Abstract
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome, which includes dyslipidemia, central obesity, hypertension, and insulin resistance. These diseases collectively and individually increase the risk of cardiovascular disease. Nonalcoholic steatohepatitis (NASH) is a subset of NAFLD that can progress to cirrhosis in up to 30% of patients and lead to decompensated liver disease requiring liver transplantation in many patients. Insulin resistance is the pathophysiological hallmark of NASH and addressing insulin resistance is an important aspect of NASH management. Lifestyle modifications with diet and exercise improve insulin sensitivity and are the cornerstone of therapy, but are often difficult to maintain long term. Not surprisingly, insulin-sensitizing agents have been a focus of pharmacologic investigation in NASH. Insulin sensitizers such as the thiazolidinediones, biguanides, glucagon-like peptide-1 receptor agonists, and the dipeptidyl peptidase IV inhibitors, also known as incretins, will be discussed with respect to their mechanism of action and how these drugs might target aspects of NASH pathophysiology. Finally, we will summarize the available clinical data and review both the risks and benefits of insulin sensitizers in the treatment of NASH.
Keywords: exenatide, insulin resistance, metformin, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, pioglitazone, rosiglitazone, sitagliptin, thiazolidinediones, vitamin E
Background
Obesity has reached epidemic proportions in the United States over the past decade and is responsible for more disease and death than any other single factor [Sturm, 2003]. The increased prevalence of nonalcoholic fatty liver disease (NAFLD) is directly related to the rise in obesity. NAFLD affects up to 24% of Americans overall and up to 93% of people with obesity [Sass et al. 2005]. Not surprisingly, it is the most common cause of abnormal liver tests in the United States [Ruhl and Everhart, 2003]. NAFLD represents the hepatic manifestation of the metabolic syndrome that includes dyslipidemia, central obesity, hypertension, and insulin resistance. It encompasses a spectrum of disease that ranges from simple hepatic steatosis to hepatic steatosis with inflammation and cellular ballooning, termed nonalcoholic steatohepatitis (NASH), with varying degrees of fibrosis [Sass et al. 2005]. Among patients with NASH, anywhere from 15% to 20% will develop cirrhosis that may result in decompensated liver disease or hepatocellular carcinoma, often necessitating liver transplantation [Sass et al. 2005; Angulo and Lindor, 2002]. The mechanisms and risk factors underlying this progression in a subset of patients are not fully understood.
One of the challenges in understanding the pathophysiology of NAFLD, as well as in designing clinical trials, is that what we now refer to as NAFLD represents a heterogeneous phenotype. Varied metabolic abnormalities may lead to hepatic steatosis and a person’s ability to break down or safely store fat in the liver. These abnormalities, in addition to the coexistence of other metabolic diseases or the presence of cirrhosis, add further complexity. Heterogeneity of the NAFLD phenotype may explain in part why clinical trials have had difficulty showing broad efficacy. Indeed, pharmacologic interventions have demonstrated a variable effect on NASH: the subtype of NAFLD that is most likely to result in significant liver disease.
Epidemiological studies have demonstrated that mortality among community-diagnosed patients with NAFLD is higher than the general population and is associated with older age, impaired fasting glucose, and cirrhosis [Adams et al. 2005a]. In addition, mortality is significantly higher in patients with NASH and is more often due to cardiovascular disease or nonliver malignancy than cirrhosis [Caldwell and Argo, 2010; Ekstedt et al. 2006]. NAFLD and NASH are highly correlated with body mass index (BMI) and obesity; insulin resistance has been shown to be an independent risk factor for the development of NASH, regardless of BMI [Chitturi et al. 2002; Marchesini et al. 1999]. Since insulin resistance is the pathophysiological hallmark of NASH, there has been considerable emphasis on the study of interventions to improve insulin sensitivity. Several large, well-designed clinical trials now offer additional insight into the therapeutic role of insulin-sensitizing agents in the treatment of NASH.
Owing to the increasing prevalence of NAFLD and its association with other metabolic disorders, it is important that clinicians have a solid understanding of its clinical spectrum of disease presentation as well as available therapeutic options. This review will address insulin resistance in NASH and the role of insulin sensitizers in the treatment of biopsy-proven NASH.
Nonalcoholic steatohepatitis and insulin resistance
The observation that there was an association between diabetes and fatty liver disease was made over 60 years ago [Zimmerman et al. 1950]. Since then, multiple studies have confirmed this association and demonstrated that insulin resistance underlies the foundation of NASH [Chitturi et al. 2002; Pagano et al. 2002]. The prevalence of insulin resistance in the general population approaches 45%, however in patients with NASH insulin resistance or the metabolic syndrome are present in up to 95% of patients [Bloom et al. 2008; Marchesini et al. 2003]. Not surprisingly, as the degree of insulin resistance worsens so does the severity of NASH [Kotronen et al. 2007; Bugianesi et al. 2006; Marchesini et al. 2003]. In addition, insulin resistance predicts the presence of NASH, even in people with normal liver enzymes [Fracanzani et al. 2008]. Thus, insulin resistance is a logical therapeutic target.
The pathogenesis of NASH is complex and beyond the scope of this review. Therefore, we will focus on the role of insulin resistance specifically. Specifically, inappropriate lipolysis in the fed state elevates free fatty acids and subsequently increases hepatic fatty acid uptake; insulin resistance impairs fatty acid oxidation, fatty acid export from the liver in the form of very-low-density lipoproteins (VLDLs) and promotes de novo lipogenesis. Furthermore, inflammatory factors, originating largely from adipose tissue, worsen insulin resistance and promote liver injury (Figure 1). Thus, a vicious cycle is created in which hepatic lipid accumulation leads to insulin resistance and insulin resistance further promotes hepatic steatosis and inflammation, which in turn exacerbate the insulin resistant state [Bugianesi et al. 2010].
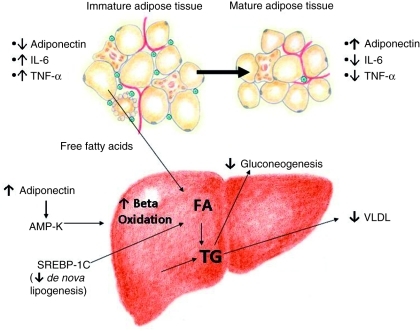
Figure 1.
