Abstract
It is clear that the microenvironment or niche plays an important role in determining the fate of stem cells: being stem cells or differentiated. However, the intrinsic pathways controlling the fate of adult stem cells in different niches are largely unknown. This study was to explore the role of β-catenin/Tcf4/survivin signaling in determining the fate of human corneal epithelial stem cells in different media. We observed that the low calcium serum-free media, especially CnT-20, promoted proliferative capacity, colony forming efficiency and stem cell-like phenotype of human corneal epithelial cells (HCECs) when compared with the cells cultured in a high calcium serum-containing medium SHEM. Three key factors in Wnt signaling, β-catenin, Tcf4 and survivin, were found to be expressed higher by HCECs grown in CnT-20 than those cultured in SHEM, as evaluated by real-time PCR, Western blotting and immunostaining. Transfection of siRNA-Tcf4 at 10-50 nM knocked down Tcf4, and also significantly suppressed its down stream molecule survivin at both mRNA and protein levels in HCECs. Furthermore, Tcf4 silencing significantly suppressed the proliferative capacity of HCECs, measured by WST-1 assay, compared with the control groups, untreated or transfected with non-coding sequence siRNA-fluorescein. These findings demonstrate that low calcium serum free media promote ex vivo expansion of corneal epithelial progenitor cells that retain a less differentiated phenotype and high proliferative capacity via β-catenin/Tcf4/survivin signaling, a novel intrinsic pathway. This study may have high impact and clinic implication on the expansion of corneal epithelial stem cells in regenerative medicine, especially for ocular surface reconstruction.
Keywords: adult stem cell, stem cell niche, corneal epithelium, β-catenin, Tcf4, survivin
INTRODUCTION
The ocular surface is an ideal region to study epithelial stem cell biology because of the unique spatial arrangement of stem cells and transient amplifying cells [1-4]. The corneal epithelial stem cells have been identified to reside in the basal layer of limbal epithelium over last two decades. Limbal epithelial stem cells exhibit unique characteristics that satisfy the widely accepted criteria for defining adult stem cells, including (1) slow cycling or long cell cycle time during homeostasis in vivo; (2) small size and poor differentiation with primitive cytoplasm; (3) high proliferative potential after wounding or placement in culture; (4) ability for self-renewal and functional tissue regeneration (see review articles by[5-8]). Both intrinsic and extrinsic signals regulate stem cell fate including adult stem cells. Through interaction with intrinsic signals, the extrinsic niche or the stem cell microenvironment is believed to be important in maintaining the “stemness” of the stem cells, including corneal epithelial stem cells [9-12]. For example, it is well known that low calcium, serum-free culture media can provide an ideal niche in vitro to maintain or promote progenitor cell properties, such as proliferative capacity and undifferentiation status [13-16], while high calcium and serum-containing media promote cell differentiation [17-19]. However, the underlining molecular mechanisms by which the niche determines the stem cell fate are far from being completely elucidated.
Wnt signaling pathway has been recognized to control a variety of functions and properties in various types of stem cells. Wnt signaling can be activated by niche factors to maintain stem cells in a self-renewing state [20-22]. During tissue development and regeneration, Wnt signals ensure the proper balance between proliferation and differentiation [23-25]. Wnt proteins are active in a variety of stem cells, including embryonic, hematopoietic, neural and mammary stem cells, as well as corneal epithelial stem cells [20, 26, 27]. The hallmark of the Wnt signaling pathway is the accumulation of the junctional protein β-catenin in the cytoplasm, which then translocates to the nucleus to trigger the β-catenin/Tcf enhancer factor transcriptional machinery, and upregulate target genes, such as survivin and c-myc [28-30]. A classic example of the importance of this pathway is in the digestive tract, where in the crypt of the colon the loss of transcription factor T cell factor 4 (Tcf4), a key factor of canonical Wnt signaling pathway, leads to depletion of stem cells [30, 31]. After activation by β-catenin/Tcf4 complex, survivin enhances cell proliferation while protecting cells from apoptosis [32, 33]. Recently, Tcf4 and Tcf3 have been found to play a vital role in long-term maintenance and wound repair of both epidermis and hair follicles [34]. However, the role of the Wnt pathway, particularly, β-catenin/Tcf4/survivin signaling in maintaining the properties of adult stem cells has not been elucidated. The purpose of present study was to explore the important role of Tcf4 signaling in determining the fate of corneal epithelial stem cells, using an in vitro culture model with different media providing niche factors: low calcium and serum free versus high calcium and serum containing.
MATERIALS AND METHODS
Materials and reagents
Cell culture dishes, plates, centrifuge tubes, and other plastic ware were purchased from Becton Dickinson and Company (Franklin Lakes, NJ). Nunc Lab-Tec II eight-chamber slides were from Nalge Nunc International Corp (Naperville, IL). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). CnT-20 and CnT-50 progenitor media were from Chemicon International (Temecula, CA). Dulbecco modified Eagle's medium (DMEM), Ham F-12, Keratinocyte-SFM (KSFM) and Defined KSFM (D-KSFM), amphotericin B, gentamicin, 0.25% trypsin/EDTA solution, mouse monoclonal antibody (mAb) against connexin 43 (Cx43), and fluorescein Alexa-Fluor 488 conjugated secondary antibodies (Donkey anti-Goat IgG, Goat anti-rabbit or Goat anti-mouse IgG) were from Invitrogen Corp (Carlsbad, CA). Human AE5/keratin (K) 3 mAb and goat antibodies against human Tcf4 and survivin were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Rabbit antibodies against β-catenin and β-actin were from Cell Signaling Technology (Beverly, MA). Human p63 (4A4), integrin β1 and EGFR mAbs were from Lab Vision (Fremont, CA). HRP conjugated secondary antibodies (goat anti-mouse, goat anti-rabbit and rabbit anti-goat for western blot were from Thermo Scientific (Fremont, CA). Ready gels, Precision Plus Protein Unstained Standards and Precision Protein Streptactin-AP Conjugate came from Bio-Rad (Hercules, CA). The BCA protein assay kit was from Pierce Chemical (Rockford, IL). RNeasy® Mini kit, siRNA-F and HiperFect transfection reagent were from Qiagen (Valencia, CA). Ready-To-Go You-Prime First-Strand Beads were from GE Healthcare (Piscataway, NJ). TaqMan® Gene Expression Assay, real-time PCR Master Mix, and Silencer Select® pre-designed small interfering RNA (siRNA) were from Applied Biosystems (Foster City, CA). WST-1 proliferative assay kit was from Roche Molecular Biochemicals (Mannheim, Germany). Human insulin, transferrin, sodium selenite, hydrocortisone, epidermal growth factor (EGF), cholera toxin A subunit, propidium iodide (PI) and all other reagents came from Sigma-Aldrich (St. Louis, MO).
