Abstract
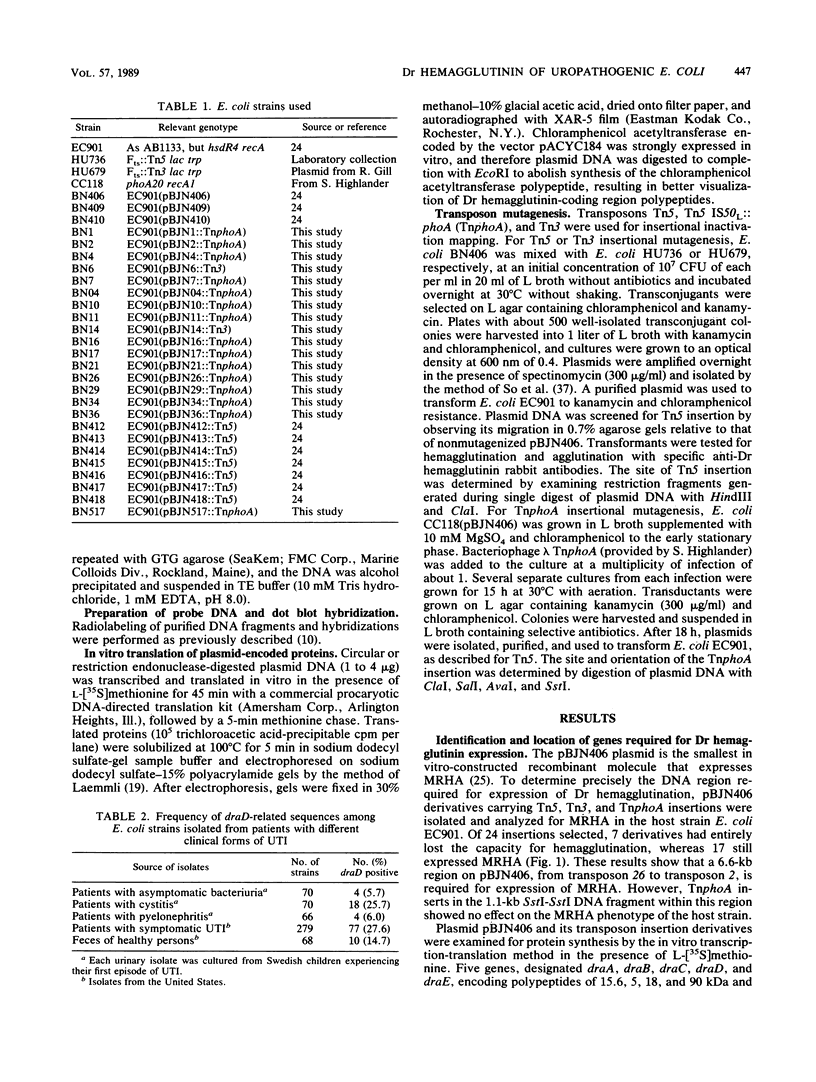
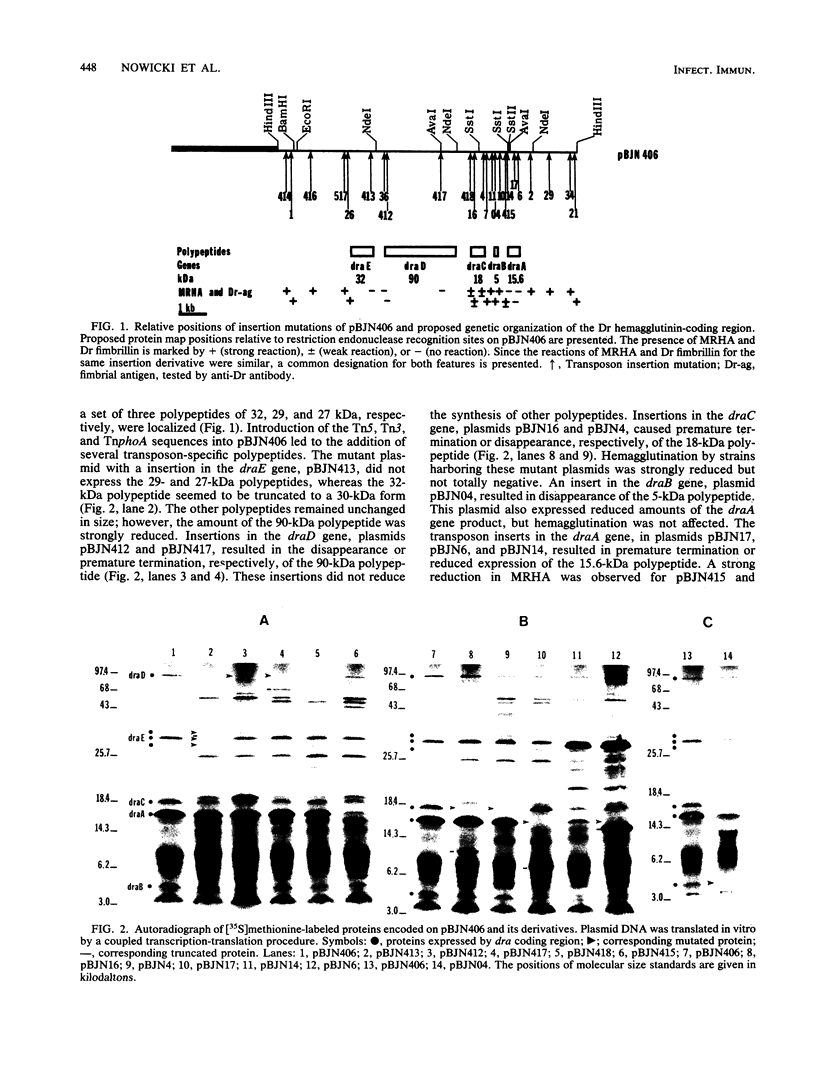
The genetic organization and epidemiology of Dr hemagglutinin was studied. Plasmids derived from pBJN406 and carrying transposon inserts were analyzed for their abilities to confer the mannose-resistant hemagglutination phenotype and expression of plasmid-encoded proteins. The 6.6-kilobase DNA fragment expressed five polypeptides with molecular masses of 15.5, 5, 18, 90, and 32 kilodaltons encoded by the draA, draB, draC, draD, and draE genes, respectively. Four genes, draA, draC, draD, and draE, were required for full mannose-resistant hemagglutination expression. Mutation in the draA gene, previously identified as encoding fimbrillin, resulted in loss of the adherence phenotype. We screened 658 strains isolated from patients with urinary tract infections (UTI) or from fecal samples for the presence of DNA sequences homologous to the draD gene. A significantly higher frequency of draD-related sequences was found among Escherichia coli strains from patients with cystitis than among strains from patients with other clinical forms of UTI. Association of draD-related sequences with O75 and other serotypes was observed. A possible role of Dr hemagglutinin as a virulence factor in lower UTI is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Hacker J., Juarez A., Hughes C., Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Norgren M., Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987 Apr 24;49(2):241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982 Nov;38(2):739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Pierce J. K. Organization of genes responsible for the production of mannose-resistant fimbriae of a uropathogenic Escherichia coli isolate. Infect Immun. 1983 Dec;42(3):900–906. doi: 10.1128/iai.42.3.900-906.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Mooi F. R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull S. I., Bieler S., Hull R. A. Restriction fragment length polymorphism and multiple copies of DNA sequences homologous with probes for P-fimbriae and hemolysin genes among uropathogenic Escherichia coli. Can J Microbiol. 1988 Mar;34(3):307–311. doi: 10.1139/m88-056. [DOI] [PubMed] [Google Scholar]
- Hull S., Clegg S., Sanborg Eden C., Hull R. Multiple forms of genes in pyelonephritogenic Escherichia coli encoding adhesins binding globoseries glycolipid receptors. Infect Immun. 1985 Jan;47(1):80–83. doi: 10.1128/iai.47.1.80-83.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Jarchau T., Goebel W. Large, unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coli O6 strain 536. J Bacteriol. 1986 Oct;168(1):22–30. doi: 10.1128/jb.168.1.22-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Then I., Müller D., Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect Immun. 1988 Mar;56(3):640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberg H., Hellström M., Jodal U., Leffler H., Lincoln K., Svanborg Edén C. Virulence-associated traits in Escherichia coli causing first and recurrent episodes of urinary tract infection in children with or without vesicoureteral reflux. J Infect Dis. 1984 Oct;150(4):561–569. doi: 10.1093/infdis/150.4.561. [DOI] [PubMed] [Google Scholar]