Adipose tissue and hepatic effects of the thiazolidinediones (TZDs). The TZDs have a complex and incompletely understood mechanism of action. In the setting of obesity and insulin resistance, visceral adipose tissue is characterized by an abundance of premature adipocytes and macrophages. This favors the production of several proinflammatory adipocytokines, including interleukin-6 and tumor necrosis factor-α (TNF-α) that promote cellular injury and further antagonize insulin action. Adiponectin is a protein hormone primarily secreted by mature adipose tissue that counteracts the effects of TNF-α and improves insulin sensitivity. Premature adipocytes within visceral adipose tissue produce very little adiponectin. TZDs promote maturation and redistribution of adipose tissue resulting in a reduction in macrophage infiltration and number of immature adipocytes. This diminishes the secretion of proinflammatory adipocytokines and increases adiponectin levels. Furthermore, direct effects on hepatic lipid metabolism increase beta oxidation, and decrease de novo lipogenesis, which then results in decreased very-low-density lipoprotein secretion and gluconeogenesis. AMP-K, 5′adenosine monophosphate-activated protein kinase; FA, fatty acid; SREBP-1c, sterol regulatory element binding protein-1c; TG, triglyceride; VLDL, very-low-density lipoprotein.
Improving insulin sensitivity as a treatment for nonalcoholic steatohepatitis
Weight loss and exercise can be effective in improving insulin sensitivity and should be part of any NAFLD treatment regimen [Promrat et al. 2010]. The metabolic benefits, including the effects of weight loss on NAFLD as a result of lifestyle modification or bariatric surgery, are discussed elsewhere [Promrat et al. 2010; Pillai and Rinella, 2009; Clark, 2006].
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) was established by the National Institute of Diabetes and Digestive and Kidney Diseases in 2002 to assess the natural history, pathogenesis, and therapy of this disease. A significant body of literature has resulted from this effort.
The NAFLD Activity Score was developed to quantify treatment effect in clinical trials [Kleiner et al. 2005]. Most clinical trials have studied the effects of two classes of insulin sensitizers on NASH: the thiazolidinediones (TZDs – troglitazone, rosiglitazone and pioglitazone), and the biguanides (metformin).
NASH is considered by many to be a component of the metabolic syndrome, a cluster of closely associated abnormalities related to insulin resistance [Marchesini and Marzocchi, 2007]. Lifestyle intervention, metformin, and the TZDs can help prevent the progression of glucose intolerance and the onset of type 2 diabetes [Gerstein et al. 2006; Knowler et al. 2002; Tuomilehto et al. 2001]. Aside from any potential direct beneficial effects on the liver, pioglitazone has also been shown to reduce the rate of progression to diabetes in patients with a history of gestational diabetes [Xiang et al. 2006]. In addition, pioglitazone is associated with an 18% reduction in death, myocardial infarction, or cerebrovascular accident [Lincoff et al. 2007]. The beneficial cardiovascular effects of pioglitazone are likely due to its positive effect on the lipid profile associated with the metabolic syndrome [raise high-density lipoprotein (HDL), lower triglycerides (TGs)] [Spanheimer et al. 2009].
thiazolidinediones
Among insulin-sensitizing drugs, TZDs are a therapeutic class that are selective agonists for peroxisome proliferator-activated receptor-γ (PPAR-γ), a transcription factor that regulates gene expression in liver, adipose, vascular endothelium, and muscle tissue. TZDs improve insulin sensitivity at the level of adipose tissue, muscle, and liver, and are now widely used for treatment of type 2 diabetes. The currently available TZDs include rosiglitazone and pioglitazone.
The TZDs overall have a complex and incompletely understood mechanism of action. They stimulate maturation of visceral fat, and hence change the adipocytokine profile secreted by adipose tissue. TZDs lead to an increase in adiponectin levels, which counteracts proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and promotes beta oxidation of fatty acids via 5′adenosine monophosphate-activated protein kinase (AMP-K) activation [Coletta et al. 2009; Bajaj et al. 2004]. The increase in beta oxidation, in conjunction with a reduction in de novo lipogenesis, decreases gluconeogenesis [Lutchman et al. 2006; Bajaj et al. 2004] (Figure 1).
Clinical trials of TZD use in nonalcoholic steatohepatitis
TZDs are the most well-studied compounds to date for the treatment of NASH (Table 1). Troglitazone was the first TZD to be tested for NASH in an open-label pilot clinical trial [Caldwell et al. 2001]. While treatment with troglitazone resulted in a biochemical response, severe hepatotoxicity in some patients led to its removal from the market [Adams et al. 2005b]. Hepatotoxicity has not been demonstrated with newer compounds, which have been extensively studied in both NAFLD and NASH [Siebler and Galle, 2006]. This review focuses on the use of TZDs in biopsy-proven NASH.
Table 1.
Studies of thiazolidinediones in adult patients with biopsy-proven nonalcoholic steatohepatitis.
| Study | n | Design | % Diabetes mellitus | Drug* | Daily dose (mg) | Compared with | Duration | Liver enzymes | Liver fat by imaging | Histology |
|---|---|---|---|---|---|---|---|---|---|---|
| Caldwell et al. [2001] | 10 | Open label, single arm | 10 | Troglitazone | 400 | Baseline | <6 months | Improved | NA | Unchanged |
| Neuschwander- Tetri et al. [2003a, 2003b] | 30 | Open label, single arm | 50 | Rosiglitazone | 8 | Baseline | 48 weeks | Improved | Improved (CT) | Improved steatosis, inflammation |
| Promrat et al. [2004] | 18 | Open label, single arm | 0 | Pioglitazone | 30 | Baseline | 48 weeks | Improved (normalized in 95%) (NS) | Improved (MRS) | Improved steatosis, inflammation and fibrosis |
| Sanyal et al. [2004] | 20 | Open label, RCT | 0 | Pioglitazone + vitamin E | 30 | Vitamin E | 6 months | Improved | NA | Improved steatosis and inflammation. Fibrosis improved (NS) |
| Belfort et al. [2006] | 55 | Blinded, RCT | 48 | Pioglitazone | 45 | Placebo | 6 months | Improved | Improved (MRS) | Improved steatosis and inflammation. Fibrosis improved (NS) |
| Aithal et al. [2008] | 74 | Blinded, RCT | 0 | Pioglitazone | 30 | Placebo | 12 months | Improved (NS) | NA | Improved hepatocellular injury and fibrosis; steatosis and inflammation improved (NS) |
| Idilman et al. [2008] | 74 | Open label, RCT | NR | Rosiglitazone | 8 | Metformin or diet + exercise | 48 weeks | NA | Improved steatosis | |
| Ratziu et al. [2008] | 63 | Blinded, RCT | 31 | Rosiglitazone | 8 | Placebo | 12 months | Improved (normalized in 38%) | NA | Improved steatosis, necroinflammation, NAS and fibrosis improved (NS) |
| Sanyal et al. [2010] | 74 | Blinded, RCT | 0 | Pioglitazone | 30 | Placebo | 24 months | Improved | NA | Improved steatosis and inflammation (NS) |
All patients in the study arm were also placed on lifestyle modifications with diet and exercise.