Human corneal epithelial cell (HCEC) cultures in different media
Primary HCECs were cultured from donors' limbal tissue explants using a previously described protocol [35, 36]. In brief, the limbal ring is cut into 12-16 pieces with similar size of approximately 2×2mm each. Two pieces with the epithelium side up were directly put into a well of 6-well plate or one piece per chamber in 8-chamber slides. Low calcium and serum free progenitor cell culture media, CnT-20, CnT-50, KSFM and D-KSFM, and high calcium serum-containing supplemental hormonal epithelial medium (SHEM) were used for cultures at 37°C under 5% CO2 and 95% humidity. The media were changed every 2-3 days.
Corneal epithelial cell growth was carefully observed and photographed through a Nikon TE200 inverted phase microscope with a Nikon DXM1200 digital camera. Only the epithelial cultures without visible fibroblast contamination were used for this study. When grown to 90% confluence, the cultures were photographed and trypsinized with 0.25%trypsin/0.03%EDTA; and the cells were seeded into a new plate at a density of 2×104 cells/cm2 for serial passages.
Colony forming efficiency (CFE) and growth capacity
To evaluate proliferative capacity of corneal epithelial cells in different culture media, the CFE was assessed in cultures in CnT-20 or SHEM using a previous method [37-39] with modification. Primary human corneal epithelial cells were seeded in triplicate at 500 cells/cm2 into six-well culture plates without 3T3 fibroblasts or any other cells as a feeder layer. Colonies with more than eight viable cells were counted manually under an inverted phase microscopy at days 6 and 8. Experiment was repeated at least three times. The CFE in SHEM or CnT-20 was calculated as a percentage of the number of colonies generated by the number of epithelial cells plated in a well. The growth capacity was evaluated on day 14 when cultured cells were stained with 1% rhodamine.
RNA interference
To explore the functional role of Tcf4 signaling, RNA interference experiments were performed using our previous method [40, 41] with modification. In brief, primary HCECs at a density of 5×104 cells/cm2 were transfected with annealed double-stranded siRNA specific for Tcf4 (siRNA-Tcf4, ID. s13863 containing a pool of 3 target-specific 20-25 nt siRNAs) at different concentrations (10nM, 25nM, 50nM), with a non-coding sequence siRNA-fluorescein (siRNA-F, UUCUCCGAACGUGUCACGU) as a negative control (also serve as visible monitor for transfection efficiency) using fast-forward transfection method with HiperFect reagent according to a manufacturer's protocol. The transfection efficiency in HCECs with different concentrations of siRNA-F (10, 25 and 50 nM) after 24 hours were 81.4±3.5%, 83.5±4.1% and 87.2±4.3%, respectively, as analyzed by flow cytometry. After incubation for additional 24-72 hours, the cells were collected for RNA extraction or protein lysate preparation for further evaluation. The cell viability after transfection for 4 days was more than 90%, as assessed by a 0.2% trypan blue exclusion test and morphological observation.
WST-1 cell proliferation assay for Tcf4 knock-down HCECs
Primary HCECs were transfected by siRNA-Tcf4 at final concentrations at 10nM, 25nM and 50nM, with a non-coding sequence siRNA-fluorescein (siRNA-F) as a negative control, using HiperFect reagent according to a manufacturer's protocol. In brief, siRNA-Tcf4 mixed with HiPerFect transfection reagent was spotted in the wells of 96-well plate, and incubated for 5-10 min at room temperature. Primary HCECs in CnT-20 were seeded at density of 6,000/well on the top of the siRNA–Hiperfect reagent complex and cultured for 48 hours. WST-1 proliferative assay [42, 43] was assessed according to the manufacturer's protocol. Briefly, 10μl of cell proliferation agent WST-1 was added to each well containing 100 μl cell culture. The cells were incubated for additional 2 hours at 37°C in a 5% CO2 atmosphere. The plate was measured at 450nm with a reference wavelength 690 nm in an Infinite M200 multimode microplate reader (Tecan US, Durham NC). The experiment for cell proliferation assays were repeated 5 times.
Immunofluorescent staining and laser scanning confocal microscopy (LSCM)
Immunofluorescent staining was performed following a previously reported method [44]. In brief, cells were fixed with methanol at 4°C for 10 minutes and permeabilized with 0.2% Triton-X in PBS for 10 minutes. After blocked with 20% of animal serum in PBS for 30 minutes, a primary antibody was applied and incubated for two hours at room temperature. Alexa Fluor 488 conjugated secondary antibody was then applied for one hour in dark, followed by counterstaining with a DNA binding dye propidium iodide (PI, 1μg/mL in PBS) for 5 minutes. A cover slip was applied with Antifade Gel/Mount (Fisher, Atlanta, GA). Slides were examined and photographed with the LSCM (LSM 510, Zeiss, Thornwood, NY).
