Abstract
The microtubule cytoskeleton is known to play a role in cell structure and serve as a scaffold for a variety of active molecules in processes as diverse as motility and cell division. The literature on the role of microtubules in signal transduction, however, is marked by inconsistencies. We have investigated a well-studied signaling pathway, TNF-α-induced NF-κB activation, and found a connection between the stability of microtubules and the regulation of NF-κB signaling in C2C12 myotubes. When microtubules are stabilized by paclitaxel (taxol), there is a strong induction of NF-κB even in the absence of TNF-α. Although there was no additive effect of taxol and TNF-α on NF-κB activity suggesting a shared mechanism of activation, taxol strongly induced the NF-κB reporter in the presence of a TNF receptor (TNFR) blocking antibody while TNF-α did not. Both TNF-α and taxol induce the degradation of endogenous IκBα and either taxol or TNF-α induction of NF-κB activity was blocked by inhibitors of NF-κB acting at different sites in the signaling pathway. Both TNF-α and taxol strongly induce known NF-κB chemokine target genes. On the other hand, if microtubules are destabilized by colchicine, then the induction of NF-κB by TNF-α or taxol is greatly reduced. Taken together, we surmise that the activity of microtubules is at the level of the TNFR intracellular domain. This phenomenon may indicate a new level of signaling organization in cell biology, actively created by the state of the cytoskeleton, and has ramifications for therapies where microtubule regulating drugs are used.
Keywords: Cytoskeleton, TNF-α receptor, Myotubes, Taxol, Colchicine
Introduction
In signal transduction, the cytoskeleton is for the most part thought of as making mechanical coupling possible or as an extensive surface area on which biological activities take place [1]. The filamentous organization of all cells contains the same basic elements creating the cytoskeleton, which consists of three basic types of filaments: microtubules, microfilaments, and intermediate filaments. The natural history of microtubules is something that has been determined biochemically and consists of a dynamic process in which mature microtubules are constantly being formed from tubulin precursors and then broken down by processes linked to movement and cell division [2]. Several compounds exist which can inhibit the formation of microtubules (e.g. colchicine and vinblastine) or stabilize their formation, inhibiting their normal recycling (e.g. paclitaxel/taxol) [3]. Mostly for their effects on cell proliferation, these compounds are widely used in the treatment of cancer.
One well-studied signal transduction pathway is that leading to the activation of nuclear factor κB (NF-κB). A canonical example of this pathway is its induction by tumor necrosis factor alpha (TNF-α), an inflammatory catabolic cytokine involved in systemic diseases including cancer. Diagrams that describe models of the activation of this system show the cell membrane on which receptors in the pathway reside and the nuclear membrane through which the active components move yet leave a description of the cytoskeleton blank and without purpose [4]. Correlations between microtubule-affecting drugs and effects on cell signaling for the NF-κB signaling pathway have been described. However, the literature on the involvement of microtubules in the TNF-α-induced NF-κB signaling pathway presents a conflicting set of data. Conclusions have been drawn showing that both inhibition [5] and stabilization [6] of microtubules lead to the induction of NF-κB.
In the present paper, we provide data that support the idea that microtubules are an essential component of TNF-α-induced NF-κB signal transduction and gene expression. We focus on skeletal muscle cells which have a well-developed cytoskeleton and a very strong NF-κB response. By using pharmacological, biochemical and molecular biological tools we show that microtubules are required for the NF-κB arm of TNF-α signaling. We present a model for the action of microtubules in regulating NF-κB induction possibly at the activation complex of the TNF receptors.
Materials and methods
Cells and chemicals
C2C12 and A9 cells were obtained from American Type Culture Collection (Manassas, VA). HeLa cells were from DSMZ (Braunschweig, Germany). TNF-α (mouse recombinant), human TNFα, colchicine, vinblastine, and taxol (paclitaxel, Taxus sp.) were purchased from Sigma (St. Louis, MO) and Calbiochem (San Diego, CA). Helenalin, andrographolide, and wedelolactone were from Calbiochem. A blocking antibody to mouse TNFR1 was an Armenian hamster monoclonal from R&D Systems (Minneapolis, MN). A polyclonal TNF receptor activating antibody was purchased from Abcam (#7365). An antibody against IκBα (#sc-321) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
Cells were grown in DMEM with added 10% FCS and antibiotics. For myoblast differentiation, cells were grown in DMEM with 2% horse serum.