CT, computed tomography; MRS, magnetic resonance spectroscopy; NA, not assessed; NAS, Nonalcoholic fatty liver disease Activity Score; NR, not reported; NS, nonsignificant; RCT, randomized controlled trial.
Pioglitazone
An early pilot study using pioglitazone examined 18 patients with NASH who did not have diabetes who received 30 mg of pioglitazone daily for 48 weeks. The authors reported that pioglitazone normalized serum aminotransferases and led to a significant histological response in the majority of treated patients [Promrat et al. 2004]. Sanyal and colleagues also showed promising results in another pilot trial comparing pioglitazone plus vitamin E with vitamin E alone for 6 months in patients with NASH and without diabetes [Sanyal et al. 2004]. While both groups had a reduction in hepatic steatosis, only the pioglitazone plus vitamin E group had a statistically significant reduction in hepatic inflammation. Although encouraging, these results were tempered by the small sample size and the lack of a control placebo group.
Accumulating pilot data on the efficacy of TZDs in NASH prompted several randomized-controlled trials (RCTs). Belfort and colleagues published the first double-blind, placebo-controlled trial using diet plus 45 mg of pioglitazone per day compared with diet plus placebo for 6 months in 55 patients with glucose intolerance or diabetes and NASH [Belfort et al. 2006]. Compared with those receiving placebo, patients receiving pioglitazone had statistically significant improvements in their serum aminotransferases as well as in all metabolic and histological variables, except for fibrosis (p = 0.08). Normalization of serum aminotransferases correlated with improved hepatic insulin sensitivity and adiponectin levels. Increases in adiponectin seen in this group were inversely related to a reduction in hepatic fat content measured by spectrometry. Aithal and colleagues published a similar trial in 74 patients with biopsy-proven NASH and without diabetes who were randomized to diet plus exercise, and either placebo or 30 mg of pioglitazone per day [Aithal et al. 2008]. A total of 61 patients had post-treatment liver biopsies. Pioglitazone was associated with decreased alanine aminotransferase (ALT) levels, improved insulin sensitivity, and improved histological necroinflammatory markers. Interestingly, fibrosis also improved significantly in the pioglitazone group, a finding that has not yet been replicated in another RCT [Aithal et al. 2008].
The largest trial completed to date on the role of TZDs in patients with biopsy-proven NASH and without diabetes or cirrhosis is the PIVENS (Pioglitazone, Vitamin E, or placebo for Nonalcoholic Steatohepatitis) trial [Sanyal et al. 2010]. A total of 247 patients were randomized to receive either 30 mg of pioglitazone per day, 800 IU of vitamin E per day or placebo for 96 weeks. The primary outcome was an improvement in histological features of NASH as assessed via the use of the NAFLD Activity Score [Kleiner et al. 2005]. Importantly, the primary outcome required an improvement by one or more points in the hepatocellular ballooning score. Primary comparisons were made only between pioglitazone and placebo or vitamin E and placebo. Although the pioglitazone group did not reach the prespecified level of significance for the primary outcome (p < 0.025), pioglitazone use was associated with highly significant reductions in steatosis, inflammation, and hepatocellular ballooning, as well as with improvements in insulin resistance and liver enzymes. Furthermore, a greater proportion of patients receiving pioglitazone (47%) versus placebo (21%) had complete resolution of steatohepatitis on end-of-treatment biopsy (p = 0.001). The failure of the pioglitazone group to meet the primary endpoint could be explained by a disproportionate misclassification of the presence of ballooning in the pioglitazone group compared with placebo or vitamin E. On central pathology review of deeper sections, it was noted that 28% of patients within the pioglitazone group – versus 17% in the placebo group and 18% in the vitamin E group – were misclassified on enrollment as having cellular ballooning, a finding necessary for study eligibility. This misclassification made it more difficult for the group to meet the primary endpoint, which required an improvement in ballooning. Post hoc sensitivity analysis using “no worsening” (rather than improvement) of hepatocellular ballooning was then associated with a near 50% improvement in the pioglitazone group. In addition, when patients who were misclassified initially were removed from the analysis, the pioglitazone group demonstrated greater histological improvement (47%) versus placebo (23%, p = 0.002). Notably, pioglitazone was not associated with a significant improvement in the mean fibrosis score, nor in portal inflammation, which has been linked to advanced disease [Brunt et al. 2009]. Overall, the PIVENS trial offers useful insight into the role of TZDs, specifically pioglitazone, in the treatment of NASH. Pioglitazone treatment improved serum aminotransferases and liver histology compared with placebo. These results must be tempered by the overall small number of patients (34%) who experienced histological improvement in PIVENS, as well as by the potential long-term side effects of TZD use.
Rosiglitazone
In contrast to clinical trials with pioglitazone, results from clinical trials using rosiglitazone have been less encouraging. Neuschwander-Tetri and colleagues published an open-label uncontrolled trial using rosiglitazone 4 mg twice daily in 30 patients with biopsy proven NASH who were overweight for 48 weeks [Neuschwander-Tetri et al. 2003b]. There was significant improvement in ALT, insulin sensitivity, liver steatosis, and inflammation, but no change in hepatic fibrosis. However, a follow-up study showed that ALT levels increased on withdrawal of the drug, and weight gain – a known side effect of the TZDs – continued despite cessation of the drug [Neuschwander-Tetri et al. 2003a, 2002]. The FLIRT (Fatty Liver Improvement with Rosiglitazone Therapy) trial examined rosiglitazone or placebo in 63 patients without dietary intervention. Among histologic outcomes, only steatosis was significantly improved after 1 year of treatment [Ratziu et al. 2008]. This result suggests that dietary modification is needed in addition to pharmacological therapy in order to achieve histological benefit when using rosiglitazone.
In the open-label FLIRT-2, patients completing the FLIRT trial were placed on rosiglitazone for 2 additional years; despite a continued improvement in insulin sensitivity and aminotransferases, rosiglitazone did not further improve liver histology [Ratziu et al. 2010]. These data suggest that long-term therapy with rosiglitazone may be required for sustained histological improvement but offers no additional histological benefit over time.
A recent meta-analysis evaluated the five high-quality RCTs [Sanyal et al. 2010, 2004; Aithal et al. 2008; Ratziu et al. 2008; Belfort et al. 2006] discussed previously (n = 354, four pioglitazone RCTs, one rosiglitazone RCT) and concluded that TZDs improve histological steatosis and inflammation, but not fibrosis [Musso et al. 2010]. The presence of diabetes, implementation of lifestyle intervention, different drug dose, or trial duration did not affect the results. TZDs consistently improved hepatic, muscle, and adipose tissue insulin resistance and reduced plasma glucose and hemoglobin A1c in patients with glucose intolerance. The benefit–safety profile of TZDs, as well as the possible eventual benefit on hepatic fibrosis, warrants further assessment in larger RCTs of longer duration.