- Low D., David V., Lark D., Schoolnik G., Falkow S. Gene clusters governing the production of hemolysin and mannose-resistant hemagglutination are closely linked in Escherichia coli serotype O4 and O6 isolates from urinary tract infections. Infect Immun. 1984 Jan;43(1):353–358. doi: 10.1128/iai.43.1.353-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Barrish J. P., Korhonen T., Hull R. A., Hull S. I. Molecular cloning of the Escherichia coli O75X adhesin. Infect Immun. 1987 Dec;55(12):3168–3173. doi: 10.1128/iai.55.12.3168-3173.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Holthöfer H., Saraneva T., Rhen M., Väisänen-Rhen V., Korhonen T. K. Location of adhesion sites for P-fimbriated and for 075X-positive Escherichia coli in the human kidney. Microb Pathog. 1986 Apr;1(2):169–180. doi: 10.1016/0882-4010(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Moulds J., Hull R., Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988 May;56(5):1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984 Nov;160(2):691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Organization of fimbriate cells in colonies of Escherichia coli strain 3040. J Gen Microbiol. 1985 May;131(5):1263–1266. doi: 10.1099/00221287-131-5-1263. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Truong L., Moulds J., Hull R. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am J Pathol. 1988 Oct;133(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Vuopio-Varkila J., Viljanen P., Korhonen T. K., Mäkelä P. H. Fimbrial phase variation and systemic E. coli infection studied in the mouse peritonitis model. Microb Pathog. 1986 Aug;1(4):335–347. doi: 10.1016/0882-4010(86)90066-5. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Klemm P., Korhonen T. K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-D-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K. M., Nowicki B., Leinonen M. Role of type 1 and S fimbriae in the pathogenesis of Escherichia coli O18:K1 bacteremia and meningitis in the infant rat. Infect Immun. 1988 Apr;56(4):892–897. doi: 10.1128/iai.56.4.892-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Dallas W. S., Falkow S. Characterization of an Escherichia coli plasmid encoding for synthesis of heat-labile toxin: molecular cloning of the toxin determinant. Infect Immun. 1978 Aug;21(2):405–411. doi: 10.1128/iai.21.2.405-411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984 Nov;46(2):401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Hull R., Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983 Oct;42(1):178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Krenn B. E., Klaasen P. Organization and expression of genes involved in the biosynthesis of K99 fimbriae. Infect Immun. 1984 Feb;43(2):508–514. doi: 10.1128/iai.43.2.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I., van Megen I., Hoekstra W., Bergmans H. Molecular organisation of the genes involved in the production of F7(2) fimbriae, causing mannose-resistant haemagglutination, of a uropathogenic Escherichia coli 06:K2:H1:F7 strain. Mol Gen Genet. 1984;194(3):528–533. doi: 10.1007/BF00425570. [DOI] [PubMed] [Google Scholar]