Treatments
For NF-κB activity experiments, unless otherwise noted, cells were transfected with a plasmid in which three copies of an Igk-derived NF-κB response element sits in a basal IL-2 promoter and directs the transcription of luciferase from pGL3 (Promega) [7]. In other cases, cells were also transfected with a human RelA (p65) synthesizing plasmid [8] or a plasmid directing synthesis of human constitutively active (c.a.) IKKβ [9]. For transfection, effectene (Qiagen) was used as per the manufacturer's protocol for 24-well plates, except in the case of myoblasts the transfection was set up in differentiation medium and differentiated for at least 2 days before treating with test compounds. Andrographolide, helenalin, or wedelolactone were preincubated with cultures for 3 h and then followed with no treatment or further incubation with TNF-α or taxol for 4 h prior to processing for luciferase. For the test of TNFR1 blocking antibody, transfected C2C12 cells were either untreated or pretreated with 20 µg/ml of the antibody for 1 h followed by continued treatment without or with 0.1 or 1 ng/ml TNFα or 20 µM taxol for a subsequent 4 h and then taken for luciferase assay. For experiments on HeLa cells and the cells directly compared to them, the treatment methods were the same as that described by Rosette and Karin [10].
Luciferase assay
At the time of harvest, the medium was removed from the cultures and 200 µl of Passive Lysis Buffer (Promega) was added per well. This was allowed to sit for 15 min and then the plate was put into a −80 °C freezer. The next day, the plate was thawed and the cells were scraped into Eppendorf tubes and vortexed then spun to remove debris. The supernatant was assayed by mixing 5 µl of the test solution with 95 µl of luciferase substrate buffer (Promega) and light emission was read on a Turner Designs TD-20/20 luminometer. As an internal control, some transfections also included a second Renilla-expressing plasmid and were assayed with the Promega Stop and Glo reagent for its detection.
Microtubule immunochemistry
Cells were grown in a 24-well plate, then treated after 2 days of differentiation with various compounds, or left untreated and fixed with neutral buffered formalin 4% for 20 min at room temperature. Then the cultures were washed with PBS–Tween before incubating with blocking solution (LiCor) overnight. The next day, they received primary antibody, mouse monoclonal to α-tubulin, for 1 to 4 h at room temperature, washed 3× with PBS–Tween, then a secondary goat anti-mouse antibody with Texas red conjugated for 1 h. After a last set of washes, coverslips were mounted with ProLong Gold Antifade Reagent and left overnight (all antibodies and antifade reagent were from Molecular Probes, Eugene, OR). The following day images were taken with a SPOT camera (Diagnostic Instruments, Inc., Michigan) on a Nikon TS100 inverted microscope.
Western blotting
For Western blotting, cell extracts were electrophoresed on SDS–polyacrylamide gels and electroblotted onto PDVF membranes. Membranes were blocked with LiCor blocking buffer and incubated with primary antibodies and then with secondary antibodies which were labeled with near IR fluorescent tags. The resulting blots were then scanned on a LiCor Odyssey machine which creates a visual image as well as quantitating the amount of antibody stained protein over five logs of dynamic range.
Quantitative PCR
For quantitative PCR, the Taqman system (Applied Biosystems, Inc.) was used. C2C12 cells were plated into 24-well plates and cultured in differentiation medium for 2 days and then untreated or treated with TNF-α (10 ng/ml) or taxol (20 µM) for 4 h. Following the treatments, the wells were harvested for RNA using the microRNA kit (Qiagen Inc.). RNA was quantitated by spectrophotometry and then used in producing cDNA with the High Capacity Reverse Transcription Kit of ABI. The resultant cDNA was used in the qPCR. The genes with ABI numbers CCl2/Mcp1 Mn00441242_m1 and Cxcl10/Ip10 Mn00445235_m1 were assayed on an ABI 7300 machine.
TNF-α assay
The ELISA MAX Set Deluxe for Mouse TNF-α was used to measure TNF-α in culture supernatants (BioLegend, San Diego, CA). The standard curve was from 0 to 500 pg/ml. The recombinant mouse TNF-α used in cell treatment served as a positive control.
