Abstract
Paraneoplastic neurological syndromes (PNSs) cover a wide range of diseases and involve both the central nervous system (CNS) and peripheral nervous system. Paraneoplastic encephalitis comprises several diseases such as paraneoplastic cerebellar degeneration (PCD), limbic encephalitis (LE), paraneoplastic encephalomyelitis (PEM), brainstem encephalitis, opsomyoclonus syndrome, in addition to other even less frequently occurring entities. LE was the first historically identified CNS PNS, and similarities between other temporal lobe diseases such as herpes encephalitis have been elucidated. In the past few decades several autoantibodies have been described in association with LE. These encompass the classical ‘onconeuronal’ antibodies (abs) such as Hu, Yo, Ri and others, and now additionally abs towards either ion channels or surface antigens. The clinical core findings in LE are various mental changes such as amnesia or confusion, often associated with seizures. Careful characterization of psychiatric manifestations and/or associated neurological signs can help to characterize the syndrome and type of ab. The treatment options in LE depend on the aetiology. In LE caused by onconeuronal abs, the treatment options are poor. In two types of abs associated with LE, abs against ion channels and surface antigens (e.g. NMDA), immunomodulatory treatments seem effective, making these types of LE treatable conditions. However, LE can also occur without being associated with cancer, in which case only immunomodulation is required. Despite effective treatments, some patients’ residual deficits remain, and recurrences have also been described.
Keywords: cancer, ion channel antibodies, limbic encephalitis, NMDA, onconeuronal antibodies, paraneoplastic disease, paraneoplastic encephalitis, surface antibodies
Introduction
Limbic encephalitis (LE), as first described by Corsellis [Corsellis et al. 1968], is characterized by an acute or subacute onset, memory loss, psychiatric features and often seizures. Psychopathologically it resembles Wernicke Korsakow’s syndrome, or herpes encephalitis. The descriptions of many clinical aspects of LE have been extrapolated from herpes encephalitis and several distinctive features exist [Gable et al. 2009]. The neuropathology is located predominately in the mesial temporal lobes and other parts of the limbic system, which can now be visualized with new MRI techniques. The neuropathological features are inflammation of the brain parenchyma and sometimes also the cortex with oedema [Ulrich et al. 1967]. Variants with pure sclerosis of Ammon’s horn have also been described [Shinohara et al. 2005].
The classical concept of LE has recently undergone a major revision. First it was understood that many cases of LE were not paraneoplastic and second that patients with LE may have clinical symptoms indicating the involvement of brain regions other than the limbic system. It was subsequently learned that the clinical syndrome is correlated with the associated autoantibodies. Several classes of antibodies (abs) have been described in association with LE [Graus et al. 2010]: the classical onconeuronal abs that are directed against intracellular antigens and those directed against surface proteins (voltage-gated potassium channel [VGKC] complex, NMDA receptor [NMDAR], AMPA receptor [AMPAR], γ-aminobutyric acid (b) [GABA (b)]).
The distinction between the different causes of LE is important for the patient, as there is a marked difference in therapeutic response: LE associated with the classical onconeuronal abs is unresponsive to treatment, while LE in association with abs against surface proteins may respond to immunomodulation. In this paper we review the current knowledge of LE.
History
The first description of a paraneoplastic neurological syndrome (PNS) involving the brain hemisphere was made by Brierley and colleagues half a century ago [Brierley et al. 1960]. Until then it was assumed that a PNS could involve the nervous system only caudally to the basal ganglia. After Brierley and colleagues’ description of three adult patients with a subacute encephalitis, other cases were identified. However the definition of paraneoplastic limbic encephalitis (PLE) was made by Corsellis and colleagues 8 years later [Corsellis et al. 1968].
Corsellis and colleagues’ [Coresellis et al. 1968] cases were two men aged between 50 and 60, who seemed to suffer from depression and irritability for some weeks, marking the preceding event. The hallmark of presentation was a loss of recent memory which, in the first patient seemed to increase over a period of months. Memory of past events was not impaired and judgement and reason were intact. This aspect of seemingly unimpaired judgement, contrasting with the loss of recent memory, often resulting in a loss of orientation, has also been emphasized by other authors [Störring et al. 1962].
Another feature was seizures, which originally suggested a focal brain disease. Both patients underwent an autopsy, which ruled out brain metastasis and confirmed an underlying lung cancer. Neuropathology showed lymphocytic perivascular cuffing and infiltration in many parts of the brain, including the cortex. The mesial-temporobasal lobe was severely affected, due to the distribution, and revealed similarities with herpes encephalitis.
Several more recent descriptions of LE in the context of PNS have been published [Vedeler and Storstein, 2009; Voltz et al. 1999; Dalmau and Posner, 1997] and, more recently, diagnostic criteria for the diagnosis of PLE have been defined by Graus and colleagues [Graus et al. 2004].
Limbic encephalitis and clinical features
The key features in LE are an acute or subacute onset of recent memory disorder associated with seizures. Patients may also present with psychiatric manifestations that can vary in the different syndromes. As reported by Kayser and colleagues, a combination of distinct psychiatric disturbances with neurological symptoms and other findings seem to be distinctive for the different types of LE [Kayser et al. 2010]. The psychopathology of LE is often vaguely described in the neurological literature although descriptions of temporal lobe pathology with references to possible mechanisms in schizophrenia are on the rise in the psychiatric literature [Zandi et al. 2010].
Paraneoplastic limbic encephalitis ‘plus’
The involvement of extralimbic areas may occur in patients with a core clinical picture of LE. These types of LE could be termed ‘LE plus’. In relation to other parts of ‘encephalitic’ involvement, brainstem, diencephalic and cortical involvement have been described. Additional central nervous system (CNS) dysfunctions can be found in the brainstem, cerebellum and spinal cord (myelitis) representing the clinical spectrum of encephalomyelitis. The PNS Euronetwork database [Giometto et al. 2010] enrolled 104 patients with a clinical picture of LE, 55 of whom had additional symptoms. Some of the extralimbic symptoms may be helpful in diagnosing specific encephalitis subtypes. For example, in NMDA encephalitis, changes were detected in levels of consciousness [Irani et al. 2010a], together with extrapyramidal symptoms and hypoventilation [Saiz et al. 2009], suggesting brainstem involvement. Conversely, hyponatremia, causing additional neurological and internal medical symptoms, seems to be a feature of encephalitis associated with anti-VGKC complex abs. The widening of the clinical spectrum of LE has recently supported the idea of moving from a clinical to an ab-based classification of this group of patients.