Total RNA Extraction, Reverse transcription (RT), and Quantitative Real-Time PCR
Total RNA was extracted from HCECs using a Qiagen RNeasy® Mini kit according to manufacturer's protocol, quantified by NanoDrop® ND-1000 Spectrophotometer, and stored at −80 °C. The first strand cDNA was synthesized by RT from 1μg of total RNA using Ready-To-Go You-Prime First-Strand Beads, and the real-time PCR was performed in the Mx3005PTM system (Stratagene) [45, 46]. TaqMan® Gene Expression Assays include β-catenin (Assay ID Hs99999168_m1), Tcf4 (Hs00162613_m1), survivin (Hs00153353_m1), p63 (Hs00186613_m1), EGFR (Hs00193306_m1), integrin β1 (Hs00236976_m1), Cx43 (Hs00748445_s1), K3 (Hs00365080_m1) and GAPDH (Hs99999905_m1). The thermocycler parameters were 50 °C for 2min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1min. A non-template control was included to evaluate DNA contamination. The results were analyzed by the comparative threshold cycle (CT) method and normalized by a housekeeping gene GAPDH [47, 48].
Western Blotting Assay
Western blot analysis was performed by a previous method [49, 50]. In brief, HCECs cultured in CnT-20 or SHEM were lysed with RIPA buffer. Equal amount (30μg/well) of cell extract protein, measured by a BCA protein assay kit, was mixed with 6×SDS reducing sample buffer and boiled for 5 minutes before loading. The proteins were separated on an SDS polyacrylamide gel and transferred electronically to PVDF membranes. The membranes were blocked with 5% nonfat milk in TTBS (50 mM Tris [pH 7.5], 0.9% NaCl, and 0.1% Tween-20) for 1 hour at room temperature and incubated with primary antibodies to β-cantenin (1:1000), Tcf4 (1:100), survivin (1:100) and β-actin (1:2000) at 4°C overnight. After three times washes with TTBS, the membranes were incubated with HRP conjugated rabbit anti-goat, goat anti-mouse or goat anti-rabbit IgG (1:2000) for 1h at room temperature. The signal bands were detected with an ECL Plus chemiluminescence reagent (GE Healthcare), and the images were acquired by a Kodak Imaging Station 2000R (Eastman Kodak, New Haven CT).
Statistical analysis
The Student's t-test or analysis of variance (ANOVA) with Tukey's post-hoc testing was used for statistical comparisons. p≤ 0.05 was considered statistically significant. All of these tests were performed using the GraphPad Prism 5.0 software (Graph-Pad Prism, Inc., San Diego, CA, http://www.graphpad.com).
RESULTS
Proliferative capacity of human corneal epithelial cells promoted by low calcium serum free media
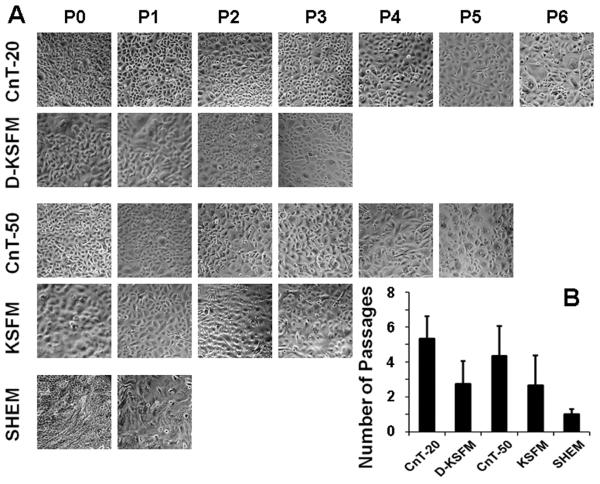
Primary HCECs were established from limbal explants using five different culture media, four low calcium and serum free progenitor cell culture media (CnT-20, CnT-50, KSFM and D-KSFM) and a high calcium serum-containing SHEM. All media were capable of supporting primary epithelial cell growth. When cells grew to subconfluent (80-90% confluence), serial passages were performed. As shown in Fig. 1. HCECs grown in SHEM, a high calcium and serum containing medium, could not be passaged more than once or twice although SHEM supported rapid and abundant cell growth in the primary culture. In contrast, higher proliferative capacity was observed in HCECs cultured in all low calcium and serum free media. For examples, cells cultured in fully defined CnT-20 were capable of being passaged six or more times, and cells grown in CnT-50 that contains bovine pituitary extracts (BPE) could be serially passaged at least 5 passages. HCECs in defined D-KSFM or BPE-containing KSFM were able to be passaged 3-4 times. CnT-20 was chosen for further studies to represent a low calcium and serum free in vitro niche known to promote epithelial progenitor cells.
Fig. 1.
Serial passaging capacity of HCECs in different media. A. Representative phase images showing primary HCECs (P0) from limbal explants and their serial passages (P1, P2… or P6) in five different media at confluence; B. Each column indicates the mean number of possible passages made by HCECs in each medium from 4 separate experiments (n=4).
The growth pattern and proliferative capacity of HCECs cultured in CnT-20 or SHEM were further compared. HCECs cultured in CnT-20 spread out from explants as single cells. The cells showed typical epithelial cell morphology with a small round or oval appearance. In comparison, the cells from limbal explants cultured in SHEM expanded like a sheet and stratified after reaching confluence. All cells were tightly packed and highly elongated (Fig. 2A). To evaluate their clonal growth capacity, HCECs were seeded at a density of 500 cells/cm2 in CnT-20 or SHEM without a feeder layer to assess colony forming efficiency (CFE). SHEM could support HCECs to form a few colonies that failed to grow continually without 3T3, a feeder layer (data not shown). In contrast, HCECs in CnT-20 generated a significant number of colonies without use of a feeder layer (Fig. 2B). As shown in Fig. 2C, the CFE of HCECs in CnT-20 reached 2.5±1.3 % at day 6 and 5.4±1.2 % at day 8. Even without any feeders, the colonies in CnT20 expanded rapidly to almost confluence on day 14 (Fig. 2D). The CFE numbers and clonal growth capacity of HCECs in CnT20 was similar to that observed with isolated corneal epithelial progenitor cells grown on a 3T3 feeder layer in previously reported studies by our group [36, 38, 39, 51]. These results indicate that the single small round cells grown in low calcium and serum free defined CnT-20 media primarily consisted of the expanded human corneal progenitor cells, although the cell morphology in CnT-20 medium was also changing with the number of large cells increased in every passage, indicating a gradual differentiation with every passage (Fig. 1).