Statistics
All graphing was done with Prism 4.0 software (Graphpad Software, California). Error bars show standard error of the mean, and curve fitting was done by Prism with the particular curve type described in the figure legends. For statistical significance of experimental samples, unpaired t-tests were carried out by Prism software.
Results
The NF-κB reporter system and effects of microtubule modifying agents
In order to standardize the role of NF-κB in cell culture, we set up an experimental system in which we transfected an NF-κB sensitive luciferase reporter plasmid into C2C12 myoblasts and then allowed the cultures to differentiate for 2 days before treating them with TNF-α. As we evaluated the reporter system, we found that a wide range of TNF-α doses induced NF-κB (Fig. 1A). The NF-κB response as plotted on a semi log scale is linear over at least three orders of magnitude and down to 10 pg/ml. At 1 pg/ml, there was no detectable NF-κB induction. Choosing two TNF-α doses from within our plotted data, we next observed how in this system, NF-κB-dependent luciferase, increases with time (Fig. 1B). We chose a dose of 10 ng/ml and a time of 4 h for further measurements. We used this functional assay to investigate how changes to the cytoskeleton would affect NF-κB induction. We therefore tested cytoskeleton-affecting compounds that could add to or antagonize the effect of TNF-α on NF-κB activity.
Fig. 1.
Effect of TNF-α, taxol, and colchicine (cells treated for 4 h for each agent) on NF-κB reporter activity in 2-day differentiated C2C12 cells. (A) NF-κB reporter activity plotted as fold change against the log of TNF-α concentration. (B) Time course of TNF-α treatment on NF-κB reporter activity at either 5 or 10 ng/ml TNF-α. (C) NF-κB reporter activity plotted as fold change against the log of taxol concentration. (D) NF-κB reporter activity plotted as fold change against the log of colchicine concentration plus a constant level of added TNF-α (10 ng/ml). All curves are generated from Prism, sigmoidal dose–response (variable slope) formula.
