom management behavior in women infected with HIV. To date, no studies have been located that have explored the benefits of utilizing a peer-led intervention program to help facilitate symptom self-management for HIV/AIDS in an all-female sample. Therefore, the main objective of this study was to test the impact of participation in a peer-based intervention for symptom management (PRISM-HIV) for women living with HIV infection on symptom management, medication adherence, and quality of life.
Methods
Participants
Eligible participants were HIV-infected adults who self-identified as female and spoke fluent English. Anyone not meeting these criteria was excluded. Recruitment occurred from January to November 2008. The last participant completed follow-up on 12 January 2009. Participants were recruited from San Francisco Bay Area HIV outpatient clinics, HIV/AIDS specific housing, and HIV/AIDS-related community- based support/peer groups.
Intervention
The intervention tested is peer-based, HIV symptom management using the curriculum, Positive Self-Management Program (PSMP) as the content of the sessions. The PSMP was designed at Stanford University in 1997 and uses Social Cognitive Theory as its theoretical framework (Gifford, 1999; Gifford et al., 1998). The PSMP program contains seven, two-hour, scripted sessions that were delivered by two trained peer leaders to a group of approximately 10 participants each week for seven weeks. The topics for each of the sessions are presented in Table 1. This intervention was pilot tested in HIV positive men in 1998 and the investigators found a significant relationship between the intervention and decreased HIV viral load, de creased symptom intensity, and increased medication adherence (Gifford et al., 1998).
Table 1.
Topics discussed at each intervention session.
| Session | Session topics |
|---|---|
| One |
|
| Two |
|
| Three |
|
| Four |
|
| Five |
|
| Six |
|
| Seven |
|
Peer leaders
Three peer leaders were identified as community leaders by HIV case managers, community leaders, and health care workers. Each peer leader completed a five-day (total of 36 hours) standardized training on the PSMP. This scripted training introduced the leaders to the material, taught them how to deliver the intervention, provided experiences to teach the material in teams, introduced teaching techniques, and offered strategies to deal with difficult group members. Each participant presented two PSMP modules while the other peer leader and trainers offered constructive feedback on the presentation (Gifford, Lorig, Laurent, & Gonzalez, 1995).
Study design
After meeting the inclusion criteria, participants gave informed consent and completed a baseline survey that included demographic, general health status, and disease management questions. Participants returning the following week were randomized into either the experimental or control condition. Those randomized to the experimental condition attended seven, peer-led group sessions over the next seven weeks and those randomized to the control condition received a copy of HIV Symptom Management Strategies: A Manual for People Living with HIV/AIDS (Wantland et al., 2008). Both groups received phone calls by the investigator reminding them to attend each session to complete four additional surveys at week 2, week 6, week 10, and week 14. These four surveys described any change in the participants’ symptom intensity, medication adherence, and quality of life throughout the intervention and follow-up period. Participants were paid $15 for completing each survey for a total of $75. An additional $40 bonus was paid to intervention group participants who completed all intervention sessions. The Institutional Review Boards at the University of California, San Francisco, Alta Bates Medical Center, and Alameda County Medical Center approved all study procedures.
Study outcomes
The main study outcomes were change in HIV-related symptom intensity, highly active anti-retroviral therapy (HAART) medication adherence, and quality of life.
Symptom intensity
Symptom intensity was assessed by the HIV Sign and Symptom CheckList-Revised. This checklist identifies the 72 most commonly experienced symptoms, and their intensity, among HIV-infected adults (Holzemer et al., 1999). Each of the 72 symptoms is scored on a scale of zero to three. A score of zero indicates that in the past 24 hours, the participant has not experienced that symptom, and a score of three that the participant severely experienced that symptom. The mean daily symptom intensity score is computed by summing every symptom intensity and dividing by 72. The total score reliability estimate in previous studies (Holzemer et al., 1999) and in the present study was 0.97.
Medication adherence
Medication adherence was assessed with the Revised AIDS Clinical Trials Group (ACTG) Reasons for Non-Adherence to Medications (ACTGrev) and with change in CD4 cell count and HIV viral load. The ACTGrev is a self-report measure of reasons for missing medications (Chesney et al., 2000; Holzemer et al., 2006). The revised instrument has two factors with a total of nine items: pill taking problems (five items) and forgetfulness (four items). Each factor or subscale can be summed separately and then collectively to create the total score. Cronbach’s alphas for the two subscales ranged from 0.8 to 0.9 (Holzemer et al., 2006). Additionally, three items were added to quantify the extent of each participant’s HIV-medication adherence within the past seven days. The reliability of these items was 0.9 in the present study. In addition, all participants were asked to bring in their current CD4 and viral load values at baseline and at the 14-week follow-up. These lab values were done at the request of their primary care provider.