Limbic encephalitis and imaging
In 1988 Kohler and colleagues described the first case of increased temporal lobe signal on T2-weighted MRI sequences in patients during the acute phase of LE [Kohler et al. 1988]. It was shown that these alterations correspond to the presence of an inflammatory infiltrate at the autopsy examination and that the lesion may evolve into hippocampal sclerosis. Today it is known that in the acute phase of the disease increased signal activity on T2 or fluid-attenuated inversion recovery (FLAIR) MRI sequences is present in 70–80% of cases. Fluoro-deoxyglucose positron emission tomography (FDG-PET) may detect the presence of hypermetabolism before the development of MRI abnormalities and may be modified with the clinical course of the disease, while MRI abnormalities remain unchanged [Dirr et al. 1990].
Limbic encephalitis and EEG
EEG is not really useful for the diagnosis. It is needed to detect the presence of epileptic discharge in the temporal lobe or to show diffuse slow-wave activity. Although it is not a diagnostic tool, it can be very useful in distinguishing between a confusional state and a partial complex seizure. Recently it has been also reported that EEG is indicated in patients with LE because the rhythmic delta activity found in comatose patients with anti-NMDAR ab encephalitis may represent a pattern of status epilepticus [Kirkpatrick et al. 2010].
Tumour types
In PLE a tumour is detected in about 60% of cases. In the PNS Euronetwork database [Giometto et al. 2010] a tumour was detected in 51 cases of LE. In 40 patients the tumour was identified after neurological syndrome onset, with a median time of 2 months (range 0.5–23 months). The most frequent tumour type was small cell lung cancer (SCLC; n = 23) followed by testicular (n = 9) and breast (n = 4) tumours. Other malignancies were gastrointestinal (n = 3), ovarian (n = 2), thymoma (n = 1) and neuroblastoma (n = 1). Two cases were of unknown primary origin.
Classification of Limbic encephalitis by antibodies
Recently the classification of LE changed from being clinical to being based on the associated abs [Graus et al. 2010], as reported in the following. The abs associated with LE may be divided into classical onconeuronal abs that are typically directed against intracellular antigens and associated with PLE, and abs directed against surface antigens that may be found in PLE, but more frequently occur in patients with autoimmune but nonparaneoplastic LE. There are also reports of LE associated with other abs that cannot classified into the two groups reported above.
Classical onconeuronal antibodies
This group encompasses PLE that may respond to tumour treatment (as LE associated with anti-Ma2), while the response to immunomodulatory therapy is low (Tables 1 and 2).
Table 1.
Onconeuronal antibodies: psychiatric and neurological manifestations.
| Onconeuronal antibody | Psychiatric manifestation | Neurologic findings | MRI | Treatment |
|---|---|---|---|---|
| Hu | Short-term memory loss, ‘confusion’, Wernicke Korsakow-like manifestation | Seizures | Mesial temporal lobe changes, extralimbic manifestations | Immunomodulation, steroids, cyclophosphamide tumour treatment |
| Ma2 | Short-term memory loss, less confusion Panic reactions, ‘nervous breakdown’, obsessive–compulsive disorders | Brainstem involvement, hypothalamic dysfunction, diencephalic syndrome, sleep dysregulation (excessive daytime sleepiness) | Mesial temporal lobe | Improvement and stabilization can occur following tumour treatment |
| CV2/CRMP | Cognitive defects, manic mood, obsessive compulsive disorders, Depression, Personality changes | Chorea extrapyramidal disorders, apraxia, optic neuropathy, disorder of smell and taste | Changes may occur | Unclear* |
| Anti-amphiphysin | Short-term memory loss, ‘confusion’, Wernicke Korsakow-like | Myoclonus, rigidity, stiff person syndrome | Unclear* |
PubMed search with CV2, CRMP5, amphiphysin with ‘limbic’ and ‘treatment’ yielded no results, with only reviews available.
Table 2.
Psychiatric and neurologic symptoms in NMDA, AMPA and GABA (b) surface antibodies.
| Antibody | Psychiatric manifestation | Neurologic findings | MRI | Treatment |
|---|---|---|---|---|
| NMDA | Psychosis, anxiety, bizarreness, delusions, paranoid behaviour ‘catatonic state’ | Reduced level of consciousness seizure hypoventilation autonomic disturbances dyskinesias Movement disorders | Rare | Treatment either of tumour or immunotherapy |
| AMPA | Memory loss confusion, aggression | Common but not necessarily | Tumour treatment or immunotherapy. Relapse possible | |
| GABA (b) | Psychosis, hallucinations, confusion | Seizures | Common (small number of cases) | Improvement due to tumour treatment, or immunotherapy. |
Anti-Hu
Anti-Hu abs are usually detected in patients with multifocal neurological involvement referred to as anti-Hu syndrome [Luchinetti et al. 1998; Dalmau et al. 1992]. In some cases there could be more focal neurological involvement and LE may dominate the clinical picture. Patients are usually older than 40 years and smokers. Clinically, seizures, short-term memory deficits and confusion appear. A depressive phase can precede the onset of LE. MRI often reveals changes in the mesial temporal lobe that may evolve into atrophy. The cerebrospinal fluid (CSF) has moderate inflammatory changes, with lymphocytic infiltration. Oligoclonal bands may be positive. In about the 70% of patients with LE and anti-Hu abs the associated tumour is SCLC, while it has been shown that only 50% of patients with LE and SCLC harbour anti-Hu abs. It does, however, seem that harbouring anti-Hu abs is a negative prognostic factor. Some patients may present only with a clinical picture of temporal seizures or epileptic status. A case of organic epilepsy associated with anti-Hu abs and SCLC [Fadul et al. 2005] has also been reported recently. Anti-Hu associated LE responds neither to tumour therapy nor to immune suppression
Anti-Ma2
Ma2 abs can appear in association with limbic or brainstem encephalitis [Barnett et al. 2001; Dalmau et al. 1999]. Hallucinations, memory loss and seizures are the characteristic findings. MRI changes occur in the mesial temporal lobe, and hypothalamus. Patients are usually males younger than 40 years of age.