Safety of thiazolidendiones in nonalcoholic steatohepatitis
TZDs have been relatively well tolerated in NASH clinical trials. None of these published trials have reported an increased incidence of congestive heart failure, bone fractures, or increased cardiovascular mortality; however, safety data were limited by short trial duration. Moreover, none of the current studies were designed to examine primary outcomes of mortality or morbidity.
Despite a concern for hepatotoxicity raised by an earlier drug of the same class, troglitazone, no cases of severe hepatitis or exacerbation of NASH have been noted in clinical studies of pioglitazone and rosiglitazone. However, there have been a few isolated case reports possibly linking these medications to cholestatic liver injury [May et al. 2002; Al-Salman et al. 2000; Forman et al. 2000]. The most common side effect of TZDs is weight gain (mean 3–5 kg), which occurs in about 60–70% of patients [Musso et al. 2010]. In addition, the weight gain seen with TZD use remains even after discontinuation of the drug [Lutchman et al. 2007]. However, despite this increase in weight the metabolic benefits achieved with prior TZD treatment persist [Lutchman et al. 2007]. This paradox can be largely explained by the important actions of PPAR-γ agonists, such as the TZDs, on adipose tissue. These agents promote adipocyte maturation and decrease macrophage infiltration of adipose tissue, which results in decreased production in proinflammatory cytokines, and increased adiponectin that improves insulin sensitivity (Figure 1). While fat mass does increase, adipose tissue distribution shifts away from the central deposition to a more diffuse distribution in the subcutaneous compartment, resulting in a more favorable metabolic profile [Smith et al. 2005; Carey et al. 2002].
Heart failure is a serious adverse event associated with the TZDs. Both rosiglitazone and pioglitazone have received a black box warning from the United States Food and Drug Administration (FDA) because of increased risk of congestive heart failure. However, there does not appear to be an increase in mortality from heart failure in patients receiving TZDs, and pioglitazone has actually been shown to decrease mortality from ischemic cardiovascular events, the leading cause of death in patients with NASH [Mannucci et al. 2010; Erdmann et al. 2007].
Furthermore, differences between rosiglitazone and pioglitazone, particularly with respect to lipid metabolism, may explain discrepant outcomes in NASH clinical trials and cardiovascular risk. Pioglitazone has favorable effects on the dyslipidemia characteristic of the metabolic syndrome (low HDL, high TG). While rosiglitazone has no effect on TG or HDL levels and even raises small low-density lipoprotein (LDL), pioglitazone increases HDL while lowering TG, LDL and de novo lipogenesis [Beysen et al. 2008; Goldberg et al. 2005]. These differences may in part explain the adverse cardiac effects linked to rosiglitazone [Nissen, 2007; Nissen and Wolski, 2007]. In 2007, rosiglitazone received a black box warning for myocardial ischemia in addition to congestive heart failure.
A few studies have also linked pioglitazone and rosiglitazone to an increased risk of osteoporosis and fractures in men and women with diabetes [Aubert et al. 2010; Home et al. 2009; Meier et al. 2008; Kahn et al. 2006]. Thus, all patients on TZDs need appropriate monitoring for the development of osteoporosis and counseling on risk-reducing behaviors, such as daily weight-bearing exercise and calcium and vitamin D supplementation.
Finally, there have been recent reports of a possible increased incidence of bladder cancer in patients receiving TZDs. These isolated reports must be tempered by other data suggesting that PPAR-γ agonists may have anticancer properties [Takashima et al. 2001; Sato et al. 2000]. In addition, epidemiological studies have shown a 33% reduction in lung cancer risk among patients receiving TZDs compared with patients not taking TZDs after adjusting for confounding interactions (relative risk 0.67; 95% confidence interval 0.51 to 0.87) [Govindarajan et al. 2007]. Therefore, the effect of TZDs on overall cancer risk is largely unknown and the current data are inconclusive.
In summary, while some adverse effects such as heart failure are common to both pioglitazone and rosiglitazone, lipid metabolic effects and risk of myocardial infarction or death, are not. The lipid metabolic effects of pioglitazone are cardioprotective, while those of rosiglitazone are not [Spanheimer et al. 2009; Nissen and Wolski, 2007]. Overall, the safety and tolerability of pioglitazone is predictable, and adverse events are not treatment limiting.
Metformin
Metformin belongs to a class of insulin-sensitizing drugs known as the biguanides. Biguanides improve insulin sensitivity via activation of the AMP-K pathway and hence decrease gluconeogenesis. Furthermore, biguanides decrease intestinal glucose absorption and increase insulin mediated glucose uptake in skeletal muscle [Zhou et al. 2001] (Figure 2). Metformin has been recognized since the 1950s, but it did not receive approval by the United States Food and Drug Administration (US FDA) for type 2 diabetes until 1994. Metformin is generally well tolerated, and its role inducing weight loss has been postulated to be beneficial in patients with NASH [Loomba et al. 2009]. However, metformin is contraindicated for use in patients with renal insufficiency or with congestive heart failure because of the risk of lactic acidosis.
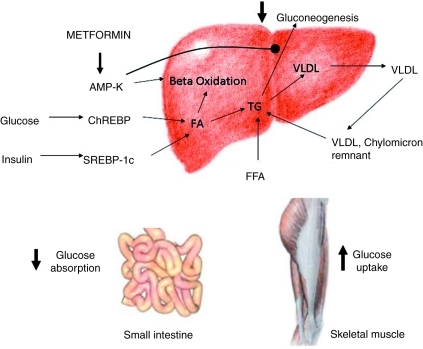
Figure 2.
Mechanisms of action of metformin that promote increased insulin sensitivity. Metformin's primary effect on hepatic lipid metabolism is via 5’adenosine monophosphate-activated protein kinase (AMP-K), resulting in increased beta oxidation and a reduction in hepatic gluconeogenesis. Other effects such as decreased glucose absorption from the gut and increased glucose uptake by skeletal muscle indirectly reduce hyperinsulinemia. ChREPB, carbohydrate responsive element binding protein; FA, fatty acid; FFA, free fatty acid; SREBP-1c, sterol regulatory element binding protein-1c; TG, triglyceride; VLDL, very-low-density lipoprotein.
Several studies have tested the effect of metformin on NASH [Loomba et al. 2009; de Oliveira et al. 2008; Idilman et al. 2008; Uygun et al. 2004; Marchesini et al. 2001] (Table 2). However, only four high-quality RCTs (n = 115) have been conducted. These studies had an open-label design and were heterogeneous in patient population, duration, and design [Haukeland et al. 2009; Shields et al. 2009; Idilman et al. 2008; Uygun et al. 2004]. A recent meta-analysis, which included published data from these four trials, concluded that 6–12 months of metformin plus lifestyle intervention did not improve liver histology or aminotransferases compared with lifestyle intervention alone, independently of dose, treatment duration, or diabetic state [Musso et al. 2010].
