Abstract
Background
Cat ownership is inversely associated with atopy and asthma in some areas of the world, but the relevance of cat ownership to allergic disease in the inner city is less known.
Objective
We sought to evaluate the relationship between cat ownership and the development of early sensitization and wheeze.
Methods
By using a prospective birth cohort study, Dominican and African American mothers living in New York City underwent repeated questionnaires about their child from birth to age 5 years. Sera collected from children at ages 2 (n = 323), 3 (n = 336), and 5 (n = 242) years were assayed for anti-cat IgE and anti–Fel d 1 IgG antibodies.
Results
Cat ownership was a significant risk factor for the development of anti-cat IgE by age 2 years (risk ratio [RR], 6.4; 95% CI, 1.9-22) but not for anti-cat IgE development between the ages of 2 and 5 years (RR, 0.88; 95% CI, 0.24-2.3). Current wheeze was significantly more common among those children with anti-cat IgE at ages 3 (RR, 3.5; 95% CI, 2.1-6.0) and 5 (RR, 3.4; 95% CI, 2.3-4.9) years. Cat ownership was inversely associated with current wheeze at age 5 years among children without anti-cat IgE (RR, 0.26; 95% CI, 0.083-0.81). Among children with anti-cat IgE, a similar trend was observed (RR, 0.57; P = .044, Fisher exact test), although one with borderline statistical significance.
Conclusions
Despite a positive association with sensitization, cat ownership in this inner-city cohort was inversely associated with wheeze, potentially suggesting an IgE-independent protective mechanism in this community.
Keywords: Cat, asthma, allergy, wheeze, inner-city, rhinitis, IgE, IgG, Fel d 1
Allergen exposure is necessary for the development of allergic sensitization. For individuals with allergic sensitization, continued exposure to allergen exacerbates allergic disease. This dogma of allergic asthma has been suggested with ambient exposure to dust mite in most, but not all, studies.1-7 However, the dose-related immune response to allergens derived from cat exposure appears to be less straightforward. In several studies outside the United States, where the prevalence of cat ownership is low or rare (<5%), positive associations between either pet allergen in house dust7,8 or pet ownership9,10 and sensitization have been reported. However, many cross-sectional and longitudinal studies in areas with a greater frequency of pet ownership, such as the suburban United States, Sweden, and New Zealand, have demonstrated an inverse association between pet ownership and sensitization, asthma, or both, referred to as a “pet protective effect.”11-15 These different observations in the effect of cat ownership might reflect variations in community allergen exposure levels, pet-keeping practices, or the influence of additional concurrent environmental exposures.
The importance of cat exposure to US inner-city communities, where the prevalence of asthma is high and pet ownership is low when compared with the suburbs,16 has not been examined in depth. A previous cross-sectional study of adult asthmatic patients found that sensitization to cat was associated with asthma prevalence in New York City (NYC).17 This finding has not yet been confirmed in a longitudinal study, nor has the pet protective effect been evaluated in a strictly inner-city community.
We hypothesized that cat ownership would protect against the development of anti-cat IgE antibodies and wheeze symptoms in the first 5 years of life in an inner-city cohort. Using a prospective birth cohort study of children living in NYC neighborhoods with high asthma prevalence, our objectives were to (1) determine the levels of cat (Fel d 1) allergen in the house dust; (2) assess whether cat ownership was associated with sensitization to cat, wheeze, and rhinitis symptoms; and (3) determine the association between sensitization to cat allergen and wheeze and rhinitis symptoms in the first 5 years of life.
METHODS
Study cohort
As part of the Columbia Center for Children’s Environmental Health program, nonsmoking pregnant African American or Dominican women between the ages 18 and 35 years who were living in Northern Manhattan and the South Bronx were enrolled, as described previously.18-21 Exclusion criteria included a diagnosis of diabetes or HIV infection, reported illicit drug use, and residency in NYC for less than 1 year before pregnancy. Between the years 1998 and 2006, 2844 mothers were screened during pregnancy, and 725 were fully enrolled, which was defined as participation in prenatal assessments and cord blood collection at birth. Mothers who were screened were not fully enrolled because of several factors, most commonly (1) a refusal by the mother or father to participate in the study, (2) a loss of contact with the participant, (3) a lack of notification by the participant at the time of delivery or a delivery at a different hospital, and (4) failure to meet the study inclusion criteria.