Excessive daytime sleepiness, narcolepsy, loss of libido and weight gain can be suggestive of hypothalamic involvement. Testicular germ-cell or seminoma or breast carcinoma [Sutton et al. 2000] have been described as associated cancers. Compared with the other LEs caused by onconeuronal abs, tumour treatment and/or immunotherapy can be beneficial [Hoffmann et al. 2008; Rosenfeld et al. 2001]. The association of a tumour of the testis and Ma2 encephalitis is strong, and even orchiectomy has been advised in suspected cases [Mathew et al. 2007].
Anti-CV2/CRMP5
The ab CV2 is directed against the collapsin response mediator protein (CRMP5) and may be associated with different PNSs including LE [Antoine et al. 1993]. Anti-CV2/CRMP5-associated LE is, however, a rare disease that involves males and females of median age equally [Knudsen et al. 2007]. There is more frequently an association with hyperkinetic movement disorders, most notably chorea [Vernino et al. 2002]. The most frequently associated tumours are SCLC and thymoma, but a testicular tumour may also be detected, as reported by Kellinghaus and colleagues [Kellinghaus et al. 2007]. Recently Knudsen and colleagues reported the case of a 52-year-old patient with subacute cognitive decline associated with thymoma harbouring abs directed against the collapsin response mediator protein types 3 and 4 (CRMP3 and CRMP4) [Knudsen et al. 2007]. These proteins have 50% homology with the CRMP5. No systematic series of treatment responses could be identified.
Anti-Ri
Anti-Ri abs are typically found in patients with opsoclonus–myoclonus syndrome and ataxia associated with SCLC or breast cancer. There are few case reports of LE associated with anti-Ri abs [White and Beringer, 2010; Launary et al. 2008; Harloff et al. 2005]. Recently an encephalitic syndrome characterized by jaw dystonia and laryngospasm, also associated with ataxia and other CNS signs, was described in association with anti-Ri abs [Pittock et al. 2010]. Neuropathological investigations included the brainstem in two patients and showed only mild inflammation with cell loss, pointing to chronic encephalomyelitis. Treatment is symptomatic (botulinum toxin) and in some cases tumour chemotherapy and immunomodulation (steroids, intravenous immunoglobulins [IVIg], etc.) have been applied.
Anti-amphiphysin
The spectrum of neurological syndromes that may related to anti-amphiphysin abs was extended by Dropcho in 1996 when he reported the association of this ab with a clinical picture of PEM in three patients with SCLC [Dropcho, 1996]. Subsequently other cases were reported in which patients presented a clinical picture not unlike that identified in patients with anti-Hu abs. It is important to observe that myoclonus and rigidity of the limbs and trunk may be present in patients with PEM associated with anti-amphiphysin abs, but are not usually observed in patients with anti-Hu abs.
In the literature 23 cases of LE associated with anti-amphiphysin abs have been reported, but detailed clinical information is available only for one case described by Dorresteijn and colleagues [Dorresteijn et al. 2002]. The larger series described by Pittok and colleagues reports on 19 patients with LE but 12 of them also had other associated autoantibodies [Pittok et al. 2005]. Four cases of LE associated with amphiphysin are collected in the PNS Euronetwork database (three males and one female, median age 72 years). An associated tumour was found in three cases (two lung cancers and one breast cancer). Three patients died due to the progression of the neurological condition and none improved with tumour therapy.
Surface antibodies
LE associated with surface abs is first an autoimmune disease that responds to immunomodulatory treatment and may have a relapsing–remitting course. In some cases it is associated with a tumour and LE may be considered paraneoplastic in origin. Some of these abs were first defined as antineutrophil abs [Ances et al. 2005; Vitaliani et al. 2005] because of their appearance on immunohistochemistry. Recently many of the targets have been identified, but this group will probably grow in the future.
Anti-VGKC complex antibodies
VGKCs were first described in conjunction with neuromyotonia [Kleopa et al. 2006; Thieben et al. 2004; Vincent et al. 2004; Pozo-Rosich et al. 2003; Hart et al. 1997].
Anti-VGKC abs have subsequently been found in patients with a combination of neuromyotonia, autonomic dysfunction, sleep disorders [Montiel et al. 2008] and cognitive impairment, referred to as Morvan’s syndrome [Liguori et al. 2001], and in patients with LE [Reid et al. 2009] or isolated epilepsy. LE associated with anti-VGKC abs differs from the other types of LE because hypersomnia and hyponatremia may be present. Moreover the clinical syndrome may have a relapsing–remitting course and usually responds to immunomodulatory treatment with plasma exchange, IVIg or steroids.
In the majority of cases with anti-VGKC the pathogenesis seems to be autoimmune not paraneoplastic. However in 25–30% of cases a tumour may be detected (mainly thymoma or SCLC). It has also been reported that lung cancer, VGKC abs and other abs may be detected in patients. This ab, initially called AGNA, is directed against the protein Sox 1 and seems to be strictly associated with lung cancer in patients with neurological syndromes associated with abs directed against ion channels [Sabater et al. 2008; Zuliani et al. 2007; Graus et al. 2005].
Recently it has been reported that VGKC are not the real target of the abs as previously assumed. Irani and colleagues reported that the sera of patients with anti-VGKC identified by the radioimmunoassay (RIA) technique did not bind to Kv1.1-transfected cells [Irani et al. 2010b]; rather they bound to two proteins which are part of the dendrotoxin-labelled VGPC complex used in the serological assay. One of these proteins was contactin-associated protein-2 (Caspr2), which colocalized with Kv1.1 and Kv1.2 at the neuronal juxta-paranode and is essential for VGKC clustering. It also seems that abs directed against Caspr2 are more frequently associated with Morvan syndrome and thymoma. The second protein to be identified was leucine-rich glioma inactivated 1 (Lgi1), which is a secreted protein that colocalizes with Kv1.1 subunits in CNS presynaptic terminals and serves as the ligand of two epilepsy-related proteins, ADAM 22 and ADAM 23. Abs directed against LGI1, as confirmed by Lai and colleagues, seem to be the main target in patients with autoimmune nonparaneoplastic LE [Lai et al. 2010]. These two recent publications indicate that the terms LE associated with anti-VGKC abs should probably be avoided, with preference being given to the definition of LE with anti-VGKC complex abs.
