Abstract
Objectives: We aim to demonstrate that endoscopic ultrasound (EUS)-guided transgastric pancreaticography/drainage of the pancreatic duct is feasible and successful in healing a persisting pancreaticocutaneous fistula.
Methods: By means of a case report, we describe the following alternative therapeutic procedure. A 76-year-old male had: (1) 10 surgical interventions because of necrotizing acute pancreatitis with a persisting pancreaticocutaneous fistula (volume 200–300 ml/day); (2) an unsuccessful attempt of transpapillary drainage (disrupted duct after necrosectomy). He then underwent a EUS-guided transluminal pancreaticography/drainage of the pancreatic duct. A transgastric puncture was performed followed by, insertion of a guide wire into the dilated tail segment, and expansion of the gastropancreaticostomy using a 10-Fr retriever. A 10-Fr Amsterdam prosthesis was then placed through the guide wire.
Results: The procedure was both a technical and clinical success as indicated by fistula occlusion and sufficient internal drainage of the pancreatic juice via the gastropancreaticostomy. No severe complications such as bleeding, perforation stent occlusion or migration were observed during the 15-month follow-up.
Conclusions: Transgastric pancreaticography and EUS-guided drainage of the enlarged pancreatic duct are elegant and feasible alternative options for the treatment of specific pancreatic lesions such as persisting pancreaticocutaneous fistula (complication after necrotizing pancreatitis), after pancreatic resective surgery, chronic pancreatitis and anomaly of the congenital pancreatic or postoperative gastrointestinal anatomy. Moreover, the procedure may represent a valid tool to avoid surgery and more invasive interventions.
Keywords: pancreaticocutaneous fistula, disconnected pancreatic tail syndrome, retention of the pancreatic duct, endoscopic ultrasound (EUS), translumenal, pancreaticography, drainage of the pancreatic duct, pancreatico-gastrostomy
Introduction
Pancreaticography followed by transpapillary drainage of the retained pancreatic duct represents a valid option when the papilla of Vater cannot be reached or a catheter cannot be introduced through the papilla due to postinflammatory alterations or postoperative changes [Will, 2008; Erickson, 2007; Kahaleh et al. 2007; Shami and Kahaleh, 2007; Buscail et al. 2006; Will et al. 2007, 2005].
The aim of this case report was to describe a novel technique adapted to case-specific requirements to achieve drainage of the dilated pancreatic duct in a disconnected pancreatic tail syndrome with persisting postoperative pancreaticocutaneous fistula. The procedure consists of endoscopic ultrasound (EUS)-guided transgastric pancreaticography and drainage of the dilated pancreatic duct. The latter represents the access site when a conventional endoscopic retrograde cholangiopancreatography (ERCP)-guided approach cannot be considered an option.
Methods
Patient
A 76-year-old male patient was referred because of a persisting pancreaticocutaneous fistula (volume 200–300 ml/day) as a result of a postinflammatory complication. This occurred after 10 surgical interventions for necrosectomy and programmed lavage due to necrotizing acute pancreatitis and peritonitis. The recent medical history was significant for a temporary multiorgan failure because of sepsis due to infected pancreatic necroses and a 6-month hospital stay. As the attempt to drain the pancreatic duct (disruption was identified after pancreatic necrosectomy) was unsuccessful, the patient underwent transgastric pancreaticography and EUS-guided drainage of the pancreatic duct (disconnected pancreatic tail syndrome). The patient was asked to sign a consent form which included a list of alternative treatment options and complications.
Procedure
The patient was positioned on a fluoroscopy table and underwent an EUS (Hitachi Medical Systems, Lübbecke, Germany) using a therapeutic longitudinal scanner (ECOSCAN Hitachi Ultraschall, Berlin, Germany) after unsuccessful conventional ERCP (under periinterventional administration of antibiotics). After EUS-based identification of the pancreatic duct (head segment with enlarged calibre) and of a pancreaticocutaneous fistula (Figure 1a–c), the duct was punctured using a 19-G needle (Cook Deutschland, Mönchengladbach, Germany, Figure 2a). The pancreatic juice was aspirated and the duct was imaged with contrast medium using fluoroscopy (Figure 2a). An attempt was performed to insert from the pancreatic side a 0.035-inch guide wire (Boston Scientific, Ratingen, Germany) into the pancreatic duct directed to the papilla of Vater. However, this was unsuccessful despite the different directions and angles attempted during the introduction (Figure 2b). Therefore, the wire was introduced within the tail segment of the duct (Figure 2c) followed by expansion of the gastropancreaticostomy using a 10-Fr prosthetic retriever (Figure 2d) and placement of a 10-Fr Amsterdam prosthesis (Medi-Globe, Achenmühle, Germany) via the guide wire (Figure 2e) and under a gastroscopy-based view. Subsequent control pancreaticography (immediately after the interventional procedure) and abdominal ultrasound (on the 4th postinterventional day; Figure 3a and b), revealed a smaller calibre of the pancreatic duct, immediate decrease and cessation of the segregation via the fistula (on the 4th postinterventional day).