Table 2.
Studies of metformin in adult patients with biopsy-proven nonalcoholic steatohepatitis.
| Study | n | Design | % Diabetes mellitus | Drug* | Daily dose (g) | Compared with | Duration | Liver enzymes | Liver fat by imaging | Histology |
|---|---|---|---|---|---|---|---|---|---|---|
| Marchesini et al. [2001] | 20 | Open label, single arm | 0 | Metformin | 1.5 | Baseline | 4 months | Improved | Decreased hepatomegaly (US) | Not assessed |
| Uygun et al. [2004] | 36 | Open label, RCT | 0 | Metformin | 1.5 | Diet and exercise | 6 months | Improved | Improved (US) | Improved necroinflammation (NS) |
| de Oliveira et al. [2008] | 20 | Open label, single arm | 80 | Metformin + NAC | 0.85–1 | Baseline | 12 months | Improved | NA | Improved steatosis, fibrosis and NAS |
| Idilman et al. [2008] | 74 | Open label, RCT | NR | Metformin | 1.7 | Rosiglitazone or diet and exercise | 48 weeks | Improved (NS) | NA | Improved steatosis |
| Loomba et al. [2009] | 28 | Open label, single arm | 29 | Metformin | 2 | Baseline | 12 months | Improved | NA | Improved steatosis, inflammation and NAS |
| Haukeland et al. [2009] | 48 | Open label, RCT | 27 (48% IGT) | Metformin | 2.5–3 | Diet and exercise | 6 months | Improved (NS) | Unchanged | Unchanged |
| Shields et al. [2009] | 19 | Open label, RCT | 0 | Metformin, long acting | 0.5–1 | Diet and exercise | 12 months | Improved (NS) | NA | Improved (NS) |
All patients in the study arm were also placed on lifestyle modifications with diet and exercise.
IGT, impaired glucose tolerance; NA, not assessed; NAC, n-acetylcysteine; NAS, Nonalcoholic fatty liver disease Activity Score; NR, not reported; NS, nonsignificant; RCT, randomized controlled trial; US, ultrasound.
Two RCTs (n = 144) evaluated the effect of metformin on radiological and biochemical indices of NAFLD alone [Nar and Gedik, 2009; Bugianesi et al. 2005] Bugianesi and colleagues showed that metformin normalized aminotransferases in 69% versus 31% in the dietary therapy group (p = 0.0003) [Bugianesi et al. 2005]. However, in the trial by Nar and Gedik, biochemical and radiological improvement was nonsignificant compared with diet plus exercise [Nar and Gedik, 2009]. Overall, when added to lifestyle intervention, metformin enhanced weight loss (mean weight loss 4.3–7.9%) and improved insulin sensitivity and plasma glucose levels. In addition, when ‘responders’ were compared with ‘nonresponders’ it was notable that all responders had lost weight, suggesting that the benefit derived in these patients could have been a result of weight loss rather than the metabolic effects of metformin [Bugianesi et al. 2005]. In addition, Loomba and colleagues demonstrated a similar effect in patients with NASH, showing a strong association between weight loss and improvements in NASH activity index and ALT levels (both p < 0.01) [Loomba et al. 2009]. Despite its weight loss-promoting and insulin-sensitizing properties, metformin showed no significant differences in liver histology.
Although there is no definitive RCT in adults comparing metformin with placebo for the treatment of NASH, such a trial was recently completed in the pediatric population. The Treatment of NAFLD in Children (TONIC) trial, sponsored by the NASH CRN, is the largest RCT investigating the effects of metformin in a NASH population, and was recently presented in abstract form [Lavine et al. 2010a]. The study randomized 173 children aged 8–17 years with biopsy-confirmed NAFLD and elevated serum ALT to 9 weeks of treatment with metformin, vitamin E or placebo [Lavine et al. 2010b]. Compared with placebo, neither vitamin E (p = 0.26) nor metformin (p = 0.83) was associated with a sustained reduction in serum ALT or an improvement in histology. Results of this trial suggest that metformin is not beneficial in the treatment of either NAFLD or NASH, although the generalizability of this study to an adult population is limited.
An important study delineated essential differences between rosiglitazone and metformin that gives insight into why metformin has not demonstrated convincing efficacy in the treatment of NASH [Tiikkainen et al. 2004]. While metformin reduced insulin resistance compared with rosiglitazone, it did not reduce hepatic fat content or increase adiponectin levels. Adiponectin has been shown to be inversely related to the severity of insulin resistance and NASH severity; lower adiponectin levels may be independently associated with NASH and correlate with more advanced NASH [Hui et al. 2004; Targher et al. 2004]. Importantly, adiponectin counteracts the effects of TNF-α, which may have a role in the development of liver injury in patients with NASH [Hui et al. 2004]. In addition, an open-label RCT (n = 64) compared the combination of rosiglitazone plus metformin with each agent alone in patients with NASH [Omer et al. 2010]. After 1 year, steatosis and necroinflammation significantly improved with rosiglitazone, but not with metformin. The combination of both drugs enhanced weight loss over rosiglitazone alone, but conferred no additional benefit to liver histology and glucose metabolism. Given the disparate effects on NASH between metformin and the TZDs it is apparent that improving insulin sensitivity alone is not sufficient to reverse the histological features of NASH. Taken together, and considering the mechanism of action of metformin, the results from several clinical trials and the results of the well-designed TONIC trial suggest that metformin does not appear to be an effective pharmacological treatment for NASH independent of its effect on body weight.
Other insulin-sensitizing drugs
Glucagon-like peptide 1 agonists
Other insulin-sensitizing agents that show promise in the treatment of NASH are under investigation or are in the early phases of clinical development for this indication. Of these, exenatide is the best studied and is currently FDA approved for use as an injectable adjunctive therapy in patients with type 2 diabetes. Exenatide is a synthetic analog of exendin-4, a 39-amino-acid agonist of the glucagon-like peptide 1 (GLP-1) receptor. Exendin-4 is present in the saliva of the Gila monster, Heloderma suspectum, and shares 53% sequence identity with GLP-1 [Eng et al. 1992]. GLP-1, a gastrointestinal hormone secreted by the L cells of the intestine, regulates blood glucose primarily via stimulation of glucose-dependent insulin release. Therefore, exenatide does not function as a direct insulin sensitizer, but rather induces clinically significant weight loss, which may lead to an insulin-sensitizing effect. In a murine model of fatty liver, administration of exendin-4 significantly reduced glucose levels, improved insulin sensitivity, and reduced hepatic steatosis [Ding et al. 2006]. In an open-label, uncontrolled clinical trial using exenatide to assess drug safety in patients with diabetes, patients were noted to have had improved aspartate aminotransferase (AST) and insulin sensitivity over the 3.5-year follow-up period [Klonoff et al. 2008]. In addition, those with elevated ALT at baseline (n = 116) had significant reductions in ALT, and 41% normalized ALT levels with treatment, independent of weight loss. A recent case report of a male patient with diabetes and NAFLD treated for 44 weeks with exenatide showed improvement in serum aminotransferases and hepatic steatosis [Tushuizen et al. 2006]. Finally, an open-label, prospective case series measuring the effect of exenatide on the hepatic histology of eight patients with type 2 diabetes and biopsy-proven NAFLD demonstrated improvement in serum aminotransferases, although no significant improvement in histopathology was demonstrated [Kenny et al. 2010]. Several clinical trials are currently underway that could provide more insight into the potential benefits of exenatide for the treatment of NASH (ClinicalTrials.gov, identifiers: NCT01208649, NCT00650546, NCT00529204).