Anti-NMDAR
Anti-NMDAR abs were first identified in young women with ovarian teratoma and a clinical picture of a potentially reversible encephalitis [Dalmau et al. 2007; Vitaliani et al. 2005]. The clinical syndrome has an acute–subacute onset often antedated by a flu-like episode. Patients present with often severe psychiatric symptoms, including abnormal behaviour, personality changes, delirium that may evolve to a well-defined psychosis; some patients may be seen for the first time in a psychiatric unit. Memory problems and seizures are also present. Moreover, other clinical symptoms may be present that indicate involvement of areas outside the limbic system, particularly involvement of the brainstem and basal ganglia. Some patients may deteriorate with a reduced level of consciousness, even progressing to a catatonic state. Neurologically, tonic–clonic seizures [Bayreuther et al. 2009], autonomic instability and dyskinesias [Kleinig et al. 2008], particularly oral, can occur. Hypoventilation can be a threatening additional finding and was observed in 66% of one case series [Dalmau et al. 2008].
Younger people are mostly affected, including children [Wong-Kisiel et al. 2010; Dale et al. 2009; Florance et al. 2009; Schimmel et al. 2009]. The syndrome has also been reported to occur during pregnancy [Ito et al. 2010]. Around 55% of affected women aged over 55 years have an ovarian teratoma but other tumours and haematological diseases may also be identified [Zandi et al. 2009]. In NMDA-associated PNS encephalitis, tumour removal can have a positive effect [Shindo et al. 2009] and immunotherapy may be beneficial.
Anti-AMPA (GluR1/2)
This is a rare syndrome, in which abs are directed against the subunits GluR1 and GluR2 receptor [Bataller et al. 2010]. Clinically, this syndrome is a classical LE with memory loss, psychiatric symptoms and seizures [Bataller et al. 2010; Lai et al. 2009]. In the majority of patients a tumour can be found particularly in the lung, breast or thymus. This syndrome responds to immunomodulation but a relapse may occur, even during treatment [Lai et al. 2009].
Anti-GABA(b) receptors
Another type of surface ab recognizes the B1 subunit of the GABA (b) receptor [Lancaster et al. 2010]. This receptor has an inhibitory function and has been associated with seizures and memory dysfunction. Only one case series has been reported. In this series a tumour was identified in seven of 15 cases and was an SCLC in five cases. Tumour treatments or immunosuppression can induce remission in most patients.
Other types of LE
Several other types of encephalitic syndromes have been described and although their description is purely observational, they can provide interesting aspects on the pathomechanism of immune-mediated or paraneoplastic encephalitis.
Ophelia syndrome
Ophelia syndrome has been described by Carr [Carr, 1982]. Degenerative hippocampal changes have been described as a paraneoplastic syndrome [Shinohara et al. 2005]. In this regard no association with abs or response to therapy is known. Neuropathologically, degenerative changes without inflammation have been described. This so-called ‘Ammon’s horn sclerosis’ has also been described in earlier observations [Ulrich et al. 1967].
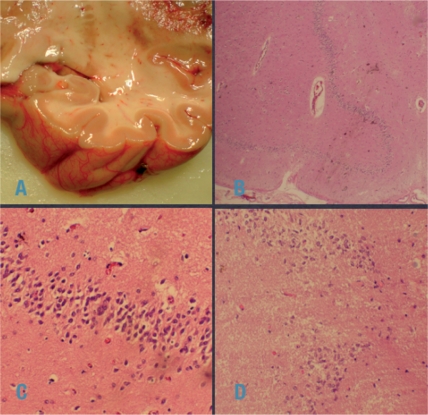
The following is a case vignette of a patient with LW, and autoptic noninflammatory Ammon’s horn sclerosis (see also Figure 1):
A 50-year-old patient was diagnosed with a primary CNS lymphoma (PCNSL). She was treated with chemotherapy, and responded well. During treatment she had several seizures, and mental changes were noted. She was referred to the neurology department as a patient with acute ‘dementia’. Psychopathologically, loss of recent memory had occurred, and she presented with a Korsakow-like syndrome. LE was suspected, but several MRI studies of the temporal lobe were negative. CSF had mild pleocytosis and an elevated protein level. Testing for onconeuronal abs and VGKC were negative; surface abs were not tested.
Treatment with steroids and IVIg was not successful. The patient was referred to a nursing home. Several months later she was readmitted after a new series of seizures. At this time a parietal lobe recurrence of the PCNSL had occurred. The patient deteriorated and subsequently died.
The autopsy demonstrated pneumonia as the cause of death and the PCNSL recurrence was confirmed. The Ammon’s horn was not atrophic (Figure 1A) and low power magnification (Figure 1B) showed diffuse cell loss, which was confirmed by large power views (Figure 1C and D). No inflammation, be it parenchymal or around the vessels, was noted at any time. The topical distribution confirms the anatomical site of LE. Retrospectively, it remains unclear whether this type of Ammon’s horn sclerosis is the residual state of an inflammation, or a primary sclerosis.>
Figure 1.
Illustration of the case vignette. The Ammon’s horn was not atrophic (A). Low power magnification (B): diffuse cell loss, confirmed by large power views (C, D). No inflammation either parenchymal or around vessels was detected. (Images courtesy of Professor M Klimpfinger, Institute Pathology, KFJ Hospital, Vienna).
Anti-GAD antibodies
We include encephalitis associated with anti-GAD abs in this group because it is a predominantly a nonparaneoplastic form of encephalitis, but the target antigen is intracellular.
Abs against glutamic acid decarboxylase (GAD) are predominantly found in patients with stiff person syndrome [Matà et al. 2010]. However, an association with ataxia, seizures, LE [Malter et al. 2010] and possibly psychosis has also been observed. Patients with LE associated with anti-GAD are usually young adults. A Japanese group reported on a patient with severe seizures and bilateral frontal lesions on MRI, who responded well to plasma exchange [Kobayakawa et al. 2010]. Steroids also seem to be effective [Akman et al. 2009].
Progressive encephalomyelitis with myoclonus and rigidity
Progressive encephalomyelitis with myoclonus and rigidity (PEMR) is a rare encephalitic syndrome involving the limbic system, brainstem and spinal cord. Clinically painful muscle cramps, axial rigidity, ataxia, myoclonus and dysautonomia appear. High titres of GAD abs can be detected in the serum, together with anti-amphipysin abs; an association with breast cancer has also been described [Meinck and Thompson, 2002; Marchiori et al. 2001].