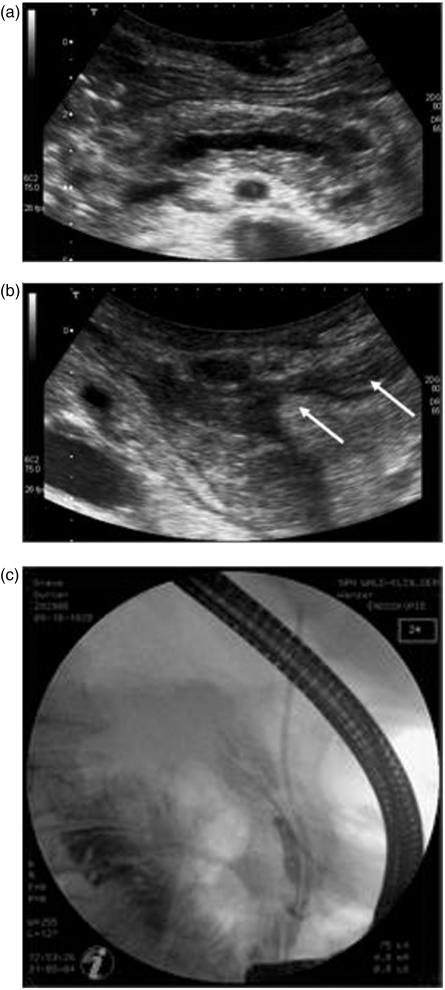
Figure 1.
Initial steps of the endoscopic intervention to image and drain the dilated pancreatic duct (76-year-old male patient). (a) Transabdominal ultrasound reveals an enlarged calibre of the pancreatic duct within the body and tail of the pancreas. (b) Ultrasound demonstrates the region of the former pancreatic tail and pancreaticocutaneous fistula (white arrows). (c) Pancreaticography shows only the pancreatic duct in the body of the pancreas and a disrupted duct without opacification of the duct within body and tail.
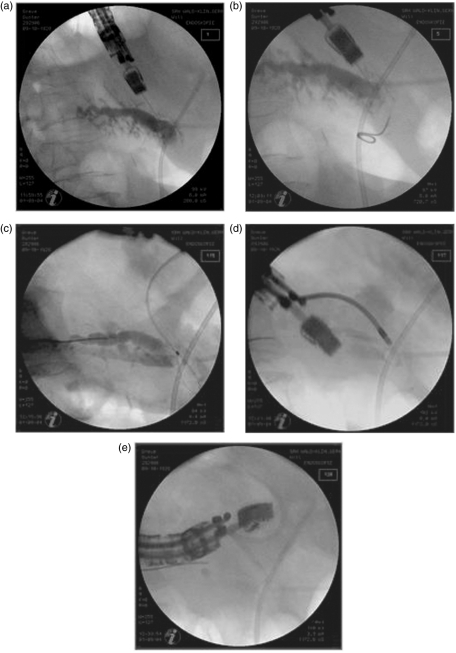
Figure 2.
Pancreaticography-based control view during the intervention. (a) EUS-guided transgastric puncture of the pancreatic duct using a 19-G needle. (b) Unsuccessful insertion of the guide wire into the pancreatic duct (attempt to direct it into the papilla of Vater from the pancreatic site). (c) Placement of the guide wire within the tail segment of the pancreatic duct. (d) Expansion of the gastropancreaticostomy using a 10-Fr retriever. (e) Placement of a 10-Fr Amsterdam prosthesis via the guide wire.
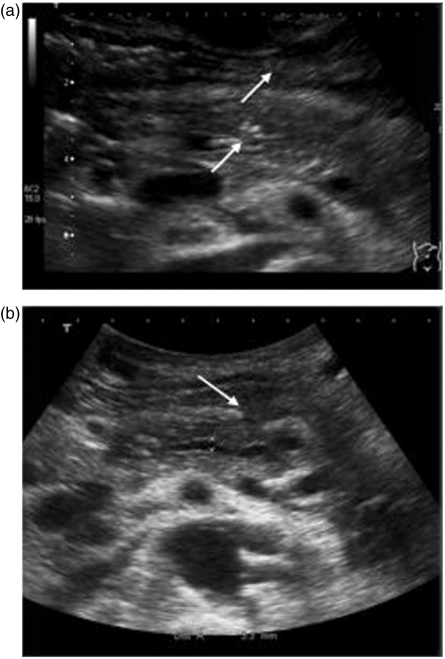
Figure 3.