Fig. 2.
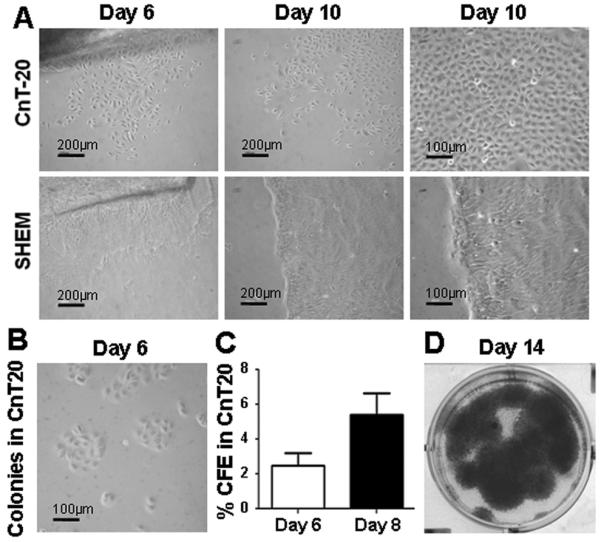
Growth pattern and capacity of HCECs in CnT-20 or SHEM medium. A. Representative phase images showing primary HCECs (P0) growing from limbal explants in CnT-20 or SHEM at Days 6 and 10 (with low and high magnification); B. Clonal growth of HCECs at day 6 in CnT-20 without feeder layer; C. Percentage colony-forming efficiency (CFE) of HCECs at days 6 and 8 in CnT-20 without feeder layer. Shown as mean ± standard deviation, n=3; D. Representative cultures stained with 1% rhodamine showing growth capacity of HCECs at day 14 in CnT-20 without feeder layer.
Progenitor phenotype of human corneal epithelial cells cultured in low calcium serum free media
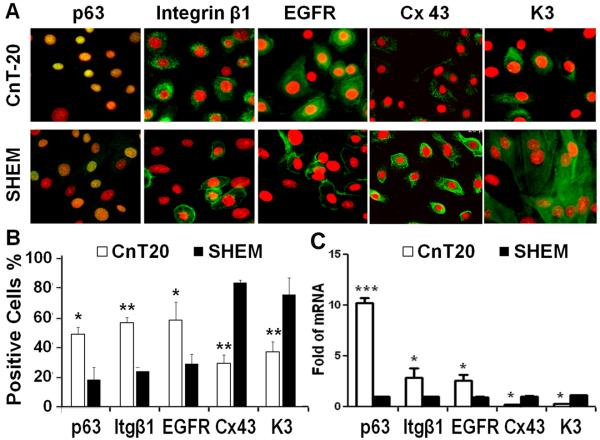
Phenotypic characterization of the primary HCECs cultured in CnT-20 and SHEM was assessed using immunofluorescent staining with stem cell associated markers, p63, integrin β1 and EGFR, as well as with differentiation markers, connexin 43 and K3. As shown in Fig. 3 A and B, HCECs in CnT-20 possessed a significantly higher percentage of p63-positive (p<0.05, n=3), integrin β1-positive (p<0.01, n=3) or EGFR-positive cells (p<0.05, n=3) than the cells cultured in SHEM. In contrast, the percentages of positively immunoreactive cells for connexin 43 and corneal epithelial specific marker K3 were significantly lower in HCECs cultured in CnT-20 than in SHEM (both p<0.01, n=3).
Fig. 3.
Cell phenotype of HCECs grown in CnT-20 or SHEM medium. A. Representative images of Immunofluorescent staining of stem cell associated markers, p63, Integrin β1 (Itg β1) and EGFR, as well as differentiation markers, Connexin (Cx) 43 and K3 in primary HCECs cultured in CnT-20 or SHEM; Color code: Red is PI; Green is p63, Itg β1, EGFR, Cx43 or K3; and Yellow is green p63 + red PI. B. Percentages of positive cells stained for each marker in primary cultures grown in CnT-20 and SHEM; C. Quantitative real-time PCR data showing the expression levels (relative fold of mRNA) of these markers by HCECs in CnT-20 or SHEM. Data shown as mean ± standard deviation, n=3; *p <0.05, **p < 0.01, ***p < 0.001.
The expression of these markers was further confirmed at the transcriptional level by reverse transcription and quantitative real-time PCR, as shown in Fig. 3C. With GAPDH as an internal control, the mRNA expression of stem cell associated markers, p63, integrin β1 and EGFR, were significantly higher by the cells cultured in CnT-20 than in SHEM. In particular, levels of p63 mRNA were 10 fold higher (p<0.001, n=3) in HCECs cultured in CnT20 than those in SHEM. Interestingly, levels of mRNA transcripts for the differentiation markers, connexin 43 and K3, were detected at much lower levels (both p<0.01, n=3) in the cells cultured in CnT-20, compared to cells in SHEM. These data confirmed the progenitor cell phenotype of HCECs cultured in CnT20, as revealed by immunofluorescent staining. Taken together, results of cell growth, passage capacity, colony forming efficiency and phenotypic markers, as shown in Figs 1-3, indicate that this low calcium serum free media can function as an in vitro niche that maintains the progenitor properties of limbal stem cell-derived corneal epithelial cells.