Other abs have been identified in encephalitis with psychiatric symptoms. These were abs against Serin/threonine kinase 2 (BRSK2) [Sabater et al. 2005] and adenylate kinase 5 [Tünzün et al. 2007]. Confusion, memory loss, delusions and agitation were described.
Differential diagnostic considerations
The clinical phenomenology of LE, although diverse for the different associated abs, can be observed in other diseases as Wernicke’s encephalopathy, viral encephalitis (HSV-1 and EV), possibly rabies [Plotkin, 2000], syphilis [Hama et al. 2008; Vieria et al. 2005] and local tumours, most notably glioma [Deramecourt et al. 2009] or gliomatosis cerebri [Simo and Dalmau, 2009]. LE has also been described in posttransplant patients [Seeley et al. 2007].
In the aged population, delirious states can be difficult to differentiate from LE. A new dimension of considerations are psychotic states, which may resemble presentations described in NMDA encephalitis.
Therapy
Steroids, immunosuppression and immunomodulation, as plasma exchange, immune adsorption or IVIg have been applied in all types of LE [Vitaliani et al. 2008]. However, there are practical implications. In each case of a possible immunomediated encephalitic syndrome, or in a suspected LE, a number of differential diagnoses have to be excluded. The detection of specific abs, such as onconeural or surface abs, usually takes time. In particular the lab workup for the surface abs is currently available in only a few centres in Europe and the treatment decision should not be delayed. The current concept is that PNS needs to be arrested at an early stage [Greenlee, 2010; Vincent, 2010]. A patient presenting with a combination of different psychiatric and neurologic symptoms can be a useful clue. For example, the combination of psychiatric delusional symptoms for several weeks, a psychotic onset, seizures and hypoventilation would be a combination pointing towards an NMDA-type of encephalitis. Pragmatically, if other treatable conditions, such as infections or tumours, have been reasonably ruled out, immunomodulation should be considered, even before a tumour search is concluded. A rational approach to tumour screening in PNS has recently been published [Titulaer et al. 2010].
In contrast to the ‘classical onconeuronal abs’, where a treatment response is infrequent, LE caused by VGKC, NMDA abs and anti-GAD abs seem to be treatment responsive. Cancer treatment and/or tumour removal also seem to have positive effects in paraneoplastic types of LE associated with voltage-gated or surface abs.
Immunosuppressive treatment in patients with cancer can, however, be a cause for concern, as tumour progression can potentially be influenced.
Owing to the rarity of these syndromes, no systematic studies are available and existing work is based on observations. Hence, the treatment of PNS encephalitis is not evidence based [Vedeler et al. 2006] and the above suggestions are a summary of existing reports.
Strategically the first level of intervention is steroids (daily gram doses similar to those used in the treatment of multiple sclerosis) and/or IVIg. A more invasive procedure is PL plasma exchange and/or immune apheresis, where abs and possibly other immune pathogenetic factors are removed. Level 2 is more extensive immunosuppression with cyclophosphamide, mycophenolate mofetil [Saidha et al. 2010] and, in selected cases, with abs such as rituximab.
In PNS with identified cancer, the tumour treatment can be beneficial. There may be an effect on the tumour per se, or immunosuppression exerted by the tumour therapy. The use of level 2 immunotherapy prior to tumour treatment is controversial and has not been resolved.
Despite the lack of evidence for immune and cancer therapy for PNS, symptomatic treatment should be provided from the beginning. Specific therapies for seizures, control of psychiatric manifestations and autonomic problems should be used.
Symptomatic therapy
Symptomatic treatment involves specific medications such as drugs for seizure control, medications to improve autonomic symptoms, and other symptom-oriented treatments as physiotherapy and occupational therapy, speech therapy and psychological support. In particular in patients with paraneoplastic encephalitis caused by onconeural abs, all symptomatic therapeutic strategies must be exploited, to improve symptoms and quality of life. In conditions caused by ion channel and surface abs, symptomatic treatment is also necessary, but substantial improvement or even cure can be expected.
Conclusion
Immune-mediated or paraneoplastic LE has core symptoms of seizure, amnestic syndromes and mental changes even appearing as psychosis. Recently it has been recognized that widespread involvement of the brain is possible in these patients and the term encephalitis is preferred to LE in these cases.
The present classification is based on the associated abs and includes LE with the classical onconeuronal, and LE with surface abs. Several other more rare ab-associated encephalitic syndromes have also emerged.
The description of the clinical neurological symptoms, most notably the psychiatric manifestations [Kayser et al. 2010], are helpful in recognizing the associated abs.
At present none of the therapies are evidence-based. The impression is that LE caused by the classical onconeuronal abs is quite resistant to treatment, while in surface ab-related encephalitis, treatment (when the case) and immunomodulation is beneficial, although relapses do occur.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors
Conflict of interest statement
None declared.