Quality of life
Quality of life was assessed with the HIV/AIDS Targeted Quality of Life Instrument, a 34-item, disease-specific instrument measuring nine dimensions of quality of life. All dimensions are transformed and scored on a linear 0–100 scale, where 0 is the worst score possible and 100 is the best score possible. Internal consistency reliability coefficients ranged from 0.83 to 0.88 for all nine dimensions (Holzemer et al., 2000).
Qualitative data
Each participant in the intervention group completed a post-intervention survey in which they listed two things they: (1) liked and did not like about the program; (2) would change and add to the program; and (3) learned from the program.
Statistical analysis
The sample size in the present study was based on the sample size in Gifford’s original study in 1998 (Gifford et al., 1998). This led to the recruitment of 89 eligible women, which provided an effect size of 0.04 with 80% power to detect change in symptom intensity between the two groups at follow-up (Hedges & Olkin, 1985).
Descriptive statistics were calculated using frequency distributions and appropriate summary statistics. The control and intervention groups were compared on major demographic variables to ensure that potentially confounding variables were equally distributed by the random assignment.
Multilevel negative binominal regression (Rabe-Hesketh & Skrondal, 2005) and multilevel linear regression analyses were used to explore changes over time across HIV/AIDS-related symptom intensity, medication adherence, HIV viral load, and quality of life between the experimental and control groups. This analytic method was selected because it can efficiently analyze all of the longitudinal data collected, even if it was not collected on each individual at each time point. This resulted in less information lost and a potentially less biased result. Post-intervention qualitative comments were summarized verbatim and themes were identified.
Analyses were performed using Stata version 10.0 (Stata Corporation) using an intent-to-treat approach. All statistical tests were considered significant if they achieved the 0.05 level with two-tailed tests.
Results
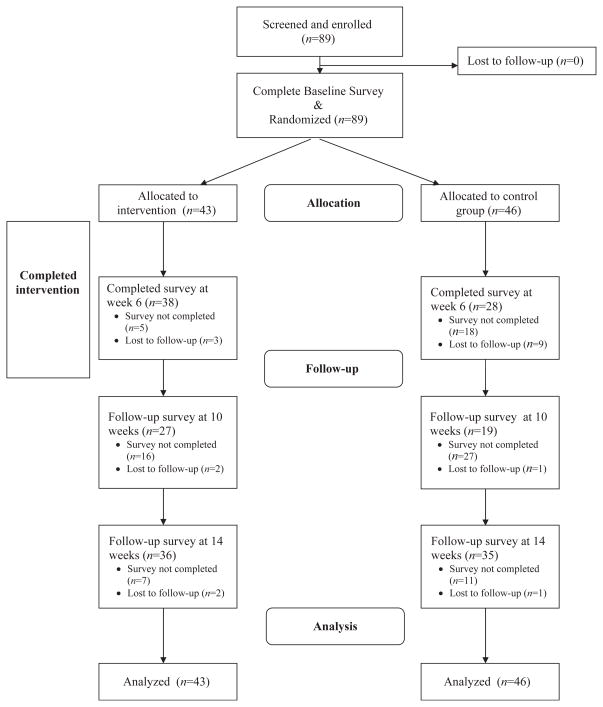
This randomized controlled study includes 89 HIV-infected women followed over 14 weeks. Eighteen participants were lost to follow-up from time of enrollment to the final survey (Figure 1). A total of 89 participants were included in the final analysis.
Figure 1.
Flowchart of participants.
The mean age of participants was 47 years (SD = 8.16 and range = 27–72). Sixty-five (73%) of the women were currently taking HAART, with a mean (± SD) total CD4 lymphocyte count of 464.4 ± 257.6 cells/μl. There were no imbalances in demographic variables between the intervention and control group at enrollment. Baseline characteristics are displayed in Table 2.
Table 2.