Expression of β-catenin, Tcf4 and survivin in human corneal epithelial progenitor cells
In order to uncover what intrinsic signaling is activated by the extrinsic factors in the niche media, we compared the expression and function of β-catenin, Tcf4 and survivin, the key factors of canonical Wnt signaling [30, 31], in HCECs cultured in CnT-20 with those grown in SHEM. As shown in Fig. 4A, the mRNA of β-catenin was highly expressed by the cells cultured in CnT-20, 4.57±0.75 (p<0.05, n=6) fold to that in SHEM. Tcf4 mRNA was elevated 3.34±0.3 fold (p<0.05, n=6) in the cells cultured in CnT-20. Interestingly, the mRNA expression of survivin, a downstream molecule of Tcf4, was also significantly higher by 5.52±1.97 fold (p<.01, n=6) in HCECs cultured in CnT-20 than in SHEM. Western blot analysis confirmed the expression pattern of β-catenin, Tcf4 and survivin by HCECs cultured in CnT-20 and SHEM, at protein levels. Fig. 4B shows representative immunoblotting results of two pairs of HCEC cell extract samples in the different media. The protein bands of β-catenin (92 kDa), Tcf4 (58 kDa) and survivin (16 kDa) were markedly higher in cells cultured in CnT-20 compared to those in SHEM, while the 45 kDa bands of β-actin protein, an internal control, were not significantly different between the cells in two conditions. Furthermore, fluorescent staining visibly displayed the higher immunoreactivities of β-catenin, Tcf4 and survivin antibodies in HCECs cultured with CnT-20 (Fig. 4C). These results indicate β-catenin/Tcf4/survivin signaling may play a role in maintaining the properties of human corneal epithelial stem cells.
Fig.4.
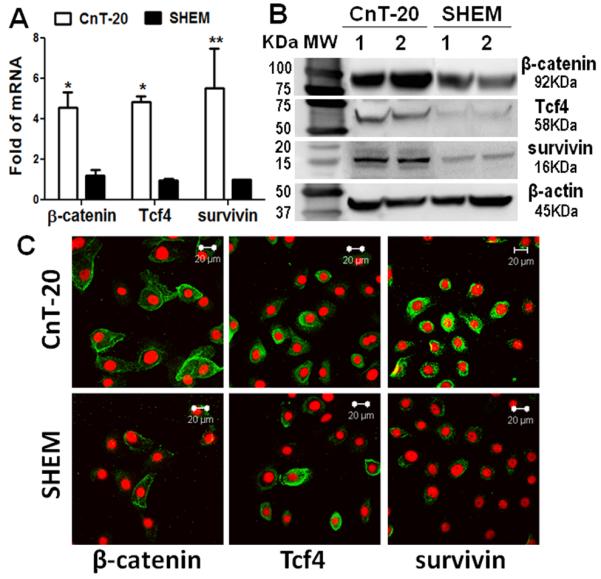
Expression of β-catenin, Tcf4 and survivin by HCECs in CnT-20 or SHEM medium. A. Real-time PCR data showing the mRNA expression of β-catenin, Tcf4 and survivin; B. Western blot results showing the protein levels of β-catenin, Tcf4 and survivin; C. Representative images showing immunofluorescent staining of β-catenin, Tcf4 and survivin (green) with Propidium Iodide (PI) (red) counterstaining.
Functional role of Tcf4 signaling in maintaining the proliferative property of human corneal epithelial progenitor cells
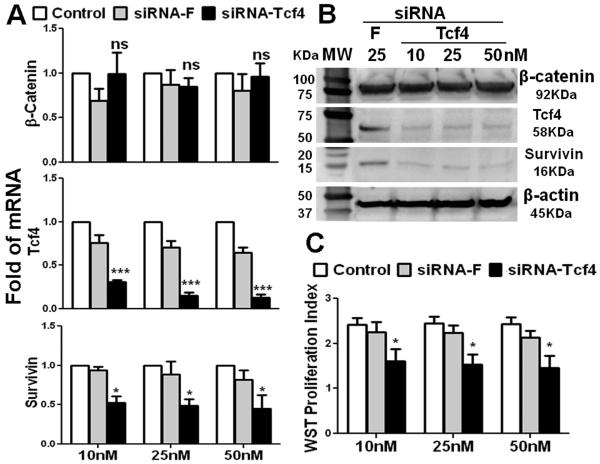
To evaluate functional role of Tcf4 signaling in human corneal epithelial stem cells, Tcf4 gene silencing experiments were performed using HCECs cultured in the corneal epithelial progenitor medium CnT-20 and transfected with a specific siRNA-Tcf4 at different concentrations. A non-coding sequence siRNA conjugated with fluorescein (siRNA-F) was transfected to HCECs in CnT20 as a negative control, as well as a visible monitor for siRNA transfection efficiency. As shown in Fig. 5A, siRNA-F transfection did not significantly alter the mRNA expression of β-catenin, Tcf4 and survivin. In HCECs transfected with siRNA-Tcf4 at 10, 25 or 50 nM, Tcf4 mRNA expression was suppressed dramatically and dose-dependently (P<0.001) by 70-85%, compared with untreated control. Interestingly, levels of survivin transcripts, a downstream molecule of Tcf4, were also suppressed dose-dependently to 53±8, 49±7 and 45±17% (all p<0.05, n=3) at 10, 25 or 50 nM of siRNA-Tcf4, respectively. However, the expression of β-catenin, a Tcf4 molecular partner, was not significantly changed in HCECs transfected with siRNA-Tcf4. The knock down of Tcf4-survivin signaling was confirmed at the protein level by Western blot analysis. As shown in Fig. 5B, the Tcf4 and survivin protein bands were dramatically reduced by Tcf4 silencing at all doses (10-50nM) of siRNA-Tcf4 tested, compared with that in HCECs transfected with non-silencing siRNA-F. In contrast, the bands of β-catenin and housekeeping β-actin were not altered by Tcf4 silencing. Notably, the proliferative capacity of HCECs was impaired by Tcf4 silencing. As measured by WST-1 assay shown in Fig. 5C, the cell proliferative index was significantly suppressed by siRNA-Tcf4 but not by the non-coding sequence siRNA-F in three concentrations (10nM, 25nM and 50nM) of siRNAs tested (all p<0.001, n=5). These results indicate that Tcf4/survivin signaling may determine the proliferative capacity of human corneal epithelial progenitor cells.