Dipeptidyl peptidase IV inhibitors
Although the potential benefits of dipeptidyl peptidase IV (DPP-IV) inhibitors on NASH are preliminary, they are interesting and demonstrate a mechanism of action that could be beneficial in patients with NASH. Like the GLP-1 agonists, they act via the incretin pathway. DPP-IV is a ubiquitous enzyme expressed on the surface of most cell types that deactivates a variety of other bioactive peptides, including GLP-1. Therefore, its inhibition could potentially affect glucose regulation through multiple effects. These inhibitors, unlike other GLP-1-based therapies, can be administered orally, making them attractive candidates for NASH treatment. In addition, these drugs are cleared via the kidney and have very little hepatic metabolism, making them safe for use in patients with decreased liver function [Migoya et al. 2009]. Currently sitagliptin and saxagliptin are the only DPP-IV inhibitors available for the treatment of type 2 diabetes in the United States. In animal studies, sitagliptin has been shown to attenuate the effects of metformin, via phosphorylation of the AMP-K pathway [Choi et al. 2010]. In addition, in fructose-fed rats who develop insulin resistance and the metabolic syndrome, administration of sitagliptin decreased liver steatosis, decreased β-cell apoptosis and insulin resistance [Maiztegui et al. 2011]. There are currently no clinical studies in humans examining the effect of sitagliptin or other DDP-IV inhibitors on NASH, but results in animal studies suggest these may be promising drugs.
Summary and future directions
Recent RCTs in NASH have advanced the field and offer potential therapeutic options [Sanyal et al. 2010]. However, even the most promising of these agents is associated with significant adverse effects and none is FDA approved for NASH therapy. Long-term data are needed to better understand the risks and assess if further benefit can be achieved with extended therapy. A major limitation of the current data is that only a fraction of patients respond to therapy. Therefore, studies are needed to identify predictors of response to highlight which patients may benefit from intensive lifestyle changes or the use of specific insulin-sensitizing agents. Studies of longer duration will help to assess long-term safety, durability, and benefits of insulin-sensitizing agents on patient-oriented outcomes, including liver-related (e.g. cirrhosis), cardiovascular, and metabolic morbidity, all of which contribute to the disease burden of NASH.
The importance of lifestyle modifications with weight loss and exercise cannot be overstated, and should be part of the management of all patients with NASH, however such modifications can be difficult to sustain and in isolation may not be sufficient. If considering the use of an insulin-sensitizing agent, the available data support the use of pioglitazone in patients with biopsy-confirmed NASH, particularly those with advanced NASH, who are at the highest risk of liver-related morbidity and mortality. Careful patient selection is crucial, as is an understanding of the potential adverse effects associated with the use of pioglitazone. Side effects must be carefully explained to patients and the risks and benefits weighed prior to the initiation of any therapy. Although pioglitazone is the best studied and most effective insulin-sensitizing drug for the treatment of NASH to date, it is worth noting that only a subset of patients had a biochemical and histological improvement suggesting that while improving insulin resistance is beneficial in patients with NASH, pioglitazone may not be sufficient definitive therapy. NASH, like other complex metabolic diseases, will likely necessitate a multifaceted approach. Trials exploring the potential additive effects of insulin sensitizers with cytoprotective agents or other modalities are eagerly awaited.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest statement
None declared.
References
- Adams L.A., Lymp J.F., St Sauver J., Sanderson S.O., Lindor K.D., Feldstein A., et al. (2005a) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121 [DOI] [PubMed] [Google Scholar]
- Adams L.A., Sanderson S., Lindor K.D., Angulo P. (2005b) The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 42: 132–138 [DOI] [PubMed] [Google Scholar]
- Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I., et al. (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135(4): 1176–1184 [DOI] [PubMed] [Google Scholar]
- Al-Salman J., Arjomand H., Kemp D.G., Mittal M. (2000) Hepatocellular injury in a patient receiving rosiglitazone. A case report. Ann Intern Med 132: 121–124 [DOI] [PubMed] [Google Scholar]
- Angulo P., Lindor K.D. (2002) Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17(Suppl.): S186–S190 [DOI] [PubMed] [Google Scholar]
- Aubert R.E., Herrera V., Chen W., Haffner S.M., Pendergrass M. (2010) Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab 12: 716–721 [DOI] [PubMed] [Google Scholar]
- Bajaj M., Suraamornkul S., Piper P., Hardies L.J., Glass L., Cersosimo E., et al. (2004) Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab 89: 200–206 [DOI] [PubMed] [Google Scholar]
- Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J., et al. (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307 [DOI] [PubMed] [Google Scholar]
- Beysen C., Murphy E.J., Nagaraja H., Decaris M., Riiff T., Fong A., et al. (2008) A pilot study of the effects of pioglitazone and rosiglitazone on de novo lipogenesis in type 2 diabetes. J Lipid Res 49: 2657–2663 [DOI] [PubMed] [Google Scholar]
- Bloom S.R., Kuhajda F.P., Laher I., Pi-Sunyer X., Ronnett G.V., Tan T.M., et al. (2008) The obesity epidemic: pharmacological challenges. Mol Interv 8: 82–98 [DOI] [PubMed] [Google Scholar]