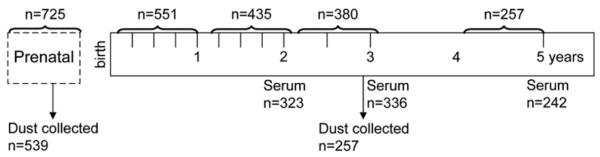
Detailed questionnaires were administered to the participants before the child was born and at ages 1, 2, 3, and 5 years. For this article, data were collected at ages 1, 2, 3, and 5 years on the children who had reached that age as part of this ongoing birth cohort (Fig 1 and Table I). This study was approved by Columbia University’s Institutional Review Board.
FIG 1.
Overview of the study. The hash marks refer to administration of symptom and exposure questionnaires. The brackets refer to the age of the child covered by the questionnaire.
TABLE I.
Demographics of cohort mothers at enrollment and children at age 5 years
| Mothers at enrollment (n = 725) | |
| Mean age (y [SD]) | 25.1 (4.9) |
| Race/ethnicity | |
| Dominican (%) | 65 |
| African American (%) | 35 |
| Asthma (%) | 25 |
| No high school degree (%) | 36 |
| Smoker in home (%) | 34 |
| Cat ownership (%) | 10 |
| Anti-cat IgE (%)* | 18 |
| Hardship last 6 mo (%)† | 45 |
| Children at age 5 y (n = 257) | |
| Male sex (%) | 47 |
| Ever lived in home with cat (%) | 25 |
| Ever lived in a home with a smoker (%) | 46 |
| Mother reported smoking during child’s lifetime (%) | 20 |
| Current wheeze at age 5 y (%)‡ | 28 |
| Ever wheeze by age 5 y (%)§ | 58 |
| Wheeze age 0-3 y but not age 5 y (%)∥ | 32 |
| Wheeze age 0-3 y and age 5 y (%)¶ | 21 |
Among the 280 mothers from whom serum was collected, 18% had anti-cat IgE (≥0.35 IU/mL).
Mother reported that in the past 6 months, she and her family could not afford needed food, rent, clothing, or medical care or that gas/electricity was suspended because of bill nonpayment.
Current wheeze at age 5 years is defined as reported wheeze in the last 12 months to the International Study of Asthma and Allergies in Childhood question.
Wheeze reported for the child during any questionnaire from birth to age 5 years.
Wheeze reported at least once between birth and age 3 years but not at age 5 years.
Wheeze reported at least once between birth and at age 5 years and at age 5 years.
Allergen measurements in the home
Dust samples were collected from the mother’s and child’s beds, respectively, during the prenatal and 3-year-old visits, as previously described.18,19,21 Fel d 1 was measured by using ELISA (Indoor Biotechnologies, Charlottesville, Va).22
Serum antibodies
IgE and IgG antibodies were measured in sera collected from the mother immediately postpartum and from the child at ages 2, 3, and 5 years (Fig 1). Specific IgE antibodies to cat dander/epithelium were measured with the ImmunoCAP system (Uppsala, Sweden). IgG antibodies to Fel d 1 were measured by means of the radioimmunoprecipitation assay, as previously described.13,23
Questionnaires
Detailed environmental exposures were assessed by questionnaire administered to the mother prenatally. At ages 1, 2, and 3 years, additional queries about environmental exposures and the child’s wheezing and rhinitis symptoms (runny nose and sneezing or itchy eyes without a cold) were made, with less detailed health follow-ups administered at 3, 6, 9, 15, 18, 21, and 30 months (Fig 1). A child was considered to have current wheeze (or rhinitis) if at least 1 episode of wheeze (or rhinitis) was reported during at least 1 of the interviews pertaining to that year of life (eg, at the 3-, 6-, 9-, or 12-month questionnaire for age 1 year). At age 5 years, the International Study of Asthma and Allergies in Childhood question regarding wheeze in the past 12 months was used to classify current wheeze.24 Cat ownership was defined as ever reporting cat ownership before the outcome of interest. Exposure to environmental tobacco smoke (ETS) likewise was defined as ever reporting a smoker in the home.