Baseline demographic characteristics by group.
| Intervention group (n = 43) | Control group (n = 46) | P-valuea | |
|---|---|---|---|
| Age at baseline in years (range) | 48.0 (27–67) | 45.9 (31–72) | 0.232b |
| Gender (%) | |||
| Female | 38 (88) | 36 (72) | |
| Transgender | 4 (9) | 10 (22) | 0.147c |
| Race (%) | |||
| African-American | 31 (72) | 37 (80.4) | |
| Hispanic/Latina | 5 (11.6) | 2 (4.4) | |
| Caucasian | 6 (14) | 4 (8.7) | |
| Native American Indian | 0 | 1 (2.2) | |
| Asian | 0 | 1 (2.2) | |
| Other | 1 (2.3) | 1 (2.2) | 0.552c |
| Marital status (%) | |||
| Married | 1 (2.3) | 2 (4.4) | |
| Single | 28 (65.1) | 33 (71.7) | |
| Separated | 3 (7.0) | 4 (8.7) | |
| Divorced | 4 (9.3) | 2 (4.4) | |
| Domestic partnership | 3 (7.0) | 3 (6.5) | |
| Other | 4 (9.3) | 2 (4.4) | 0.851c |
| Education level (%) | |||
| Eleventh grade or less | 15 (34.9) | 19 (41.3) | |
| High school or GED | 18 (41.9) | 19 (41.3) | |
| Two years of college/AA | 8 (18.6) | 5 (10.9) | |
| College (BS/BA) | 2 (4.7) | 2 (4.4) | |
| Master’s degree | 0 | 1 (2.2) | |
| Doctorate | 0 | 0 | 0.797c |
| Currently work (%) | 4 (9.3) | 5 (10.9) | 0.806d |
| Income adequacy (%) | |||
| Enough | 12 (27.9) | 14 (30.4) | |
| Barely adequate | 24 (55.8) | 26 (56.5) | |
| Totally inadequate | 7 (16.3) | 6 (13.0) | 0.900d |
| Has health insurance (% yes) | 41 (95.4) | 43 (93.5) | 1.00c |
| Pregnant (% yes) | 1 (2.3) | 3 (6.5) | 0.617c |
| Has children (% yes) | 27 (62.8) | 27 (58.7) | 0.693d |
| Co-morbidities (%) | |||
| Diabetes | 16 (37.7) | 12 (26.1) | 0.637d |
| Hypertension | 12 (27.9) | 11 (23.9) | 0.940d |
| Depression | 12 (27.9) | 8 (17.4) | 0.518d |
| HIV RNA (1000/mL) | 2.061 | 5.396 | 0.209b |
| Baseline CD4 cells/μ1 | 495.1 (SD=277.9) | 434.6 (SD=236.1) | 0.319b |
| HIV duration – years | 12.8 | 12.4 | 0.670b |
| Current HAART use (% yes) | 30 (69.8) | 35 (76.1) | 0.502d |
| Year started HAART (range) | 1999 (1996–2008) | 2001 (1996–2008) | 0.348b |
Statistical tests were considered significant if they achieved the 0.05 level with two-tailed tests.
Student’s t-test: Differences in baseline mean demographics for continuous variables were analyzed using student’s t-test.
Fisher’s exact test: Differences in baseline mean demographics for categorical variables, when any of the cells had less than five occassions, were analyzed using Fisher’s exact test.
Pearson chi-square test: Differences in baseline mean demographics for categorical variables were analyzed using Pearson’s chi-square test.
Impact on symptom intensity
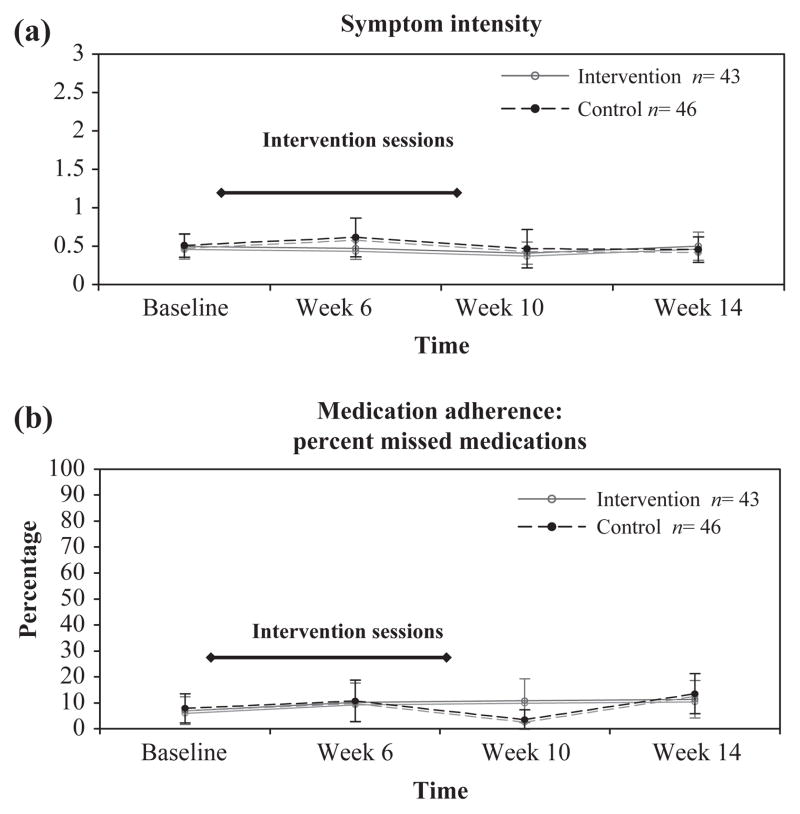
The Sign and Symptom Checklist for Persons with HIV Disease revised (SSC-HIV(rev)) mean scores with 95% confidence intervals by group at baseline and weeks 4, 8, and 12 are presented in Figure 2(a). Random-effects negative binominal regression indicated no significant difference in change between groups across time for total symptom intensity score (Table 3). The mean total symptom intensity score demonstrated a downward linear trend in the intervention group, compared with an upward trend in the control group. This trend was observed when the intervention sessions were occurring (Figure 2). However, this difference between groups over time was not statistically significant.