Control abdominal ultrasound on the 4th postinterventional day. (a) Regular position of the stent. (b) Smaller calibre of the pancreatic duct.
Results
The procedure was both a technical and clinical success as indicated by fistula occlusion and sufficient internal drainage of the pancreatic juice via the gastropancreaticostomy. In addition, there were no severe complications such as bleeding or perforation and only a temporary and mild postinterventional pain was observed.
The further clinical course was uneventful and the patient was discharged on the 12th postinterventional day with substantial quality of life improvements. The investigations during a follow-up period of 15 months revealed no signs of stent occlusion or migration. Stent replacement was not necessary since, prior to the intervention, the patient did not show any signs or symptoms indicating recurrent problems. However, a recurrent pain was treated with analgesics. Follow-up investigations consisted of clinical examination, abdominal ultrasound and laboratory analysis of inflammatory parameters. Pancreatic enzymes were checked every 3 months to exclude recurrent pancreatitis, fistula, reobstruction and/or stent occlusion/migration. After an intermediate loss to follow-up, the patient recently visited his family practitioner in good health conditions and no complications were observed during the 4 years postintervention.
Discussion
EUS-guided identification and puncture of the pancreatic duct with an enlarged calibre may circumvent the problem of an unreachable papilla of Vater, facilitate the introduction of a catheter into the papilla and allow further manipulation such as the insertion of a guide wire into the pancreatic duct (to achieve drainage of the retained or disrupted pancreatic duct [Will, 2008; Erickson, 2007; Shami and Kahaleh, 2007; Will et al. 2007, 2005; Kahaleh et al. 2007, 2003; Buscail et al. 2006; Dewitt et al. 2004; Mallery et al. 2004; Francois et al. 2002]). In agreement with the recent literature, the EUS-guided procedure represents the appropriate treatment option for several conditions. [Gleeson and Levy, 2008; Shami and Kahaleh, 2007]. These include:
postinflammatory changes in the periampullary region, with no identifiable mouth of the papilla of Vater;
congenital anomaly (‘pancreas divisum’);
postoperative abnormalities of the gastrointestinal and periluminal anatomy after gastrectomy with esophagojejunostomy according to Roux-en-Y reconstruction, BII gastric resection or Whipple procedure;
disconnected pancreatic tail syndrome after acute pancreatitis or endoscopic/surgical necrosectomy.
It is important to note that in (ii) and (iii) the papilla cannot be reached [Gleeson and Levy, 2008; Will, 2008; Kahaleh et al. 2007; Will et al. 2007, 2005].
Recently, EUS-guided interventions at the pancreatic duct have been described as case reports [Dewitt et al. 2004; Mallery et al. 2004; Kahaleh et al. 2003; Francois et al. 2002]. At present approximately 60 EUS-guided drainages of the pancreatic duct have been reported.
Two EUS-based techniques for pancreatic duct drainage should be distinguished:
rendezvous EUS-guided endoscopic retrograde pancreatography (ERP). The technique consists of an injection of contrast medium into the pancreatic duct via EUS followed by passage of a wire through the EUS needle into the downstream duct and the papilla of Vater, and subsequent ERP;
passage of the wire into pancreatic duct under EUS control (with no contrast injection into the pancreatic duct) and placement of a stent over the wire to drain the pancreatic duct transluminally into the stomach.
In the case presented, ERCP was the initial step to achieve drainage of the pancreatic duct and a sufficient closure of the persisting pancreaticocutaneous fistula. However, this was not successful because of a disrupted pancreatic duct. Transgastric pancreaticography and EUS-guided drainage of the pancreatic duct represent a valid option to avoid surgery. These are reasonable and feasible alternative options for the treatment of specific pancreatic lesions such as pancreaticocutaneous fistula. Moreover, these procedures can be considered:
to lower pressure within the pancreatic duct;
to achieve an internal drainage of the pancreatic juice even in the case of a disconnected duct in the tail of the pancreas;
to circumvent the conventional route of drainage via the papilla of Vater; and
to finally improve quality of life.
The pancreaticocutaneous fistula can be considered a postoperative complication after necrosectomy most likely in the segment of the pancreatic corpus (maintained by a persisting lesion or interruption of the pancreatic duct or parenchyma after necrosectomy).