Statistics
Geometric means (95% CIs) were calculated for Fel d 1 allergen, with concentrations less than the limit of detection assigned a value of half the limit of detection. For bivariate analyses, risk ratios (RRs) were calculated with 95% CIs. The Fisher exact and χx2 tests were used when appropriate. When evaluating anti-cat IgE as a risk factor for wheeze, the presence of antibodies at the age of analysis was used (eg, the association between anti-cat IgE at age 3 years and wheeze at age 3 years was tested). To test the association between cat ownership and the development of IgE in a longitudinal fashion, anti-cat IgE levels were categorized into 3 categories: (1) no anti-cat IgE (<0.35 IU/mL) by age 5 years, (2) anti-cat IgE (≥0.35 IU/mL) at age 2 years, and (3) incidence of anti-cat IgE between ages 2 and 5 years. Incidence was defined as the presence of anti-cat IgE at age 5 years but not age 2 years. Because of the changing phenotype of wheeze with age (eg, associated more with atopy and more predictive of asthma as the child ages25), current wheeze was analyzed cross-sectionally at each year, with greater clinical significance attributed to findings related to current wheeze at age 5 years. Multiple logistic regression analyses for wheeze and rhinitis were performed, adjusting for ethnicity, sex, maternal asthma, and ETS in the home. The sample sizes for some of the regression analyses are slightly smaller than the bivariate analyses because of missing data on covariates. Data were analyzed with SPSS version 14.0 software (SPSS, Inc, Chicago, Ill).
RESULTS
Study population
Cat ownership was approximately 10% in the cohort at any one age; however, 25% of the children lived with a cat at some time before age 5 years (Table I). Very few of the participants reported owning multiple pets (0.5%-1.6% at any time point). Approximately 8% of the mothers reported not being able to afford their gas or electricity bill in the past 6 months. Among these mothers, the prevalence of cat ownership (3.1%) was lower compared with that of mothers who could afford gas or electricity (10.8%, P = .048).
Cat allergens in house dust and current wheeze
As reported elsewhere,26 the mean cat allergen level in homes with a cat (14.1 μg/g; 95% CI, 9.2-22) was almost 2 orders of magnitude higher than in homes without a cat (0.20 μg/g; 95% CI, 0.18-0.23). Fel d 1 was measurable (≥0.2 μg/g) in the bed dust of 46% of homes without a cat.
Children were classified into less than the limit of detection (<0.2 μg/g), low (≥0.2-<1 μg/g), or moderate/high (≥1 μg/g) exposure to Fel d 1 in the dust sample collected prenatally or at 3 years (based on the greater concentration of the 2 time points). There was no association between these categories of Fel d 1 in the bed dust and the prevalence of current wheeze at age 5 years, which was 24%, 31%, and 28%, respectively, for less than the limit of detection, low, and moderate/high Fel d 1 levels (P = .6).
Cat ownership, Fel d 1, and development of IgE and IgG
At ages 2, 3, and 5 years, respectively, 3.1%, 5.4%, and 15% of the children had measurable anti-cat IgE (≥0.35 IU/mL). Cat ownership was a significant risk factor for the development of anti-cat IgE (≥0.35 IU/mL) by age 2 years (RR, 6.4; 95% CI, 1.9-2.2) but not for incidence of anti-cat IgE between ages 2 and 5 years (RR, 0.88; 95% CI, 0.24-2.3). There were significant positive associations between Fel d 1 concentrations in the bed and the prevalence of IgE and IgG antibodies at all ages (Table II).
TABLE II.
Association between anti-cat IgE or anti–Fel d 1 IgG and cat ownership and dust Fel d 1 allergen
| Cat in home* |
Fel d 1 allergen in the bed dust at birth and age 3 y† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age (y) | Antibody response | Never | Ever | P value | <LOD | Low | Moderate/high | P value for trend |
| 2 y (n = 323)‡ | Anti-cat IgE (%) | 1.5 | 9.8 | .004 | 0.8 | 2.4 | 8.2§ | .005 |
| Anti–Fel d 1 IgG (%) | 1.9 | 18.0 | <.001 | 0.8 | 3.6 | 12.8 | <.001 | |
| 3 y (n = 336)‡ | Anti-cat IgE (%) | 3.4 | 12.3 | .006 | 3.7 | 4.8 | 10§ | .049 |
| Anti–Fel d 1 IgG (%) | 4.9 | 28.8 | <.001 | 2.2 | 8.2 | 25 | <.001 | |
| 5 y (n = 241)‡ | Anti-cat IgE (%) | 12.4 | 20.3 | .13 | 7.0 | 18 | 23§ | .003 |
| Anti–Fel d 1 IgG (%) | 7.2 | 37.5 | <.001 | 3.0 | 17 | 32 | <.001 | |
LOD, Limit of detection.
Child lived in a home with a cat ever between the prenatal period and the age at which the outcome was assessed.