References
- Akman C.I., Patterson M.C., Rubinstein A., Herzog R. (2009) Encephalitis associated with anti-GAD antibody and common variable immune deficiency. Dev Med Child Neurol 51: 563–567 [DOI] [PubMed] [Google Scholar]
- Ances, B.M., Vitaliani, R., Taylor, R.A., Liebeskind, D.S., Voloschin, A., Houghton, D.J. et al. (2005) Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 128: 1764–1777. [DOI] [PMC free article] [PubMed]
- Antoine J., Honnorat J., Koeing F., Aguera M., Berlin M.F., Michel D. (1993) Posterior uveitis and paraneoplastic encephalomyelitis with auto-antibodies reacting against cytoplasmic protein of brain and retina. J Neurol Sci 163: 159–162 [DOI] [PubMed] [Google Scholar]
- Barnett M., Prosser J., Sutton I., Halmagyi G.M., Davies L., Harper C., et al. (2001) Paraneoplastic brain stem encephalitis in a woman with anti-Ma2 antibody. J Neurol Neurosurg Psychiatry 70: 222–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller L., Galiano R., García-Escrig M., Martínez B., Sevilla T., Blasco R., et al. (2010) Reversible paraneoplastic limbic encephalitis associated with antibodies to the AMPA receptor. Neurology 74: 265–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayreuther C., Bourg V., Dellamonica J., Borg M., Bernardin G., Thomas P. (2009) Complex partial status epilepticus revealing anti-NMDA receptor encephalitis. Epileptic Disord 11: 261–265 [DOI] [PubMed] [Google Scholar]
- Brierley J.B., Corsellys J.A.N., Hierons R., Nevin S. (1960) Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 83: 357–368 [Google Scholar]
- Carr I. (1982) The Ophelia syndrome: memory loss in Hodgkin’s disease. Lancet 1: 844–845 [DOI] [PubMed] [Google Scholar]
- Corsellis J.A.N., Goldberg G.J., Norton A.R. (1968) ‘Limbic encephalitis’ and it’s association with carcinoma. Brain 91: 481–495 [DOI] [PubMed] [Google Scholar]
- Dale R.C., Irani S.R., Brilot F., Pillai S., Webster R., Gill D., et al. (2009) NMDA receptor antibodies in pediatric dyskinetic encephalitis lethargica. Ann Neurol 66: 704–709 [DOI] [PubMed] [Google Scholar]
- Dalmau J., Gleichman A.J., Hughes E., Rossi J.E., Peng X., Lai M., et al. (2008) Anti-NMDA- receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7: 1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J., Graus J., Rosenblum M.K., Posner J. (1992) Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine 71: 59–72 [DOI] [PubMed] [Google Scholar]
- Dalmau J., Gultekin S.H., Voltz R. (1999) A novel neuron-testis specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain 122: 27–39 [DOI] [PubMed] [Google Scholar]
- Dalmau J., Posner J.B. (1997) Paraneoplastic syndromes affecting the nervous system. Semin Oncol 24: 318–328 [PubMed] [Google Scholar]
- Dalmau J., Tuzun E., Wu H.Y., Masjuan J., Rossi J.E., Voloschin A., et al. (2007) Paraneoplastic anti-Nmethyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 61: 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deramecourt V., Bombois S., Debette S., Delbeuck X., Ramirez C., Reyns N., et al. (2009) Bilateral temporal glioma presenting as a paraneoplastic limbic encephalitis with pure cognitive impairment. Neurologist 15: 208–211 [DOI] [PubMed] [Google Scholar]
- Dirr L.Y., Elster A.D., Donofrio P.D., Smith M. (1990) Evolution of brain MRI abnormalities in limbic encephalitis. Neurology 40: 1304–1306 [DOI] [PubMed] [Google Scholar]
- Dorresteijn L.D., Kappaelle A.C., Reiner W.O., Gijtenbek J.M. (2002) Anti-amphiphysin associated limbic encephalitis: a paraneoplastic presentation of small cell carcinoma. J Neurol 249: 1307–1308 [DOI] [PubMed] [Google Scholar]
- Dropcho E.J. (1996) Antiamphiphysin antibodies with small cell lung carcinoma and paraneoplastic encephalomielitis. Ann Neurol 39: 659–667 [DOI] [PubMed] [Google Scholar]
- Fadul C.E., Stommel E.W., Dragnev K.H., Dalmau J.O. (2005) Focal paraneoplastic limbic encephalitis presenting as orgasmic epilepsy. J Neuro-Oncol 72: 195–198 [DOI] [PubMed] [Google Scholar]
- Florance N.R., Davis R.L., Lam C., Szperka C., Zhou L., Ahmad S., et al. (2009) Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 66: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable M.S., Gavali S., Radner A., Tilley D.H., Lee B., Dyner L., et al. (2009) Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis 28: 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giometto B., Grisold W., Vitaliani R., Graus F., Honnorat J., Bertolini G., et al. (2010) Paraneoplastic neurologic syndrome in the PNS Euronetwork database. Arch Neurol 67: 330–335 [DOI] [PubMed] [Google Scholar]
- Graus F., Delattre J.Y., Antoine J.C., Dalmau J., Giometto B., Grisold W., et al. (2004) Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 75: 1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F., Saiz A., Dalmau J. (2010) Antibodies and neuronal autoimmune disorders of the CNS. J Neurol 257: 509–517 [DOI] [PubMed] [Google Scholar]
- Graus F., Vincent A., Pozo-Rosich P., Sabater L., Saiz A., Lang B., et al. (2005) Anti-glial nuclear antibody marker of lung cancer-related paraneoplastic neurologica syndromes. J Neuroimmunol 165: 166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee J.E. (2010) Treatment of paraneoplastic neurologic disorders. Curr Treat Options Neurol 12: 212–230 [DOI] [PubMed] [Google Scholar]
- Hama K., Ishiguchi H., Tuji T., Miwa H., Kondo T. (2008) Neurosyphilis with mesiotemporal magnetic resonance imaging abnormalities. Intern Med 47: 1813–1817 [DOI] [PubMed] [Google Scholar]
- Harloff A., Hummer S., Kleinschidt M., Rauer S. (2005) Anti-Ri antibodies and limbic encephalitis in a patient with carcinoid tumor of the lung. J Neurol 252: 1404–1405 [DOI] [PubMed] [Google Scholar]
- Hart I.K., Waters C., Vincent A., Newland C., Beeson D., Pongs O. (1997) Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 41: 238–246 [DOI] [PubMed] [Google Scholar]
- Hoffmann L.A., Jarius S., Pellkofer H.L., Schueller M., Krumbholz M., Koenig F. (2008) Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J Neurol Neurosurg Psychiatry 79: 767–773 [DOI] [PubMed] [Google Scholar]