- Brunt E.M., Kleiner D.E., Wilson L.A., Unalp A., Behling C.E., Lavine J.E., et al. (2009) Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): A histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 49(3): 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugianesi E., Gentilcore E., Manini R., Natale S., Vanni E., Villanova N., et al. (2005) A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 100: 1082–1090 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Marchesini G., Gentilcore E., Cua I.H., Vanni E., Rizzetto M., et al. (2006) Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology 44: 1648–1655 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Moscatiello S., Ciaravella M.F., Marchesini G. (2010) Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des 16: 1941–1951 [DOI] [PubMed] [Google Scholar]
- Caldwell S., Argo C. (2010) The natural history of non-alcoholic fatty liver disease. Dig Dis 28: 162–168 [DOI] [PubMed] [Google Scholar]
- Caldwell S.H., Hespenheide E.E., Redick J.A., Iezzoni J.C., Battle E.H., Sheppard B.L. (2001) A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol 96: 519–525 [DOI] [PubMed] [Google Scholar]
- Carey D.G., Cowin G.J., Galloway G.J., Jones N.P., Richards J.C., Biswas N., et al. (2002) Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected]. Obes Res 10: 1008–1015 [DOI] [PubMed] [Google Scholar]
- Chitturi S., Abeygunasekera S., Farrell G.C., Holmes-Walker J., Hui J.M., Fung C., et al. (2002) NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 35: 373–379 [DOI] [PubMed] [Google Scholar]
- Choi M.K., Jin Q.R., Ahn S.H., Bae M.A., Song I.S. (2010) Sitagliptin attenuates metformin-mediated AMPK phosphorylation through inhibition of organic cation transporters. Xenobiotica 40(12): 817–825 [DOI] [PubMed] [Google Scholar]
- Clark J.M. (2006) Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol 40(Suppl. 1): S39–S43 [DOI] [PubMed] [Google Scholar]
- Coletta D.K., Sriwijitkamol A., Wajcberg E., Tantiwong P., Li M., Prentki M., et al. (2009) Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 52: 723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira C.P., Stefano J.T., de Siqueira E.R., Silva L.S., de Campos Mazo D.F., Lima V.M., et al. (2008) Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res 38: 159–165 [DOI] [PubMed] [Google Scholar]
- Ding X., Saxena N.K., Lin S., Gupta N.A., Anania F.A. (2006) Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt M., Franzen L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G., et al. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873 [DOI] [PubMed] [Google Scholar]
- Eng J., Kleinman W.A., Singh L., Singh G., Raufman J.P. (1992) Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 267: 7402–7405 [PubMed] [Google Scholar]
- Erdmann E., Charbonnel B., Wilcox R.G., Skene A.M., Massi-Benedetti M., Yates J., et al. (2007) Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: Data from the PROactive study (PROactive 08). Diabetes Care 30: 2773–2778 [DOI] [PubMed] [Google Scholar]
- Forman L.M., Simmons D.A., Diamond R.H. (2000) Hepatic failure in a patient taking rosiglitazone. Ann Intern Med 132: 118–121 [DOI] [PubMed] [Google Scholar]
- Fracanzani A.L., Valenti L., Bugianesi E., Andreoletti M., Colli A., Vanni E., et al. (2008) Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48: 792–798 [DOI] [PubMed] [Google Scholar]
- Gerstein H.C., Yusuf S., Bosch J., Pogue J., Sheridan P., Dinccag N., et al. (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 368: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Goldberg R.B., Kendall D.M., Deeg M.A., Buse J.B., Zagar A.J., Pinaire J.A., et al. (2005) A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28: 1547–1554 [DOI] [PubMed] [Google Scholar]
- Govindarajan R., Ratnasinghe L., Simmons D.L., Siegel E.R., Midathada M.V., Kim L., et al. (2007) Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 25: 1476–1481 [DOI] [PubMed] [Google Scholar]
- Haukeland J.W., Konopski Z., Eggesbo H.B., von Volkmann H.L., Raschpichler G., Bjoro K., et al. (2009) Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol 44: 853–860 [DOI] [PubMed] [Google Scholar]
- Home P.D., Pocock S.J., Beck-Nielsen H., Curtis P.S., Gomis R., Hanefeld M., et al. (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet 373: 2125–2135 [DOI] [PubMed] [Google Scholar]
- Hui J.M., Hodge A., Farrell G.C., Kench J.G., Kriketos A., George J. (2004) Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 40: 46–54 [DOI] [PubMed] [Google Scholar]
- Idilman R., Mizrak D., Corapcioglu D., Bektas M., Doganay B., Sayki M., et al. (2008) Clinical trial: Insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 28: 200–208 [DOI] [PubMed] [Google Scholar]
- Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P., et al. (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443 [DOI] [PubMed] [Google Scholar]
- Kenny P.R., Brady D.E., Torres D.M., Ragozzino L., Chalasani N., Harrison S.A. (2010) Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: A case series. Am J Gastroenterol 105: 2707–2709 [DOI] [PubMed] [Google Scholar]
- Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321 [DOI] [PubMed] [Google Scholar]
- Klonoff D.C., Buse J.B., Nielsen L.L., Guan X., Bowlus C.L., Holcombe J.H., et al. (2008) Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 24: 275–286 [DOI] [PubMed] [Google Scholar]
- Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotronen A., Westerbacka J., Bergholm R., Pietilainen K.H., Yki-Jarvinen H. (2007) Liver fat in the metabolic syndrome. J Clin Endocrinol Metab 92: 3490–3497 [DOI] [PubMed] [Google Scholar]
- Lavine, J.E., Schwimmer, J.B., Molleston, J., Chalasani, N., Rosenthal, P., Murray, K.F. et al. (2010a) Vitamin E, metformin or placebo for treatment of nonalcoholic fatty liver disease in children. In: Proceedings of the The Liver Meeting, Vol. 52, Boston, MA, Hepatology.