Fig.5.
Effects of Tcf4 silencing by siRNA interference in HCECs cultured in CnT-20. A. Real-time PCR analysis showing the effects of siRNA-Tcf4 (10-50nM) transfection on mRNA expression of β-catenin, Tcf4 and survivin with untreated cells and transfected with non-coding sequence siRNA-F as controls, n=5; B. Western blot results showing the protein levels of β-catenin, Tcf4 and survivin after transfection with siRNA-Tcf4 or –F; C. WST-1 assay showing the cell proliferation indexes after siRNA-Tcf4 transfection. Data are shown the mean and standard deviation of results, ns: no significance, *p <0.05, **p <0.01, ***p <0.001, n=3, compared with siRNA-F transfection.
DISCUSSION
The maintenance of corneal epithelial health and corneal tissue repair following trauma both depend on the regenerative capacity of corneal epithelial stem cells, which are located in the basal layer of the limbal epithelium. It is now clear that the niche plays an important role in maintenance of stem cell properties, and the different fate of stem cells, being stem cells or differentiated, can be determined by different niche [1, 6, 12, 52, 53]. It is well known that low calcium, serum free media could provide an ideal niche in vitro to maintain or promote progenitor cell properties by delaying the onset of terminal differentiation of cultured epithelial cells [13, 16, 17, 54]. However, it is not clear how niche extrinsic factors activate intrinsic factors or the signaling pathways that maintain progenitor cells properties. Wnt signaling has been implicated in stem cell and their niche, and it is likely of great importance in the interactions between stem cells and their niche micro-environment [27, 55-57]. In our present study, we explored the potential roles of β-catenin/Tcf4/survivin signaling of the canonical Wnt pathway in maintaining the properties of human corneal epithelial stem cells, which may serve as a representative model of tissue-specific adult stem cells.
As shown in Figs 1 and 2, all four low calcium, serum free media tested in this study were capable of supporting better growth, more serial passages and higher CFE of HCECs in culture, compare to high calcium, serum-containing medium SHEM, indicating that low calcium, serum free culture niche maintains the proliferative capacity of HCECs. The colony forming efficiency assay is usually used to monitor the proliferative capacity of epithelial progenitor cells, and it requires 3T3 mouse fibroblasts as a feeder layer to support clonal growth of corneal epithelial cells [14, 36, 51, 58]. However, we noted that the low calcium, serum free medium CnT-20 could support clonal growth of HCECs without a feeder layer. The CFE of HCECs cultured in CnT-20 without feeder layer was 5.4±1.2% at day 8, which reached the ranges of CFE rates (3.8-8.7%) of HCECs in SHEM with a feeder layer of 3T3 fibroblasts, as previously reported [35, 36, 38, 39]. This suggests that CnT-20 maintains the proliferative potential of human corneal epithelial cells in culture. Given the clinical use of limbal stem cells in stem cell therapy and corneal regeneration, the identification of culture conditions for HCECs that is serum free and does not require feeder cells is highly significant to the therapeutic ophthalmological field [59].
We have characterized a unique phenotype of stem cell enriched basal cells at human limbal epithelium and proposed that limbal stem cells are small primitive cells expressing three patterns of molecular markers: (1) exclusively positive for p63, ABCG2 and integrin α9 by a subset of basal cells; (2) relatively higher expression of integrin β1, EGFR, K19 and α-enolase by most basal cells, and (3) lack of expression of nestin, E-cadherin, connexin 43, involucrin, K3 and K12 [35, 39, 44, 51]. In this study, HCECs grown in CnT-20 expressed significantly higher signals of stem cell associated markers, p63, integrin β1 and EGFR, while lower levels of differentiation markers connexin 43 and K3, at both mRNA and protein levels, than the cells in SHEM. Taken together, our results demonstrated that low calcium serum free media maintain the stem cell-like phenotype and high proliferative capacity of human corneal epithelial progenitor cells in vitro.
Recent progress in cellular and molecular biology has uncovered the crucial role of Wnt signaling in proliferation, differentiation and self-renewal of stem cells. The best known Wnt signaling pathways include the Wnt/β-catenin, Wnt/planar cell polarity (PCP), and Wnt/calcium pathways [60]. The Wnt/β-catenin pathway is often called the “canonical” Wnt pathway, of which β-catenin and Tcf are key regulatory molecules. The hallmark of the Wnt signaling pathway is that the members of the Tcf family bind β-catenin and trigger Wnt target genes, such as survivin, to regulate cell functions [33, 61-63]. Survivin is the smallest member of the inhibitor of apoptosis (IAP) gene family in mammalian cells, an essential mitotic gene, localized to multiple aspects of the mitotic apparatus, and indispensable for several steps in cell division. Although it has been considered as a “cancer gene” since its discovery in 1997, survivin expression has been associated with “stemness” gene signatures of hematopoietic, mesenchymal, neuronal and skin progenitor cells. Recently, survivin has been identified as a direct transcriptional target of Wnt/β-catenin, which involves the recognition of discrete TCF-4-binding elements in the survivin promoter (see review article by [64]). β-catenin/Tcf4/survivin signaling has been found to be important in determining cell development and differentiation [33, 65, 66].