Figure 2.
Line graphs of mean scores with 95% confidence intervals for the sign and symptom checklist-HIV revised and the percentage of missed HAART doses by group and time period. (a) SSC-HIV(rev) mean scores with 95% confidence intervals by group at baseline and weeks 6, 10, and 14. Higher scores indicate worse symptom intensity in the past 24 hours when 0 = no symptoms; 1 = mild symptoms; 2 = moderate symptoms; and 3 = severe symptoms. Error bars indicate 95% confidence interval of the mean (standard error of the mean). Intervention sessions occurred during weeks 2–8. (b) Mean percentage of missed HAART doses in the past week with 95% confidence intervals at baseline and week 6, 10, and 16. Error bars indicate 95% confidence interval. Intervention sessions occurred during weeks 2–8.
Table 3.
Conditional linear growth model for selected outcomes by predictor variables.
| Outcome | β for timea (standard error) | β for group | β Time × group interaction | Overall Wald χ2 (df = 3) | P-valueb |
|---|---|---|---|---|---|
| Symptoms | |||||
| Mean total symptom intensityb | −0.048 (0.10) | −0.13 (0.27) | 0.04 (0.15) | 0.41 | 0.9378 |
| Quality of life | |||||
| •Overall Functioning | −0.25 (1.25) | −0.66 (4.87) | 0.57 (1.81) | 0.10 | 0.991 |
| •Life Satisfaction | 1.65 (1.44) | 6.78 (5.59) | −4.93 (2.02) | 6.63 | 0.085 |
| •Health Worries | 0.15 (1.30) | 2.45 (6.06) | 0.45 (1.81) | 0.54 | 0.909 |
| •Financial Worries | 1.4 (1.79) | 2.69 (7.24) | 0.63 (2.52) | 2.37 | 0.499 |
| •Medication Worries | −0.05 (0.09) | −0.179 (0.23) | 0.08 (0.12) | 0.66 | 0.884 |
| •HIV Mastery | −0.11 (0.07) | −0.53 (0.20) | −0.05 (0.12) | 25.08 | <0.005c |
| •Disclosure Worries | −0.012 (0.07) | −0.71 (0.22) | −0.01 (0.12) | 24.67 | <0.005c |
| •Trust in HCPd | 0.05 (0.10) | 0.45 (0.25) | −0.13 (0.13) | 3.98 | 0.2631 |
| •Sexual Functioning Worries | −0.24 (2.11) | 0.66 (7.68) | −1.03 (2.94) | 0.41 | 0.9375 |
| Medication adherence | |||||
| Mean percentage missed HAART medicationse | 0.18 (0.10) | −0.13 (0.428) | −0.10 (0.15) | 4.56 | 0.2068 |
All coefficients analyzed are reported using regression coefficients.
Statistical tests were considered significant if they achieved the 0.05 level with two-tailed tests.
Although these tests reached statistical significance, further inspection of the mean scores over time revealed that the difference between the two groups was at baseline and therefore not a result of the intervention.
HCP, health care provider.
HAART, highly active anti-retroviral therapy.