- Lavine J.E., Schwimmer J.B., Molleston J.P., Scheimann A.O., Murray K.F., Abrams S.H., et al. (2010b) Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials 31: 62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff A.M., Wolski K., Nicholls S.J., Nissen S.E. (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA 298: 1180–1188 [DOI] [PubMed] [Google Scholar]
- Loomba R., Lutchman G., Kleiner D.E., Ricks M., Feld J.J., Borg B.B., et al. (2009) Clinical trial: Pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 29: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutchman G., Modi A., Kleiner D.E., Promrat K., Heller T., Ghany M., et al. (2007) The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology 46: 424–429 [DOI] [PubMed] [Google Scholar]
- Lutchman G., Promrat K., Kleiner D.E., Heller T., Ghany M.G., Yanovski J.A., et al. (2006) Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: Relationship to histological improvement. Clin Gastroenterol Hepatol 4: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Maiztegui B., Borelli M.I., Madrid V.G., Del Zotto H., Raschia M.A., Francini F., et al. (2011) Sitagliptin prevents the development of metabolic and hormonal disturbances, increased beta-cell apoptosis and liver steatosis induced by a fructose-rich diet in normal rats. Clin Sci (Lond) 120: 73–80 [DOI] [PubMed] [Google Scholar]
- Mannucci E., Monami M., Di Bari M., Lamanna C., Gori F., Gensini G.F., et al. (2010) Cardiac safety profile of rosiglitazone: A comprehensive meta-analysis of randomized clinical trials. Int J Cardiol 143: 135–140 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Zoli M., Melchionda N. (2001) Metformin in non-alcoholic steatohepatitis. Lancet 358: 893–894 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Brizi M., Morselli-Labate A.M., Bianchi G., Bugianesi E., McCullough A.J., et al. (1999) Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 107: 450–455 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., et al. (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–923 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Marzocchi R. (2007) Metabolic syndrome and NASH. Clin Liver Dis 11(1): 105–117, ix [DOI] [PubMed] [Google Scholar]
- May L.D., Lefkowitch J.H., Kram M.T., Rubin D.E. (2002) Mixed hepatocellular-cholestatic liver injury after pioglitazone therapy. Ann Intern Med 136: 449–452 [DOI] [PubMed] [Google Scholar]
- Meier C., Kraenzlin M.E., Bodmer M., Jick S.S., Jick H., Meier C.R. (2008) Use of thiazolidinediones and fracture risk. Arch Intern Med 168: 820–825 [DOI] [PubMed] [Google Scholar]
- Migoya E.M., Stevens C.H., Bergman A.J., Luo W.L., Lasseter K.C., Dilzer S.C., et al. (2009) Effect of moderate hepatic insufficiency on the pharmacokinetics of sitagliptin. Can J Clin Pharmacol 16: e165–e170 [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M., Pagano G. (2010) A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 52: 79–104 [DOI] [PubMed] [Google Scholar]
- Nar A., Gedik O. (2009) The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol 46: 113–118 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A., Brunt E.M., Bacon B.R. (2002) Histological improvement in NASH following reduction in insulin resistance with 48 week treatment with the PPAR-gamma agonist rosiglitizone. Hepatology 36: 379A–379A [Google Scholar]
- Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Oliver D., Bacon B.R. (2003a) Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38: 1008–1017 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Sponseller C.A., Hampton K., Bacon B.R. (2003b) Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non-alcoholic steatohepatitis. J Hepatol 38: 434–440 [DOI] [PubMed] [Google Scholar]
- Nissen, S.E. (2007) Perspective: effect of rosiglitazone on cardiovascular outcomes. Curr Cardiol Rep 9(5): 343–344, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17877927. [DOI] [PubMed]
- Nissen S.E., Wolski K. (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471 [DOI] [PubMed] [Google Scholar]
- Omer Z., Cetinkalp S., Akyildiz M., Yilmaz F., Batur Y., Yilmaz C., et al. (2010) Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 22: 18–23 [DOI] [PubMed] [Google Scholar]
- Pagano G., Pacini G., Musso G., Gambino R., Mecca F., Depetris N., et al. (2002) Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology 35: 367–372 [DOI] [PubMed] [Google Scholar]
- Pillai A.A., Rinella M.E. (2009) Non-alcoholic fatty liver disease: Is bariatric surgery the answer? Clin Liver Dis 13: 689–710 [DOI] [PubMed] [Google Scholar]
- Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., et al. (2010) Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51: 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promrat K., Lutchman G., Uwaifo G.I., Freedman R.J., Soza A., Heller T., et al. (2004) A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 39: 188–196 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Charlotte F., Bernhardt C., Giral P., Halbron M., Lenaour G., et al. (2010) Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: Results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51: 445–453 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L., et al. (2008) Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135: 100–110 [DOI] [PubMed] [Google Scholar]
- Ruhl C.E., Everhart J.E. (2003) Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 124: 71–79 [DOI] [PubMed] [Google Scholar]
- Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., et al. (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A.J., Mofrad P.S., Contos M.J., Sargeant C., Luketic V.A., Sterling R.K., et al. (2004) A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2: 1107–1115 [DOI] [PubMed] [Google Scholar]
- Sass D.A., Chang P., Chopra K.B. (2005) Nonalcoholic fatty liver disease: A clinical review. Dig Dis Sci 50: 171–180 [DOI] [PubMed] [Google Scholar]
- Sato H., Ishihara S., Kawashima K., Moriyama N., Suetsugu H., Kazumori H., et al. (2000) Expression of peroxisome proliferator-activated receptor (PPAR)gamma in gastric cancer and inhibitory effects of PPARgamma agonists. Br J Cancer 83: 1394–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields W.W., Thompson K.E., Grice G.A., Harrison S.A., Coyle W.J. (2009) The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Ther Adv Gastroenterol 2: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler J., Galle P.R. (2006) Treatment of nonalcoholic fatty liver disease. World J Gastroenterol 12: 2161–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.R., De Jonge L., Volaufova J., Li Y., Xie H., Bray G.A. (2005) Effect of pioglitazone on body composition and energy expenditure: A randomized controlled trial. Metabolism 54: 24–32 [DOI] [PubMed] [Google Scholar]
- Spanheimer R., Betteridge D.J., Tan M.H., Ferrannini E., Charbonnel B. (2009) Long-term lipid effects of pioglitazone by baseline anti-hyperglycemia medication therapy and statin use from the PROactive experience (PROactive 14). Am J Cardiol 104: 234–239 [DOI] [PubMed] [Google Scholar]
- Sturm R. (2003) Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med 163: 2146–2148 [DOI] [PubMed] [Google Scholar]
- Takashima T., Fujiwara Y., Higuchi K., Arakawa T., Yano Y., Hasuma T., et al. (2001) PPAR-gamma ligands inhibit growth of human esophageal adenocarcinoma cells through induction of apoptosis, cell cycle arrest and reduction of ornithine decarboxylase activity. Int J Oncol 19: 465–471 [PubMed] [Google Scholar]
- Targher G., Bertolini L., Scala L., Poli F., Zenari L., Falezza G. (2004) Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clin Endocrinol (Oxf) 61: 700–703 [DOI] [PubMed] [Google Scholar]
- Tiikkainen M., Hakkinen A.M., Korsheninnikova E., Nyman T., Makimattila S., Yki-Jarvinen H. (2004) Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176 [DOI] [PubMed] [Google Scholar]
- Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P., et al. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350 [DOI] [PubMed] [Google Scholar]
- Tushuizen M.E., Bunck M.C., Pouwels P.J., van Waesberghe J.H., Diamant M., Heine R.J. (2006) Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int 26: 1015–1017 [DOI] [PubMed] [Google Scholar]
- Uygun A., Kadayifci A., Isik A.T., Ozgurtas T., Deveci S., Tuzun A., et al. (2004) Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 19: 537–544 [DOI] [PubMed] [Google Scholar]
- Xiang A.H., Peters R.K., Kjos S.L., Marroquin A., Goico J., Ochoa C., et al. (2006) Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 55: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H.J., Mac M.F., Rappaport H., Alpert L.K. (1950) Studies on the liver in diabetes mellitus. II. The significance of fatty metamorphosis and its correlation with insulin sensitivity. J Lab Clin Med 36: 922–928 [PubMed] [Google Scholar]