Using microarray analysis, we have recently observed that Tcf4 was one of the most highly up-regulated genes in human corneal epithelial progenitor cells that were isolated by rapid adhesion to collagen IV. Furthermore, β-catenin/Tcf4 signaling was found to be important in maintaining human corneal epithelial stem cell properties [48]. In our current study, the expression and function of β-catenin, Tcf4 and survivin were evaluated to investigate the underlying mechanism by which the low calcium, serum free medium CnT-20 maintains progenitor cell properties. As shown in Figs 4 and 5, human corneal epithelial progenitor cells cultured in CnT-20 expressed higher levels of these three factors at both gene transcript and protein levels. Silencing Tcf4 by siRNA transfection not only knocked down Tcf4 gene, but also blocked its downstream target molecule survivin at the transcriptional and translational levels, and furthermore significantly suppressed the proliferative capacity of HCECs cultured in CnT-20, confirmed with the WST-1 cell proliferation assay. These results strongly suggest that β-catenin/Tcf4/survivin signaling plays a vital role in maintaining corneal epithelial progenitor cell properties. However, the effect of Tcf4 silencing on the cell differentiation state is not clear and needs to be further studied. It is also important to explore how this signaling pathway is activated and which extrinsic factors contribute to stem cell maintenance.
In conclusion, these findings demonstrate that low calcium serum free media can provide a microenvironment niche for ex vivo expansion of corneal epithelium progenitor cells that retain a less differentiated phenotype and high proliferative capacity. The ability of this media to maintain the properties of human corneal epithelial progenitor cells is mediated by the β-catenin/Tcf4/survivin signaling pathway. This study may have high impact and clinic implication on the expansion of corneal epithelial stem cells in regenerative medicine, especially for ocular surface reconstruction.
ACKNOWLEDGMENTS
This study was supported by Department of Defense CDMRP PRMRP FY06 PR064719 (DQL), NEI NIH Grant EY11915 (SCP), National Natural Science Foundation of China 30901634 (RL) and 30872813 (HQ), Natural Science Foundation of Guangdong Province (9151008901000210), Lions Foundation for Sight, Research to Prevent Blindness, Oshman Foundation, William Stamps Farish Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors have no financial and commercial conflicts of interest
REFERENCES
- 1.Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 2.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 3.Lavker RM, Dong G, Cheng SZ, Kudoh K, Cotsarelis G, Sun TT. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes, Invest Ophthalmol. Vis. Sci. 1991;32:1864–1875. [PubMed] [Google Scholar]
- 4.Diaz-Flores L, Jr., Madrid JF, Gutierrez R, Varela H, Valladares F, varez-Arguelles H, Diaz-Flores L. Adult stem and transit-amplifying cell location. Histol. Histopathol. 2006;21:995–1027. doi: 10.14670/HH-21.995. [DOI] [PubMed] [Google Scholar]
- 5.Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. J. Investig. Dermatol. Symp. Proc. 2004;9:202–207. doi: 10.1111/j.1087-0024.2004.09311.x. [DOI] [PubMed] [Google Scholar]
- 6.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 7.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotsarelis G, Kaur P, Dhouailly D, Hengge U, Bickenbach J. Epithelial stem cells in the skin: definition, markers, localization and functions. Exp. Dermatol. 1999;8:80–88. doi: 10.1111/j.1600-0625.1999.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 9.German MJ, Pollock HM, Zhao B, Tobin MJ, Hammiche A, Bentley A, Cooper LJ, Martin FL, Fullwood NJ. Characterization of putative stem cell populations in the cornea using synchrotron infrared microspectroscopy. Invest Ophthalmol. Vis. Sci. 2006;47:2417–2421. doi: 10.1167/iovs.05-1254. [DOI] [PubMed] [Google Scholar]
- 10.Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Notara M, Alatza A, Gilfillan J, Harris AR, Levis HJ, Schrader S, Vernon A, Daniels JT. In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp. Eye Res. 2010;90:188–195. doi: 10.1016/j.exer.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Li D-Q, Pflugfelder SC, Huang AJW. Ocular surface epithelial stem cells. In: Low WC, Verfaillie LC, editors. Stem Cells and Regenerative Medicine. First ed. World Scientific Publishing Company; New Jersey: 2008. pp. 111–141. [Google Scholar]
- 13.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J. Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 14.Kruse FE, Tseng SC. A serum-free clonal growth assay for limbal, peripheral, and central corneal epithelium, Invest Ophthalmol. Vis. Sci. 1991;32:2086–2095. [PubMed] [Google Scholar]
- 15.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 2006;66:8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loo D, Beltejar C, Hooley J, Xu X. Primary and multipassage culture of human fetal kidney epithelial progenitor cells. Methods Cell Biol. 2008;86:241–255. doi: 10.1016/S0091-679X(08)00010-1. [DOI] [PubMed] [Google Scholar]
- 17.Bertolero F, Kaighn ME, Camalier RF, Saffiotti U. Effects of serum and serum-derived factors on growth and differentiation of mouse keratinocytes. In Vitro Cell Dev. Biol. 1986;22:423–428. doi: 10.1007/BF02623533. [DOI] [PubMed] [Google Scholar]
- 18.Vicanova J, Boelsma E, Mommaas AM, Kempenaar JA, Forslind B, Pallon J, Egelrud T, Koerten HK, Ponec M. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J. Invest Dermatol. 1998;111:97–106. doi: 10.1046/j.1523-1747.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol. Vis. Sci. 2004;45:3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- 20.Rattis FM, Voermans C, Reya T. Wnt signaling in the stem cell niche. Curr. Opin. Hematol. 2004;11:88–94. doi: 10.1097/01.moh.0000133649.61121.ec. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth MJ, Bodine DM. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 2007;17:746–758. doi: 10.1038/cr.2007.69. [DOI] [PubMed] [Google Scholar]