Impact on medication adherence
The mean percentage of missed HAART doses in the past week with 95% confidence intervals is presented in Figure 2(b). Multilevel linear regression indicated no difference between groups across time for HAART medication adherence. The mean, self-reported percentage of missed HAART doses demonstrated an upward trend in both the control and experimental groups, indicating that more doses missed (Figure 2). However, this difference between groups over time was not statistically significant.
Neither the ACTG Reasons for Missed Medications Scale nor the self-reported number of doses missed in the past week changed significantly over time. Change in CD4 lymphocyte count or viral load did not differ significantly by group.
Impact on quality of life
Multilevel linear regression indicated a significant difference between groups across time for two of the nine subscales of the HIV/AIDS-targeted Quality of Life (HAT QoL): HIV Mastery (χ2 = 25.08; p < 0.005) and Disclosure Worries (χ2 = 24.67; p < 0.005). However, further inspection of the mean differences over time revealed that the differences between the two groups were only at baseline. Consequently, the significant results cannot be attributed to the intervention. None of the other quality of life subscales yielded significant results between the experimental and control groups over time (Table 3).
Qualitative results
Overall, the qualitative comments about the intervention were very positive. The participants suggested logistical improvements that could be incorporated into the intervention. However, the three main substantive themes that emerged from intervention group participants included:
-
A strong sense that intervention taught women how to manage their symptoms. One comment that expresses this theme is:
I like the program because I have learned more ways to control any negative symptoms I have.
-
The intervention facilitated a strong sense of community, two of the comments that best exemplify this sentiment are:
I meet other women who are just like me and we learn about each other; they bring experience and they bring lots of information about HIV.
Being able to speak openly and not be ashamed of my status; seeing old friends and knowing I’m not alone.
-
Feeling that the peer leaders could be better: one comment that best expresses this theme is:
I feel the teachers should be more prepared, not just reading off papers. Students and teachers should be able to make lesson plans more interesting; they should be more professional in presentation.
In addition, the participants had many comments about potential topics to be added to the intervention. Suggested topics included more information on discordant couples and relationships, menopause, substance abuse, co-morbidities, and dealing with stress.
Discussion
Symptom management for individuals living with HIV/AIDS continues to be an important biomedical goal. However, the results of this study suggest that a peer-based symptom management intervention may not decrease total symptom intensity or improve medication adherence and quality of life in women living with HIV/AIDS, when compared to a control group who received a symptom management guide-book.
The differences between the two groups on symptom intensity was often less than 0.20 mean points at any point in time which yielded a very small effect size. Previous research with this intervention yielded an effect size of 0.61 (Gifford et al., 1998). However, they were working with a sample that was more recently diagnosed with HIV and compared the intervention group to usual care. The present sample had been living with HIV for many years and reported few symptoms at the beginning of this study (mean symptom intensity of 0.49 on the SSC-HIVrev). Consequently, this may have led to a natural floor effect among those participating in the intervention because they were unable to report lower symptom intensity given the boundaries of the scale. Additionally, a more recent trial testing the effect of the symptom management manual, used as the control condition in the present study, yield ed a 0.4 effect size (Hedges & Olkin, 1985; Wantland et al., 2008). This larger effect size for the control condition may explain the diminished effect size in symptom intensity between the intervention and control groups over time. Several other explanations exist to explain the discrepancies in effect sizes between the original and present study of the PSMP.
The original study was tested in an all-male, relatively well-educated, mainly white sample. Additionally, the original study was completed in 1996. Consequently, the intervention was tested in a very different sample in a time when HIV/AIDS self and symptom management were very different (Chou et al., 2004; Portillo et al., 2007). While the intervention has been updated in the past decade, it was updated based on new medical interventions and not to specifically reflect the needs of women living with HIV/AIDS. For example, qualitative findings suggested that the intervention content could be modified to include topics on gynecological symptoms and menopause, childbearing/rearing, stigma, and sexual negotiation (HRSA, 2005).
In recognition of these growing needs of minority women living with AIDS, investigators developed and tested the SMART/EST Women’s Project (Ironson et al., 2005; Jones et al., 2007). In contrast to the present study, professional therapists, not peers, led this intervention. This two-phase intervention was a 22-week group therapy intervention focusing on cognitive-behavioral stress management related to HIV/AIDS. The analysis of the SMART/EST intervention on HAART medication adherence indicated that participants with exposure to the group sessions had increased medication adherence compared to participants who received the educational control. The intervention also predicted an increase in coping skills related to medication adherence (Jones et al., 2007). The investigators attributed these results to an increase in social learning and emotional support which is supported by other recent literature (Brown & Vanable, 2008). However, other studies have found the positive effect of cognitive-behavioral interventions on HAART medication adherence and stress management is short-lived (Brown & Vanable, 2008; Johnson, Charlebois, Morin, Remien, & Chesney, 2007).