Fel d 1 levels in the bed dust were classified as less than the limit of detection (<0.2 μg Fel d 1/g dust), low (≥0.2-<1 μg/g), and moderate/high (≥1 μg/g) based on the higher of the 2 Fel d 1 levels measured in the bed dust samples collected prenatally and at age 3 years. For the age 2-year analyses, 42%, 28%, and 29% of the children were categorized as having less than the limit of detection, low, and moderate/high Fel d 1 levels, respectively. Similar proportions were observed at ages 3 and 5 years.
Sample sizes for the analyses of the association between Fel d 1 in dust and anti-cat IgE were slightly lower than for the analyses with cat ownership as the exposure: 292, 308, and 221 for ages 2, 3, and 5 years, respectively.
As exploratory analysis, the moderate/high group was stratified further into moderate (≥1-10 μg/g) vs high (≥10 μg/g) Fel d 1 levels. IgE antibodies were higher in the moderate compared with the high group at age 2 (11% vs 5.3%), 3 (12% vs 7.9%), and 5 (28% vs 15%) years, although none of these differences were statistically significant.
Anti-cat IgE and current wheeze and rhinitis
Children with anti-cat IgE antibodies were more likely to have current wheeze and rhinitis at ages 3 and 5 years (Table III). The association between anti-cat IgE and current wheeze at age 5 years remained significant when anti-cockroach, mouse, and dust mite IgE were included in a logistic regression model (P = .004 for anti-cat IgE), as well as when controlling for sex, ETS exposure, ethnicity, maternal asthma, and maternal IgE.
TABLE III.
RR for current wheeze and rhinitis associated with anti-cat IgE
| Age (y) | N | Prevalence of symptom (%) |
RR for association with anti-cat IgE* |
|
|---|---|---|---|---|
| Current wheeze† | 2 | 320 | 25.6 | 0.77 (0.22-2.7) |
| 3 | 334 | 16.2 | 3.5 (2.1-6.0)∥ | |
| 5 | 194‡ | 28.4 | 3.4 (2.3-4.9)∥ | |
| Current rhinitis* | 2 | 321 | 32.4 | 1.6 (0.82-3.0) |
| 3 | 333 | 28.8 | 1.8 (1.1-2.9)§ | |
| 5 | 233 | 30.0 | 2.2 (1.5-3.2)∥ |
RRs represent the risk for current wheeze or rhinitis with the presence of anti-cat IgE at each age (dichotomous variable, ≥0.35 IU/mL considered positive).
Symptoms reported at least once in the preceding 12 months.
At age 5 years, there are fewer children evaluated for current wheeze than current rhinitis because administration of the International Study of Asthma and Allergies in Childhood question regarding current wheeze was not administered to the first 39 children to reach 5 years of age.
P < .05.
P < .01.
Cat ownership, current wheeze, and rhinitis
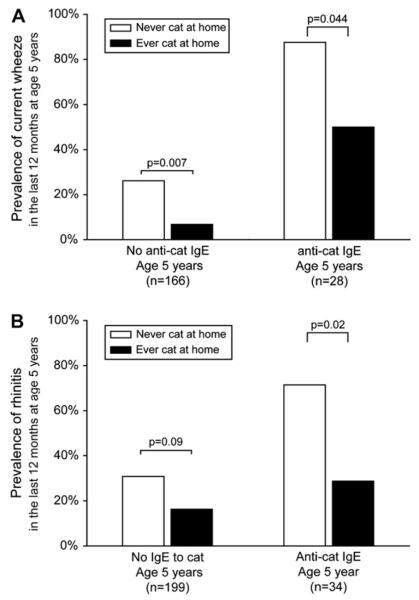
Cat ownership was inversely associated with current wheeze at age 5 years among children without anti-cat IgE (RR, 0.26; 95% CI, 0.083-0.81; P = .007). Among children with anti-cat IgE, a similar trend was observed (RR, 0.57; 95% CI, 0.32-1.03; P = .044, Fisher exact test), but the association was of borderline statistical significance (Fig 2, A). Those children who had IgE were also significantly less likely to have current rhinitis if they had a cat in the home (Fig 2, B).
FIG 2.
Prevalence of current wheeze (A) and current rhinitis (B) at age 5 years stratified by cat ownership and anti-cat IgE response. An IgE level of greater than or equal to 0.35 IU/mL was considered positive. Current wheeze and rhinitis were based on a report of symptoms at least once in the last 12 months at age 5 years.