- Irani S.R., Alexander S., Waters P., Kleopa K.A., Pettingill P., Zuliani L., et al. (2010b) Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133: 2734–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani S.R., Bera K., Waters P., Zuliani L., Maxwell S., Zandi M., et al. (2010a) N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133: 1655–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Abe T., Tomioka R., Komori T., Araki N. (2010) Anti-NMDA receptor encephalitis during pregnancy (in Japanese). Rinsho Shinkeigaku 50: 103–107 [DOI] [PubMed] [Google Scholar]
- Kayser C.G., Kohler C.G., Dalmau J. (2010) Psychiatric manifestations of paraneoplastic disorders. Am J Psychiatry 167: 1039–1050 [DOI] [PubMed] [Google Scholar]
- Kellinghaus C., Kraus J., Blaes F., Nabavi D.G., Schabitz W.R. (2007) CRMP-5 autoantibodies in testicular cancer associated with limbic encephalitis and choreiform dyskinesia. Eur Neurol 57: 241–243 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M.P., Clarke C.D., Sonmezturk H.H., Abou-Khalil B. (2011) Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti- NMDA receptor antibody encephalitis. Epilepsy Behav 20(2): 392–394 [Epub 28 December 2010] [DOI] [PubMed] [Google Scholar]
- Kleinig T.J., Thompson P.D., Matar W., Duggins A., Kimber T.E., Morris J.G., et al. (2008) The distinctive movement disorder of ovarian teratoma-associated encephalitis. Mov Disord 23: 1256–1261 [DOI] [PubMed] [Google Scholar]
- Kleopa K.A., Elman L.B., Lang B., Vincent A., Scherer S.S. (2006) Neuromyotonia and limbic encephalitis sera target mature shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain 129: 1570–1584 [DOI] [PubMed] [Google Scholar]
- Knudsen A., Bredolt A., Storstein A., Oteldal L., Dvanger S., Krossness B., et al. (2007) Antibodies to CRMP3-4 associated with limbic encephalitis and thymoma. Clin Exp Immunol 147: 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa Y., Tateishi T., Kawamura N., Doi H., Ohyagi Y., Kira J. (2010) A case of immune-mediated encephalopathy showing refractory epilepsy and extensive brain MRI lesions associated with anti-glutamic acid decarboxylase antibody. Rinsho Shinkeigaku 50: 92–97 [DOI] [PubMed] [Google Scholar]
- Kohler, J., Hufschmidt, A., Hermle, L., Volk, B., Lücking, C.H. (1988). J Neuroimmunol 20: 177–178. [DOI] [PubMed]
- Lai M., Hughes E.G., Peng X., Zhou L., Gleichman A.J., Shu H., et al. (2009) AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 65: 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E., Lai M., Peng X., Hughes E., Constantinescu R., Raizer J., et al. (2010) Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 9: 67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launary M., Bozzolo E., Vennisacc N., Delemont E., Fredenrich A., Thomas P. (2008) Paraneoplastic limbic encephalitis with positive anti-Ri antibodies and mediastinal seminoma. Rev Neurol (Paris) 164: 612–619 [DOI] [PubMed] [Google Scholar]
- Liguori R., Vincent A., Clover L., Avoni P., Plazzi G., Cortelli P., et al. (2001) Morvan's syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 124: 2417–2426 [DOI] [PubMed] [Google Scholar]
- Luchinetti C.F., Kimmel D.W., Vanda A.L. (1998) Paraneoplastic and oncologic profiles of patients seropositive for Type 1 antineuronal nuclear autoantibodies. Neurology 50: 652–657 [DOI] [PubMed] [Google Scholar]
- Malter M.P., Helmstaedter C., Urbach H., Vincent A., Bien C.G. (2010) Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 67: 470–478 [DOI] [PubMed] [Google Scholar]
- Marchiori G.C., Vaglia A., Vianello M., Bardin P.G., Giometto B. (2001) Encephalitis associated with glutamic acid decarboxylase autoantibodies. Neurology 56: 814–814 [DOI] [PubMed] [Google Scholar]
- Matà S., Muscas G.C., Cincotta M., Bartolozzi M.L., Ambrosini S., Sorbi S. (2010) GAD antibodies associated neurological disorders: incidence and phenotype distribution among neurological inflammatory diseases. J Neuroimmunol 227: 175–177 [DOI] [PubMed] [Google Scholar]
- Mathew R.M., Vandenberghe R., Garcia-Merino A., Yamamoto T., Landolfi J.C., Rosenfeld M.R., et al. (2007) Orchiectomy for suspected microscopic tumor in patients with anti-Ma2-associated encephalitis. Neurology 68: 900–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck H.M., Thompson P.D. (2002) Stiff man syndrome and related conditions. Mov Disord 17: 853–886 [DOI] [PubMed] [Google Scholar]
- Montiel P., Sellal F., Clerc C., Ricard P., Bataillard M. (2008) Limbic encephalitis with severe sleep disorder associated with voltage gated potassium channels (VGKC) antibodies (in French). Rev Neurol (Paris) 164: 181–184 [DOI] [PubMed] [Google Scholar]
- Pittock S.H., Parisi J.E., McKeon A., Roemer S.F., Lucchinetti C.F., Tan K.M., et al. (2010) Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri). Arch Neurol 67: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Pittock S.J., Luchinetti C.F., Parisi J.E., Benearroch E.E., Mokri B., Stephan C.L., et al. (2005) Amphiphisin autoimmunity: paraneoplastic accompaniments. Ann Neurol 58: 96–107 [DOI] [PubMed] [Google Scholar]
- Plotkin S.A. (2000) Rabies. Clin Infect Dis 30: 4–12 [DOI] [PubMed] [Google Scholar]
- Pozo-Rosich P., Clover L., Saiz A., Vincent A., Graus F. (2003) Voltage-gated potassium channel antibodies in limbic encephalitis. Ann Neurol 54: 530–533 [DOI] [PubMed] [Google Scholar]
- Reid J.M., Foley P., Willison H.J. (2009) Voltage-gated potassium channel-associated limbic encephalitis in the West of Scotland: case reports and literature review. Scott Med J 54: 27–31 [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.R., Eichen J.G., Wade D.F., Posner J.B., Dalmau J. (2001) Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Ann Neurol 50: 339–348 [PubMed] [Google Scholar]
- Sabater L., Gomez-Choco M., Saiz A., Graus F. (2005) BR serine/threonine kinase 2: a new autoantigen in paraneoplastic limbic encephalitis. J Neuroimmunol 170: 186–190 [DOI] [PubMed] [Google Scholar]