Qualifications of peer leaders are also a potential source of effect size discrepancies. In this study, the peer leaders were representative of the overall sample. They had a high-school diploma or General Educational Development Test (GED), identified as African-American or Latina, were single and had a history of substance abuse. While it is desirable to have peer leaders identify with the participants on these variables, the low education level was an impediment to delivering the scripted intervention as intended. For example, the peers had a hard time reading parts of the script and would often get flustered during the intervention sessions. Several of the qualitative comments supported this barrier.
Finally, one potential reason for the small effect size is that the dose was not sufficient for this population. While the intervention was ongoing, there was a small, although not significant trend toward a decrease in symptom intensity missed HIV medications in the intervention group. However, this trend disappeared when the intervention sessions concluded. These findings are compatible with recent clinical trials evaluating the effect of behavioral interventions on medication adherence (Johnson et al., 2007; Parsons, Golub, Rosof, & Holder, 2007; Sampaio-Sa et al., 2008) and is consistent with a recent review suggesting that effective medication adherence interventions tend to last longer than 12 weeks (Sergio et al., 2006). Of note, the SMART/EST trial did find a significant difference in HAART medication adherence between the intervention and control conditions. However, the dose of their intervention was much longer, both phases lasted 22 weeks, and the measurements were taken immediately after the intervention was complete (Jones et al., 2007).
The primary limitation of this study is its small sample size, which limited the ability to detect significant changes between the two groups over time. While the sample size was based on the original pilot study, the aforementioned factors may have led to a much smaller effect size. Additionally, the sample size was based on the primary outcome, change in symptom intensity, which may have limited the ability to detect changes in medication adherence and quality of life. Women also self-selected into this study, which may have biased the results.
Despite the non-significant findings and limitations in this study, previous research does suggest that peer-based interventions may work to help increase symptom management and self-care (Doull et al., 2004). Future work on similar interventions in comparable populations should consider whether the content of the intervention addresses the more general needs of people living with multiple chronic diseases and in poverty. Future work should also consider using a combination of peers and professionals to help efficiently deliver the content when other barriers exist (i.e., limited literacy). It is also advisable to consider a larger intervention dose and longer follow-up period in order to test the long-term efficacy of future interventions.
Conclusion
To the best of our knowledge, this is the first study to test an established peer-based symptom management intervention in this sample, compared to an efficacious control condition. In this sample of urbandwelling women living with HIV/AIDS, results suggest that a peer-based symptom management intervention may not decrease symptom intensity or increase medication adherence. There is some positive evidence that suggests that the intervention may increase some important aspects of quality of life. However, further research is warranted to elucidate the effect of peer-based interventions in achieving positive self-management outcomes using study designs that incorporate heterogeneous HIV+, female populations and longer follow-up periods.
Acknowledgments
I would like to thank all members of my dissertation committee: William Holzemer, Sally Rankin, Carmen Portillo and Kate Lorig. Their input and assistance with this project was invaluable. I also want to thank Bruce Cooper for his statistical support and Marla Longenecker for her editoral suppport. I also wish to thank all of the community clinic representatives who assisted in the recruitment of the participants of this study: Sona Soha and Deb Royal at the East Bay AIDS Consortium, Gwen Smith at Southeast Health Center, Roland Zepf at Ward 86 in San Francisco General Hospital and Shalini Eddens at WORLD. This project was funded by a training grant from the National Institutes of Health 1F31NR009910, by the 2008 American Nurses Foundation/Western Institute of Nursing Research Grant and by grants from the University of California, San Francisco Graduate Division and the School of Nursing Century Fund.
References
- Brown J, Vanable P. Cognitive, ÄìBehavioral stress management interventions for persons living with HIV: A review and critique of the literature. Annals of Behavioral Medicine. 2008;35(1):26–40. doi: 10.1007/s12160-007-9010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. HIV/AIDS among women. 2006 Retrieved July 13, 2006, from http://www.cdc.gov/hiv/topics/women/resources/factsheets/pdf/women/pdf.