The analyses also were stratified for atopy, which was defined as the child having any positive allergen-specific IgE (ie, >0.35 IU/mL to cockroach, Dermatophagoides farinae, mouse, cat, or dog). The nonatopic children were significantly less likely to have current wheeze (RR, 0.46; 95% CI, 0.22-0.96) or current rhinitis (RR, 0.49; 95% CI, 0.22-1.1) if there had been a cat in the home. Among atopic children, there was no association between cat ownership and current wheeze (RR, 0.84; 95% CI, 0.41-1.7) or rhinitis (RR, 0.88; 95% CI, 0.43-1.8).
Maternal asthma had a different influence on the risk for current wheeze and rhinitis associated with cat ownership. Specifically, among children without maternal asthma, a nonsignificant inverse association between current wheeze (age 5 years) and cat ownership was observed (RR, 0.58; 95% CI, 0.27-1.2). There was no association between cat ownership and current wheeze (RR, 1.02; 95% CI, 0.55-1.9) for those children with maternal asthma. Conversely, for current rhinitis, among children without maternal asthma, there was no association between cat ownership and current rhinitis (RR, 0.88; 95% CI, 0.51-1.5), and among children with maternal asthma, current rhinitis was significantly less common for children with cats at home (RR, 0.31; 95% CI, 0.11-0.90).
The association between cat ownership and the development of current wheeze and rhinitis was evaluated in adjusted logistic regression (Table IV). At age 5 years, but not at earlier ages, cat ownership was associated with a decreased risk of both current wheeze and rhinitis. Current wheeze at age 5 years remained negatively associated with cat ownership in the model when the following potential confounders were included (separately): mother reported inability to afford the electrical bill and prenatal mouse and prenatal cockroach allergens in the kitchen or bed dust. The magnitude of the association between cat ownership and current wheeze and rhinitis was not affected by inclusion of endotoxin in the model. As validation of the representativeness of the children who had reached age 5 years, the associations between cat ownership and current wheeze of all children monitored at ages 1 to 3 years were similar to those reported in Table IV when the analysis was restricted to the subset of children who had reached age 5 years (age 1 year: odds ratio [OR], 0.82 [95% CI, 0.38-1.8]; age 2 years: OR, 1.2 [95% CI, 0.51-3.0]; age 3 years: OR, 1.5 [95% CI, 0.60-3.9]).
TABLE IV.
Logistic regression models for the associations between cat ownership and current wheeze and rhinitis
| Adjusted model† |
Adjusted model including the covariate anti-cat IgE |
||||
|---|---|---|---|---|---|
| Age (y)* | n | Adjusted OR | n | Adjusted OR† | |
| Current wheeze | 1 | 583 | 1.1 (0.64-1.9) | — | — |
| 2 | 492 | 1.3 (0.71-2.4) | 278 | 2.0 (0.91-4.28) | |
| 3 | 369 | 1.7 (0.80-3.4) | 298 | 1.4 (0.58-3.3) | |
| 5 | 234 | 0.48 (0.21-1.1) | 186 | 0.16 (0.051-0.52)§ | |
| Current rhinitis | 1 | 583 | 1.0 (0.63-1.7) | — | — |
| 2 | 493 | 0.80 (0.45-1.9) | 279 | 0.61 (0.28-1.3) | |
| 3 | 367 | 1.0 (0.54-1.9) | 297 | 0.70 (0.34-1.4) | |
| 5 | 257 | 0.41 (0.19-0.89)‡ | 207 | 0.33 (0.14-0.80)‡ | |
Age of symptoms in the last 12 months (eg, current wheeze at 2 years reflects a report of wheeze in the second year of life). A report of at least 1 episode of wheeze was considered positive.
ORs are calculated by using logistic regression, including the covariates maternal asthma, sex, race/ethnicity, and ETS. The last column of ORs also include the covariate IgE to cat (≥0.35 IU/mL).
P ≤ .05.
P ≤ .01.
DISCUSSION
In this inner-city cohort cat ownership was positively associated with the development of anti-cat IgE antibodies by age 2 years. In addition, sensitization to cat was positively associated with current wheeze and rhinitis at ages 3 and 5 years. However, cat ownership was associated with protection against current wheeze and rhinitis at age 5 years. These findings, which contrast observations in suburban and rural cohorts, suggest that the natural history and clinical significance of sensitization to cat might differ between US inner-city and other cohorts.