This case represents the first interventional EUS used to achieve the closure of a pancreaticocutaneous fistula a similar procedure was used in a case with different clinical conditions [Arvanitakis et al. 2007]) to achieve the closure of a pancreaticocutaneous fistula among 2371 investigations and 457 EUS-guided interventional approaches reported in our clinic in the last 3 years. This approach can also be considered reasonable for several indications such as chronic pancreatitis, anomalies of the congenital pancreatic or postoperative gastrointestinal and peripancreatic anatomy, providing a low periinterventional risk and avoiding more traumatic surgery [Will, 2008; Kahaleh et al. 2007, 2003; Shami and Kahaleh 2007; Will et al. 2007, 2005]. However, the procedure has some limitations and can be performed only when a dilated pancreatic duct is observed. Moreover, follow-up recommendations and time intervals for control investigations have yet to be established and need further evaluation [Will et al. 2007]. Therefore, stent replacement is usually recommended when there are reoccurring signs and symptoms indicating stent occlusion or migration as revealed by repeated clinical examination, analysis of laboratory parameters and abdominal ultrasound within short-term intervals.
Conclusions
Implications for clinical care (prevention, recognition and management)
Transgastric pancreaticography and EUS-guided drainage of the enlarged pancreatic duct are elegant and feasible alternative options for the treatment of disrupted pancreatic tail syndrome with a persisting pancreaticocutaneous fistula (complication after necrotizing pancreatitis), after pancreatic resective surgery [Calasan et al. 2009], chronic pancreatitis and anomaly of the congenital pancreatic or postoperative gastrointestinal anatomy. The procedure may represent a valid tool to avoid surgery and more invasive interventions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- Arvanitakis M., Delhaye M., Bali M.A., Matos C., Le Moine O., Devière J. (2007) Endoscopic treatment of external pancreatic fistulas: when draining the main pancreatic duct is not enough. Am J Gastroenterol 102: 516–524 [DOI] [PubMed] [Google Scholar]
- Buscail L., Faure P., Bournet B., Selves J., Escourrou J. (2006) Interventional endoscopic ultrasound in pancreatic diseases. Pancreatology 6: 7–16 [DOI] [PubMed] [Google Scholar]
- Calasan I., Junger M., Schwendtner P., Pommer S., Schoenberg M.H. (2009) Pancreatic surgery in a regional hospital. Zentralbl Chir 134: 160–165 [DOI] [PubMed] [Google Scholar]
- Dewitt J., McHenry L., Fogel E., Leblanc J., McGreevy K., Sherman S. (2004) EUS-guided methylene blue pancreatography for minor papilla localization after unsuccessful ERCP. Gastrointest Endosc 59: 133–136 [DOI] [PubMed] [Google Scholar]
- Erickson R.A. (2007) EUS-guided pancreaticogastrostomy: invasive endosonography coming of age. Gastrointest Endosc 65: 231–232 [DOI] [PubMed] [Google Scholar]
- Francois E., Kahaleh M., Giovanni M., Matos C., Devierre J. (2002) EUS-guided pancreaticogastrostomy. Gastrointest Endosc 56: 128–133 [DOI] [PubMed] [Google Scholar]
- Gleeson F.C., Levy M.J. (2008) Endoscopic ultrasound (EUS) guided access and therapy of pancreatico-biliary disorders. Minerva Gastroenterol Dietol 54: 151–160 [PubMed] [Google Scholar]
- Kahaleh M., Yoshida C., Yeaton P. (2003) EUS antegrade pancreatography with gastropancreatic duct stent placement. Review of two cases. Gastrointest Endosc 58: 919–923 [DOI] [PubMed] [Google Scholar]
- Kahaleh M., Hernandez A.J., Tokar J., Adams R.B., Shami V.M., Yeaton P. (2007) EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc 65: 224–230 [DOI] [PubMed] [Google Scholar]
- Mallery S., Matlock J., Freeman M.L. (2004) EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc 59: 100–107 [DOI] [PubMed] [Google Scholar]
- Shami V.M., Kahaleh M. (2007) Endoscopic ultrasonography (EUS)-guided access and therapy of pancreatico-biliary disorders: EUS-guided cholangio and pancreatic drainage. Gastrointest Endosc Clin N Am 17: 581–593, vii–viii [DOI] [PubMed] [Google Scholar]
- Will U., Meyer F., Manger T., Wanzar I. (2005) Endoscopic ultrasound-assisted rendezvous maneuver to achieve pancreatic duct drainage in obstructive chronic pancreatitis. Endoscopy 37: 171–173 [DOI] [PubMed] [Google Scholar]
- Will U., Fueldner F., Thieme A.K., Goldmann B., Gerlach R., Wanzar I., et al. (2007) Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg 14: 377–382 [DOI] [PubMed] [Google Scholar]
- Will U. (2008) Therapeutic endosonography. Z Gastroenterol 46: 555–563 [DOI] [PubMed] [Google Scholar]