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Chou F, Holzemer WL, Portillo CJ, Slaughter R. Self care strategies and sources of information for HIV/AIDS symptom management. Nursing Research. 2004;53(5):332–339. doi: 10.1097/00006199-200409000-00008. [DOI] [PubMed] [Google Scholar]
- Doull M, O’Connor AM, Wells GA, Tugwell P, Welch V. Peer-based interventions for reducing morbidity and mortality in HIV-infected women (Protocol) Cochrane Database of Systematic Reviews. 2004;(2):Art. No.: CD004774. doi: 10.1002/14651 858.CD004774. [DOI] [Google Scholar]
- Garcia-Moreno CW, Watts C. Violence against women: Its importance for HIV/AIDS. AIDS. 2000;14(Suppl 3):S253–S265. [PubMed] [Google Scholar]
- Gifford A, Laurent D, Gonzales V, Chesney M, Lorig K. Pilot randomized trial of education to improve self-management skills of men with symptomatic HIV/AIDS. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;18(2):136–144. doi: 10.1097/00042560-199806010-00005. [DOI] [PubMed] [Google Scholar]
- Gifford A, Lorig K, Laurent D, Gonzalez V. Positive self-management program leader’s manual. Palo Alto, CA: Stanford Patient Education Research Center; 1995. [Google Scholar]
- Gifford AL. Self-management health education for chronic HIV infection. AIDS Care. 1999;11(1):115–130. doi: 10.1080/09540129948243. [DOI] [PubMed] [Google Scholar]
- Gupta GR. How men’s power over women fuels the HIV epidemic: It limits women’s ability to control sexual interactions. British Medical Journal. 2002;324(186):183–184. doi: 10.1136/bmj.324.7331.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzemer W, Bakken S, Portillo C, Grimes R, Welch J, Wantland D, et al. Testing a nurse tailored HIV medication adherence intervention. Nursing Research. 2006;55(3):189–197. doi: 10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Holzemer WL, Henry SB, Nokes KM, Corless IB, Brown M, Powell-Cope GM, et al. AIDS Care 9 Validation of the Sign and Symptom Check-list for Persons with HIV Disease (SSC-HIV) Journal of Advanced Nursing. 1999;30(5):1041–1049. doi: 10.1046/j.1365-2648.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- Holzemer WL, Henry SB, Portillo CJ, Miramontes H. The client adherence profiling-intervention tailoring (CAP-IT) intervention for enhancing adherences to HIV/AIDS medications: A pilot study. Journal of the Association of Nurses in AIDS Care. 2000;11(1):36–44. doi: 10.1016/s1055-3290(06)60420-2. [DOI] [PubMed] [Google Scholar]
- HRSA. Washington, DC: H.R.A.S. Administration; 2005. Applying elements of the chronic are model to HIV/AIDS clinical care: Moving CARE Act clients from intensive case management toward self-management; pp. 32–41. Retrieved September 1, 2009, from: http://www.maricopa.gov/Public_Health/PubDocuments/06.08.07_Over_45_Needs_Assessment.pdf. [Google Scholar]
- Hunter M. The materiality of everyday sex: Thinking beyond “prostitution”. African Studies. 2002;61(1):99–120. [Google Scholar]
- Ironson G, Weiss S, Lydston D, Ishii M, Jones D, Asthana D, et al. The impact of improved self-efficacy on HIV viral load and distress in culturally diverse women living with AIDS: The SMART/EST women’s project. AIDS Care. 2005;17(2):222–236. doi: 10.1080/09540120512331326365. [DOI] [PubMed] [Google Scholar]
- Johnson M, Charlebois E, Morin S, Remien R, Chesney M. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: The healthy living project randomized controlled study. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 2007;46(5):574–580. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MO, Stallworth T, Neilands TB. The drugs or the disease? Causal attributions of symptoms held by HIV-Positive Adults on HAART. AIDS and Behavior. 2003;7(2):109–117. doi: 10.1023/a:1023938023005. [DOI] [PubMed] [Google Scholar]
- Jones D, McPherson-Baker S, Lydston D, Camille J, Brondolo E, Tobin J, et al. Efficacy of a group medication adherence intervention among HIV positive women: The SMART/EST Women’s Project. AIDS and Behavior. 2007;11(1):79–86. doi: 10.1007/s10461-006-9165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K, Cunningham W, Spritzer K, Hays R. Changes in symptoms and health-related quality of life in a nationally representative sample of adults in treatment for HIV. Quality of Life Research. 2006;15(6):951–958. doi: 10.1007/s11136-005-6010-x. [DOI] [PubMed] [Google Scholar]