- Sabater L., Titulaer M., Saiz A., Verschuuren J., Gure A.O., Graus F. (2008) Sox1 antibodies are a markers of paraneoplastic Lambert–Eaton myastenic syndrome. Neurology 70: 928–940 [DOI] [PubMed] [Google Scholar]
- Saidha, S., Murphy, S., Ronayne, A., McCarthy, P., Hennessy, MJ., Counihan, T. (2010) Treatment of anti-glutamic acid decarboxylase antibody-associated limbic encephalitis with mycophenolate mofetil. J Neurol 257(6): 1035–1038. [DOI] [PubMed]
- Saiz A., Bruna J., Stourac P., Vigliani M.C., Giometto B., Grisold W., et al. (2009) Anti-Hu- associated brainstem encephalitis. J Neurol Neurosurg Psychiatry 80: 404–407 [DOI] [PubMed] [Google Scholar]
- Schimmel M., Bien C.G., Vincent A., Schenk W., Penzien J. (2009) Successful treatment of anti-N-methyl D-aspartate receptor encephalitis presenting with catatonia. Arch Dis Child 94: 314–316 [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Marty F.M., Holmes T.M., Upchurch K., Soiffer R.J., Antin J.H., et al. (2007) Post-transplant acute limbic encephalitis. Clinical features and relationship to HHV6. Neurology 69: 156–165 [DOI] [PubMed] [Google Scholar]
- Shindo A., Kagawa K., Li Y., Sasaki R., Kokubo Y., Kuzuhara S. (2009) Anti-N- methyl-D-aspartate receptor-related grave but reversible encephalitis with ovarian teratoma in 2 Japanese women presenting with excellent recovery without tumor resection. Eur Neurol 61: 50–51 [DOI] [PubMed] [Google Scholar]
- Shinohara T., Kojima H., Nakamura N., Ogata A., Betsuyaku T., Suzuki A., et al. (2005) Pathology of pure hippocampal sclerosis in a patient with dementia and Hodgkin's disease: the Ophelia syndrome. Neuropathology 25: 353–360 [DOI] [PubMed] [Google Scholar]
- Simo M., Dalmau J. (2009) Gliomatosis cerebri simulando una encefalitis limbica. Neurologia 24: 500–501 [PubMed] [Google Scholar]
- Störring G.E., Hauss K., Ule G. (1962) Zur topischen Diagnostik des amnestischen Symptomenkomplexes. Psychiat Neurol (Basel) 143: 161–177 [Google Scholar]
- Sutton I., Winer J., Rowlands D., Dalmau J. (2000) Limbic encephalitis and antibodies to Ma2: A paraneoplastic presentation of breast cancer. Neurol Neurosurg Psychiatr 69: 266–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieben M.J., Lennon V.A., Boeve B.F., Aksamit A.J., Keegan M., et al. (2004) Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 62: 1177–1182 [DOI] [PubMed] [Google Scholar]
- Titulaer M.J., Soffietti R., Dalmau J., Gilhus N.E., Giometto B., Graus F., et al. (2010) Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüzün E., Rossi J.E., Karner S.F., Centurion A.F., Dalmau J. (2007) Adenylate kinase 5 autoimmunity in treatment refractory limbic encephalitis. J Neuroimmunol 186: 177–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J., Spiess H., Huber R. (1967) Neurologische Syndrome als Fernwirkung maligner Tumore (Ammonshornsklerose bei Bronchuskarzinom). Schw Arch Neurol Neurochir Psychiat 99: 83–100 [PubMed] [Google Scholar]
- Vedeler C.A., Antoine J.C., Giometto B., Graus F., Grisold W., Hart I.K., et al. (2006) Management of paraneoplastic neurological syndromes: report of an EFNS Task Force. Eur J Neurol 13: 682–690 [DOI] [PubMed] [Google Scholar]
- Vedeler C.A., Storstein A. (2009) Autoimmune limbic encephalitis. Acta Neurol Scand Suppl 189: 63–67 [DOI] [PubMed] [Google Scholar]
- Vernino S., Tuite P., Adler C.H., Meschia J.F., Boeve B.F., Boasberg P., et al. (2002) Paraneoplastic chorea associated with CRMP-5 neuronal antibody and lung carcinoma. Ann Neurol 51: 625–630 [DOI] [PubMed] [Google Scholar]
- Vieria A., Matias S., Saraiva P., Goulao A. (2005) Differential diagnosis of mesiotemporal lesions; case report of neurosyphillis. Neuroradiology 47: 774–777 [DOI] [PubMed] [Google Scholar]
- Vincent A. (2010) Guest lecture. J Neurol Neurosurg Psychiatry 81: e10–e11 [Google Scholar]
- Vincent A., Buckley C., Schott J.M., Baker I., Dewar B.K., Detert N., et al. (2004) Potassium channel antibody associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 127: 701–712 [DOI] [PubMed] [Google Scholar]
- Vincent A., Irani S.R., Lang B. (2010) The growing recognition of immunotherapy responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr Opin Neurol 23: 144–150 [DOI] [PubMed] [Google Scholar]
- Vitaliani R., Mason B., Ances B., Zwerdling T., Jang Z., Dalmau J. (2005) Paraneoplastic encephalitis, psychiatric symptoms and hypoventilation in ovarian teratoma. Ann Neurol 58: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliani R., Zoccarato M., Vianello M., Giometto B. (2008) Clinical, immunological and therapeutic aspects of autoimmune encephalitis. Recent Patents on CNS Drug Discovery 3: 16–22 [DOI] [PubMed] [Google Scholar]
- Voltz R., Gultekin S.H., Rosenfeld M.R., Gerstner E., Eichen J., Posner J.B., et al. (1999) A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med 340: 1788–1795 [DOI] [PubMed] [Google Scholar]
- White D., Beringer T. (2010) Paraneoplastic limbic encephalitis in an elderly patient with small cell lung carcinoma. Ulster Med J 79: 22–24 [PMC free article] [PubMed] [Google Scholar]
- Wong-Kisiel L.C., Ji T., Renaud D.I., Kotagal S., Patterson M.C., Dalmau J., et al. (2010) Response to immunotherapy in a 20-month old boy with anti NMDA receptor encephalitis. Neurology 74: 1550–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi M.S., Irani S.R., Follows G., Moody A.M., Molyneux P., Vincent A. (2009) Limbic encephalitis associated with antibodies to the NMDA receptor in Hodgkin lymphoma. Neurology 73: 2039–2040 [DOI] [PubMed] [Google Scholar]
- Zandi M.S., Irani S.R., Lang B., Waters P., Jones P.B., McKenna P., et al. (2011) Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 258(4): 686–688 [Epub 26 October 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani L., Tavolato B., Giometto B., Vincent A., Graus F. (2007) Paraneoplastic limbic encephalitis associated with potassium channel antibodies: value of anti-glial nuclear antibodies in indentifying the tumor. J Neurol Neurosurg Psychiatry 78: 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]