The positive association between cat exposure and sensitization was opposite from our a priori hypothesis. Although many prospective studies have reported an inverse association between cats in the home and the development of sensitization,11-13,27 a positive association between cat allergen and sensitization was reported recently in 2 European prospective studies. Both found a nonlinear increase in the risk for sensitization to cat with Fel d 1 exposure at birth7,8 but no association between pet ownership and sensitization. The authors point to a relatively lower community prevalence of cat ownership as possibly leading to the difference in their findings. A cross-sectional study from Kuwait, where pet ownership was even less common, found a positive association between cat ownership and sensitization to cat.9
These studies and findings from the European Community Health Survey suggest that the community prevalence of cat ownership might influence the likelihood of the pet protective effect.28 In communities with low cat ownership, non–cat owners at high risk for atopy, in contrast to cat owners, might not be exposed to sufficient cat allergen to experience an allergic response. Cat owners might be the only ones with sufficient exposure to permit sensitization. This could explain the relationship between cat ownership and sensitization in our NYC cohort, where cat ownership is relatively infrequent. Cat ownership was a risk for sensitization by age 2 years but not subsequent incidence of sensitization over the next 3 years, when older children might become exposed to cat allergen while spending time outside of the home.
Alternatively, lower community cat allergen levels might not be sufficient to result in protection from development of sensitization among cat owners. The mean levels of domestic Fel d 1 among cat owners in communities with low cat ownership (eg, NYC, Germany, and Kuwait) have been less than levels of Fel d 1 in cat owners’ homes in communities where cat ownership was more common (eg, Virginia, Sweden, and New Zealand).15,26,27 In the recent European studies in which an increased risk for sensitization with Fel d 1 exposure was observed, few of the homes had Fel d 1 levels as high (>20 μg/g) as the protected group from the study by Platts-Mills et al.7,8 Nonlinear trends of increased risk for sensitization with cat allergen exposure in Germany and NYC (ie, decreasing with the highest exposure; see footnote in Table II) could lend support to this theory but have not been adequately evaluated in these studies because of the low number of highly exposed children.8 The reason for lower cat allergen levels in the dust of homes with cats in different communities is not clear but could be due to housing characteristics or pet-keeping habits.
Sensitization to cat was a strong risk factor for current wheeze and rhinitis in this cohort. These associations remained significant even after controlling for sensitization to cockroach and mouse, often considered the dominant allergens for inner-city asthma.29 Elsewhere, very early sensitization to cat also has recently been shown to be associated with subsequent decreased lung function at age 13 years, an association further aggravated by exposure to perennial allergens.4
A novel finding is that cat ownership appeared to protect against current wheeze and rhinitis at age 5 years, despite strong associations between (1) cat ownership and sensitization and (2) sensitization to cat and current wheeze and rhinitis. Similarly, a birth cohort study in metropolitan Boston reported a strong association between sensitization to cat and airway hyperreactivity at age 7 years (OR, approximately 15) but no association between current cat exposure and airway hyperreactivity among sensitized children.30 These findings seem to indicate that in some populations a pet protective effect might be modulated through a non–IgE-mediated pathway. The mechanism of this pathway is not known but could be due to immune responses to other concurrent exposures associated with domestic cats (eg, endotoxin) or related to the immunostimulatory capability of the cat protein Fel d 1. Reefer et al31 have demonstrated that peptides from chain 2 of Fel d 1 bind T-cell epitopes selectively to induce CD4+ cell production of IL-10, regardless of the presence of an anti-cat IgE response. Moreover, increased IL-10 production also has been observed with cat allergen immunotherapy, mediating T-cell hyporesponsiveness.31,32 These results suggest a possible role for IL-10–secreting T-regulatory cells in the control of the immune response to cat and possibly peripheral tolerance. Increased plasma IL-10 concentrations were observed during the pollen season in adults with anti-pollen IgE but without allergic rhinitis symptoms, possibly indicating a role for IL-10 in the suppression of symptoms among exposed atopic individuals.33
Endotoxin exposure related to pet ownership has been suggested as the mechanism of the pet protective effect. Although endotoxin exposure might be related to rural exposure to pets and other farm animals, Litonjua et al14 found that in Boston the effects of pet and endotoxin exposure were separate and contrary. Similarly, although endotoxin levels in this NYC cohort were related to wheeze and rhinitis,21 they neither were related to pet ownership nor affected the associations between pet ownership and wheeze/rhinitis in logistic regression.