- 22.Fleming HE, Janzen V, Lo CC, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 24.Lo CC, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, III, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 27.Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am. J. Pathol. 2005;167:381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham TA, Ferkey DM, Mao F, Kimelman D, Xu W. Tcf4 can specifically recognize beta-catenin using alternative conformations. Nat. Struct. Biol. 2001;8:1048–1052. doi: 10.1038/nsb718. [DOI] [PubMed] [Google Scholar]
- 29.Poy F, Lepourcelet M, Shivdasani RA, Eck MJ. Structure of a human Tcf4-beta-catenin complex. Nat. Struct. Biol. 2001;8:1053–1057. doi: 10.1038/nsb720. [DOI] [PubMed] [Google Scholar]
- 30.Wei W, Chua MS, Grepper S, So S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int. J. Cancer. 2010;126:2426–2436. doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- 31.Korinek V, Barker N, Moerer P, van DE, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 32.Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet. 2003;362:205–209. doi: 10.1016/S0140-6736(03)13910-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H, Zhang G, Wang Y, Xu N, He S, Zhang W, Chen M, Liu M, Quan L, Bai J, Xu N. Inhibition of ErbB2 by Herceptin reduces survivin expression via the ErbB2-beta-catenin/TCF4-survivin pathway in ErbB2-overexpressed breast cancer cells. Cancer Sci. 2010;101:1156–1162. doi: 10.1111/j.1349-7006.2010.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat. Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Jun S, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Paiva CS, Pflugfelder SC, Li D-Q. Cell size correlates with phenotype and proliferative capacity in human corneal epithelial cells. Stem Cells. 2006;24:368–375. doi: 10.1634/stemcells.2005-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 38.Li D-Q, Chen Z, Song XJ, de Paiva CS, Kim HS, Pflugfelder SC. Partial enrichment of a population of human limbal epithelial cells with putative stem cell properties based on collagen type IV adhesiveness. Exp. Eye Res. 2005;80:581–590. doi: 10.1016/j.exer.2004.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li D-Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Li D-Q, Tong L, Stewart P, Chu C, Pflugfelder SC. Targeted inhibition of p57 and p15 blocks transforming growth factor beta-inhibited proliferation of primary cultured human limbal epithelial cells. Mol. Vis. 2006;12:983–994. [PMC free article] [PubMed] [Google Scholar]
- 41.Ma P, Wang Z, Pflugfelder SC, Li DQ. Toll-like receptors mediate induction of peptidoglycan recognition proteins in human corneal epithelial cells. Exp. Eye Res. 2010;90:130–136. doi: 10.1016/j.exer.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peuster M, Fink C, von SC. Biocompatibility of corroding tungsten coils: in vitro assessment of degradation kinetics and cytotoxicity on human cells. Biomaterials. 2003;24:4057–4061. doi: 10.1016/s0142-9612(03)00274-6. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X, de Paiva CS, Rao K, Li DQ, Farley WJ, Stern M, Pflugfelder SC. Evaluation of the Transforming Growth Factor beta Activity in Normal and Dry Eye Human Tears by CCL-185 Cell Bioassay. Cornea. 2010;29:1048–1054. doi: 10.1097/ICO.0b013e3181cf98ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li D-Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li D-Q, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Yoon KC, de Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol. Vis. Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 47.Qi H, Li DQ, Bian F, Chuang EY, Jones DB, Pflugfelder SC. Expression of glial cell-derived neurotrophic factor and its receptor in the stem-cell-containing human limbal epithelium. Br. J. Ophthalmol. 2008;92:1269–1274. doi: 10.1136/bjo.2007.132431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bian F, Liu W, Yoon KC, Lu R, Zhou N, Ma P, Pflugfelder SC, Li DQ. Molecular signatures and biological pathway profiles of human corneal epithelial progenitor cells. Int. J. Biochem. Cell Biol. 2010;42:1142–1153. doi: 10.1016/j.biocel.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis. Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 50.Ma P, Bian F, Wang Z, Zheng X, Chotikavanich S, Pflugfelder SC, Li DQ. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol. Vis. Sci. 2009;50:2702–2709. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z, Evans WH, Pflugfelder SC, Li D-Q. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells. 2006;24:1265–1273. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stepp MA, Zieske JD. The corneal epithelial stem cell niche. Ocul. Surf. 2005;3:15–26. doi: 10.1016/s1542-0124(12)70119-2. [DOI] [PubMed] [Google Scholar]
- 53.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 54.Balk SD, Whitfield JF, Youdale T, Braun AC. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1973;70:675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 56.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De PM, Anti M, Van Gijn ME, Suijkerbuijk S, Van de WM, Marra G, Clevers H. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 58.Hudson DL, O'Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest. 2000;80:1243–1250. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- 59.Rama P, Matuska S, Paganoni G, Spinelli A, De LM, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 60.James RG, Conrad WH, Moon RT. Beta-catenin-independent Wnt pathways: signals, core proteins, and effectors. Methods Mol. Biol. 2008;468:131–144. doi: 10.1007/978-1-59745-249-6_10. [DOI] [PubMed] [Google Scholar]
- 61.Van de WM, Sancho E, Verweij C, de LW, Oving I, Hurlstone A, van der HK, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den BM, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 62.Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J. Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres VA, Tapia JC, Rodriguez DA, Lladser A, Arredondo C, Leyton L, Quest AF. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol. Cell Biol. 2007;27:7703–7717. doi: 10.1128/MCB.01991-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27:6276–6284. doi: 10.1038/onc.2008.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez DA, Tapia JC, Fernandez JG, Torres VA, Munoz N, Galleguillos D, Leyton L, Quest AF. Caveolin-1-mediated suppression of cyclooxygenase-2 via a beta-catenin-Tcf/Lef-dependent transcriptional mechanism reduced prostaglandin E2 production and survivin expression. Mol. Biol. Cell. 2009;20:2297–2310. doi: 10.1091/mbc.E08-09-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]