- Lorenz KA, Shapiro MF, Asch SM, Bozzette SA, Hays RD. Associations of symptoms and health-related quality of life: Findings from a National Study of Persons with HIV Infection. Annuals of Internal Medicine. 2001;134(9_Part_2):854–860. doi: 10.7326/0003-4819-134-9_part_2-200105011-00009. [DOI] [PubMed] [Google Scholar]
- Lorig K, Ritter PL, Plant K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis and Rheumatism. 2005;53(6):950–957. doi: 10.1002/art.21604. [DOI] [PubMed] [Google Scholar]
- Maman S, Mbwambo JK, Hogan NM, Kilonzo GP, Campbell JC, Weiss E, et al. HIV-Positive women report more lifetime partner violence: Findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. American Journal of Public Health. 2002;92(8):1331–1337. doi: 10.2105/ajph.92.8.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannheimer SB, Wold N, Gardner EM, Telzak EE, Huppler Hullsiek K, Chesney M, et al. Mild, Äêto, ÄêModerate Symptoms during the first year of antiretroviral therapy worsen quality of life in HIV, ÄêInfected individuals. Clinical Infectious Diseases. 2008;46(6):941–945. doi: 10.1086/528859. [DOI] [PubMed] [Google Scholar]
- Ogden J, Esim S. Reconcepualizing the care continuum for HIV/AIDS. Washington, DC: International Center for Research on Women; 2003. [Google Scholar]
- Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndrome. 2007;46(4):443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo C, Holzemer WL, Chou FY. HIV symptoms. Annual Review of Nursing Research. 2007;25:259–291. [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modelling using Stata. 1. College Station, TX: Stata press; 2005. [Google Scholar]
- Sampaio-Sa M, Page-Shafer K, Bangs berg D, Evans J, Dourado M, Teixeira C, et al. 100% adherence study: Educational workshops vs. video sessions to improve adherence among ART-NaÏve patients in Salvador, Brazil. AIDS and Behavior. 2008;12:S54–S62. doi: 10.1007/s10461-008-9414-0. [DOI] [PubMed] [Google Scholar]
- Sandelowski M, Lambe C, Barroso J. Stigma in HIV-positive women. Journal of Nursing Scholarship. 2004;36(2):122–128. doi: 10.1111/j.1547-5069.2004.04024.x. [DOI] [PubMed] [Google Scholar]
- Sergio R, Park-Wyllie LY, Bayoumi A, Tynan A-M, Antoniou T, Rourke S, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database of Systematic Review. 2006;(2):Art. No.: CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel K, Schrimshaw SW, Dean L. Symptom interpretation: Implications for delay in HIV testing and care among HIV-infected late middle-aged and older adults. AIDS Care. 1999;11(5):525–535. doi: 10.1080/09540129947686. [DOI] [PubMed] [Google Scholar]
- Sousa KH, Holzemer WL, Bakken Henry S, Slaughter R. Dimensions of health-related quality of life in persons living with HIV disease. Journal of Advanced Nursing. 1999;29(1):178–187. doi: 10.1046/j.1365-2648.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- Sowell RL, Seals BF, Moneyham L, Demi A, Cohen L, Brake S. Quality of life in HIV-infected women in the south-eastern United States. AIDS Care. 1997;9(5):510–512. doi: 10.1080/713613191. [DOI] [PubMed] [Google Scholar]
- Stata & Corporation. College Station, TX: Author; 2009. Version 10.0. [Google Scholar]
- Steinberg M, Johnson M, Schierhout G, Ndewa D. A survey of house-holds affected by HIV/AIDS in South Africa. Menlo Park, CA: The Henry Kaiser Family Foundation; 2002. Hitting home: How households cope with the impact of the HIV/AIDS epidemic. [Google Scholar]
- van Rompay KKA, Madhivanan P, Rafiq M, Krupp K, Chakrapani V, Selvam D. Human. 6. Vol. 10. A.R. Webel Resources for Health; 2008. Empowering the people: Development of an HIV peer education model for low literacy rural communities in India; p. 6. Published online 2008 April 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Servellen G, Sarna L, Jablonski KJ. Women with HIV: Living with symptoms. Western Journal of Nursing Research. 1998;20(4):448–464. doi: 10.1177/019394599802000404. [DOI] [PubMed] [Google Scholar]
- Wantland DJ, Holzemer WL, Moezzi S, Willard SS, Arudo J, Kirksey KM, et al. A randomized controlled trial testing the efficacy of an HIV/AIDS symptom management manual. Journal of Pain and Symptom Management. 2008;36(3):235–246. doi: 10.1016/j.jpainsymman.2007.10.011. [DOI] [PubMed] [Google Scholar]