A clinically important examination was testing the association between cat exposure and current wheeze stratified by sensitization to cat. Although the association among the children without anti-cat IgE was strongly inverse, the association among those children with anti-cat IgE (15% of the children) was only of borderline statistical significance (P = .044, Fisher exact test; 95% CI, 0.32-1.03). Hence definitive conclusions about whether cat ownership is protective against current wheeze among catsensitized children cannot be made from this study. However, a positive association between cat ownership and current wheeze among those sensitized also was absent, questioning the validity of the recommendation to avoid cats at young ages in an effort to reduce wheeze.
There are several limitations of our study. First, all nonintervention studies of the pet protective effect are subject to potential confounding by who chooses to own a pet.13,34 Although there were no differences in cat ownership by maternal asthma status, there were differences by race/ethnicity. Still, significant associations remained after adjustment for race/ethnicity in regression models and were observed at the same magnitude when examined in the African American group alone. Socioeconomic status was also a potential confounder because the very poor were less likely to own a cat and potentially more likely to wheeze. Inclusion of material hardship variables did not affect the association between pet ownership and allergic symptoms.
A second concern might be a possible selection bias given the difference in composition of the cohort monitored at different ages and the lack of an association with current wheeze at these ages in contrast to age 5 years. Yet when the analyses were restricted to only the children who had reached age 5 years, there were still no significant associations between current wheeze and cat ownership at ages 1, 2, or 3 years.
A third limitation was a lack of statistical power to examine dog ownership and asthma given the low frequency of dog ownership. Additionally, the low number of children in homes with very high cat allergen levels prevented comparison of these cohort children with those exposed to similarly high levels in Sweden, New Zealand, and the suburban United States. It is important to point out that in this study IgE was measured against a cat extract, whereas IgG was only measured against Fel d 1.
Finally, our sample size was insufficient to evaluate the critical time period of cat ownership (ie, first year vs later) for protecting against current wheeze and rhinitis.
In conclusion, in a cohort of inner-city children with high asthma risk, a strong positive association between sensitization to cat and current wheeze and rhinitis at ages 3 and 5 years was found. Unlike findings from other prospective studies, cat ownership was a positive risk factor for the development of sensitization early in life. Despite these associations, children in homes with a cat were less likely to have current wheeze or rhinitis at age 5 years, a finding probably more pronounced among those children who were not sensitized to cat. The current findings suggest a potential non–IgE-associated mechanism of the pet protective effect on current wheeze and rhinitis at age 5 years.
Clinical implications.
Sensitization to cat was associated with inner-city wheeze. Paradoxically, cat ownership was both positively associated with early sensitization and protective against current wheeze at age 5 years.
Acknowledgments
We thank the participating mothers and children. This work would not have been possible without the hard work and dedication of the research workers and field technicians. We also thank Lisa Naccara at the University of Virginia and Kazim Panjwani at Columbia University for assistance with assays.
Supported by the National Institute of Environmental Health Sciences (grants R03ES013308, P01 ES09600, 5 RO1 ES08977, and P30 ES009089), the US Environmental Protection Agency (grants R827027 and RD-832141), the Irving General Clinical Research Center (grant RR00645), the Bauman Family Foundation, the Gladys and Roland Harriman Foundation, the New York Community Trust, the Educational Foundation of America, the New York Times Company Foundation, the Horace W. Goldsmith Foundation, the John Merck Fund, the Johnson Family Foundation, the Marisla Foundation, and Trustees of the Blanchette Hooker Rock-efeller Fund.
Abbreviations used
- ETS
Environmental tobacco smoke
- NYC
New York City
- OR
Odds ratio
- RR
Risk ratio
Footnotes
Disclosure of potential conflict of interest: M. S. Perzanowski has received research support from the National Institutes of Health. G. L. Chew has received research support from the National Institutes of Environmental Health Sciences. I. F. Goldstein has received research support from the National Institutes of Environmental Health Sciences and the National Institutes of Health. T. A. E. Platts-Mills has served on the advisory board for Indoor Biotechnologies and has received research support from Phadia and the National Institutes of Health. R. L. Miller has received research support from the National Institutes of Health, the US Environmental Protection Agency, and the Sandler Program for Asthma Research and has served as a member of the American Thoracic Society.
The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 2.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141–6. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 3.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100(suppl):S2–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 4.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 5.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–7. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 6.Brussee JE, Smit HA, van Strien RT, Corver K, Kerkhof M, Wijga AH, et al. Allergen exposure in infancy and the development of sensitization, wheeze, and asthma at 4 years. J Allergy Clin Immunol. 2005;115:946–52. doi: 10.1016/j.jaci.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Torrent M, Sunyer J, Munoz L, Cullinan P, Iturriaga MV, Figueroa C, et al. Early-life domestic aeroallergen exposure and IgE sensitization at age 4 years. J Allergy Clin Immunol. 2006;118:742–8. doi: 10.1016/j.jaci.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 8.Lau S, Illi S, Platts-Mills TA, Riposo D, Nickel R, Gruber C, et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat-specific IgG and development of asthma in childhood—report of the German Multicentre Allergy Study (MAS 90) Allergy. 2005;60:766–73. doi: 10.1111/j.1398-9995.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mousawi MS, Lovel H, Behbehani N, Arifhodzic N, Woodcock A, Custovic A. Asthma and sensitization in a community with low indoor allergen levels and low pet-keeping frequency. J Allergy Clin Immunol. 2004;114:1389–94. doi: 10.1016/j.jaci.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Almqvist C, Egmar AC, Hedlin G, Lundqvist M, Nordvall S, Pershagen G, et al. Direct and indirect exposure to pets—risk of sensitization and asthma at 4 years in a birth cohort. Clin Exp Allergy. 2003;33:1190–7. doi: 10.1046/j.1365-2222.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- 11.Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29:611–7. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 12.Ownby D, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 and 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 13.Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166:696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 14.Litonjua AA, Milton DK, Celedon JC, Ryan L, Weiss ST, Gold DR. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002;110:736–42. doi: 10.1067/mai.2002.128948. [DOI] [PubMed] [Google Scholar]
- 15.Erwin EA, Wickens K, Custis NJ, Siebers R, Woodfolk J, Barry D, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol. 2005;115:74–9. doi: 10.1016/j.jaci.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Asthma facts. 2nd ed The City of New York Department of Health and Mental Hygiene; New York: 2003. [Google Scholar]
- 17.Lin RY, LaFrance J, Sauter D. Hypersensitivity to common indoor aeroallergens in asthmatic patients. Ann Allergy. 1993;71:33–9. [PubMed] [Google Scholar]
- 18.Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. 2001;164:995–1001. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- 19.Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111:1348–51. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein IF, Perzanowski MS, Lendor C, Garfinkel RS, Hoepner LA, Chew GL, et al. Prevalence of allergy symptoms and total IgE in a New York City cohort and their association with birth order. Int Arch Allergy Immunol. 2005;137:249–57. doi: 10.1159/000086338. [DOI] [PubMed] [Google Scholar]
- 21.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–9. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luczynska CM, Li Y, Chapman MD, Platts-Mills TA. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990;141:361–7. doi: 10.1164/ajrccm/141.2.361. [DOI] [PubMed] [Google Scholar]
- 23.Platts-Mills TA, Snajdr MJ, Ishizaka K, Frankland AW. Measurement of IgE anti-body by an antigen-binding assay: correlation with PK activity and IgG and IgA antibodies to allergens. J Immunol. 1978;120:1201–10. [PubMed] [Google Scholar]
- 24.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 25.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–76. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 26.Perzanowski MS, Ronmark E, Nold B, Lundback B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103:1018–24. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 27.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 28.Svanes C, Heinrich J, Jarvis D, Chinn S, Omenaas E, Gulsvik A, et al. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. J Allergy Clin Immunol. 2003;112:289–300. doi: 10.1067/mai.2003.1596. [DOI] [PubMed] [Google Scholar]
- 29.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Tepas EC, Litonjua AA, Celedon JC, Sredl D, Gold DR. Sensitization to aeroallergens and airway hyperresponsiveness at 7 years of age. Chest. 2006;129:1500–8. doi: 10.1378/chest.129.6.1500. [DOI] [PubMed] [Google Scholar]
- 31.Reefer AJ, Carneiro RM, Custis NJ, Platts-Mills TA, Sung SS, Hammer J, et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004;172:2763–72. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- 32.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Bubnoff D, Fimmers R, Bogdanow M, Matz H, Koch S, Bieber T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clin Exp Allergy. 2004;34:1056–63. doi: 10.1111/j.1365-2222.2004.01984.x. [DOI] [PubMed] [Google Scholar]
- 34.Almqvist C, Egmar AC, van Hage-Hamsten M, Berglind N, Pershagen G, Nordvall SL, et al. Heredity, pet ownership, and confounding control in a population-based birth cohort. J Allergy Clin Immunol. 2003;111:800–6. doi: 10.1067/mai.2003.1334. [DOI] [PubMed] [Google Scholar]