We found that taxol, a stabilizer of polymerized microtubules, could act in a manner similar to TNF-α with respect to the induction of NF-κB reporter activity (Fig. 1C). By itself, taxol did not induce any measurable increase of endogenous TNF-α (measured values were less than 0.4 pg/ml, data not shown). Next we found that colchicine, an inhibitor of microtubule polymerization, although not producing any effects of its own, was an antagonist of the TNF-α effect as shown by a dose–response curve (Fig. 1D). Colchicine is also an inhibitor of taxol-induced NF-κB induction with a slightly different effective concentration (Supplementary Fig. 1). In addition, the two inducers are not additive; when we combine treatments of cells with TNF-α and taxol, we only get the induction equal to TNF-α alone (Supplementary Fig. 2).
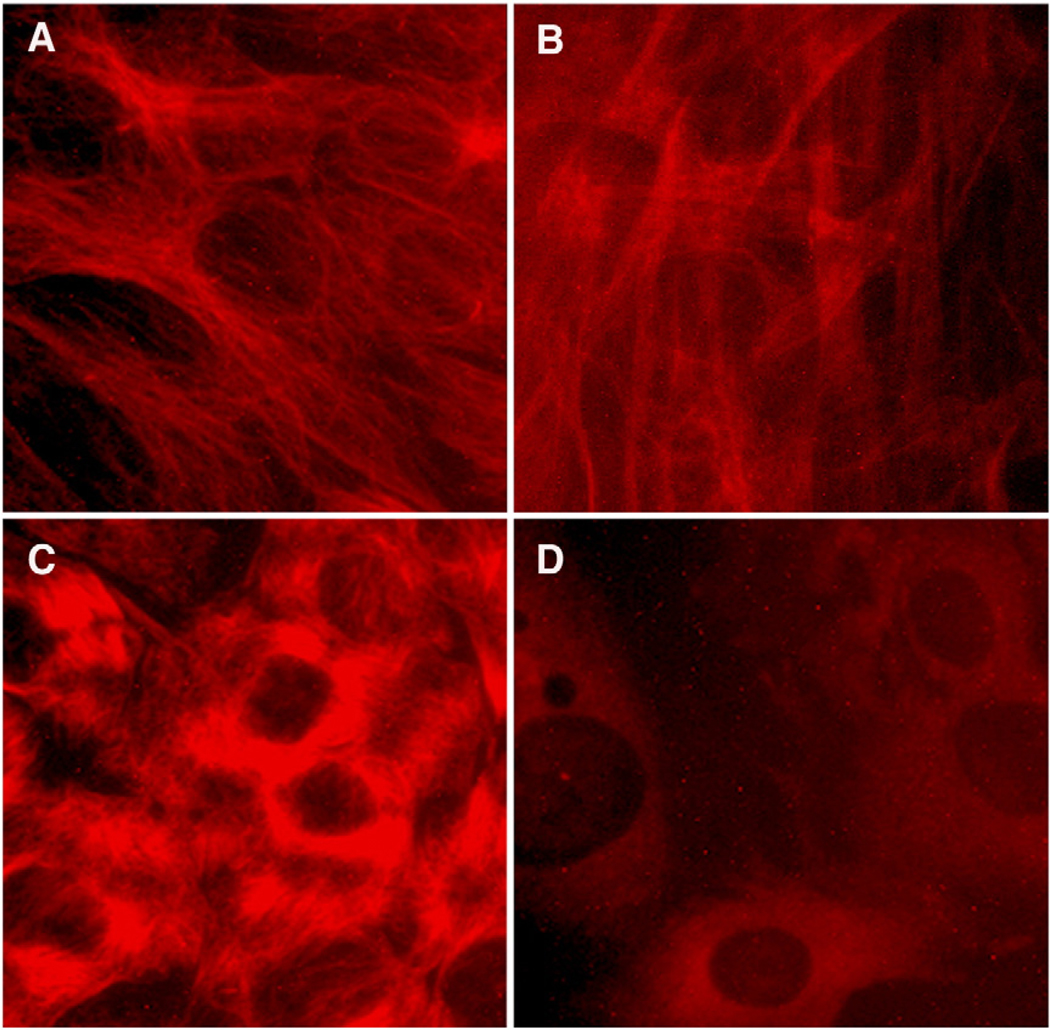
The gross visual effect of these compounds on the staining of microtubules in treated cells reveals that microtubules in the cells are normally arrayed in a fibrous network (Fig. 2A), an appearance which changes little with the addition of TNF-α (Fig. 2B). The stained microtubules become condensed to tufts with taxol treatment (Fig. 2C) or lose fibers altogether with the addition of colchicine (Fig. 2D).
Fig. 2.
Immunofluorescent images of 2-day differentiated C2C12 cells treated for 4 h with modifiers of microtubules and stained with anti-tubulin. (A) Untreated myotubes. (B) TNF-α treatment (10 ng/ml). (C) Taxol treatment (5 µM). (D) 5 µM colchicine treatment.
Taxol versus TNF-α activation of NF-κB
NF-κB signaling by TNF-α or taxol in the presence of NF-κB inhibitors
In order to discover where in the TNF-α-NF-κB signaling cascade microtubules were functioning, we used NF-κB inhibitors to assay for components of the signaling pathway that are either shared or contrasting between TNF-α and taxol. We tested several inhibitors of NF-κB induction and found that they all blocked both TNF-α and taxol activation of NF-κB reporter activity (Fig. 3). This included antagonists of p50 (andrographolide) [11], p65 (helenalin) [12], and the inhibitor kappa B kinase (IKK) (wedelolactone) [13]. This suggests that like TNF-α, taxol also activates endogenous NF-κB signaling at several levels.
Fig. 3.
NF-κB inhibitors abolish TNF-α or taxol induction of NF-κB reporter activity. Three different inhibitors were added for 3 h prior to a further 4 h treatment with TNF-α (10 ng/ml) or taxol (10 µM). Control wells received only the vehicle for the inhibitors, 0.2% DMSO. Inhibitors of p50, p65, and IKKα/β were andrographolide (100 µM), helenalin (20 µM), and wedelolactone (50 µM), respectively. All inhibitors blocked TNF-α or taxol induction of NF-κB reporter activity (P<0.05).
TNF-α and taxol induce degradation of IκBα
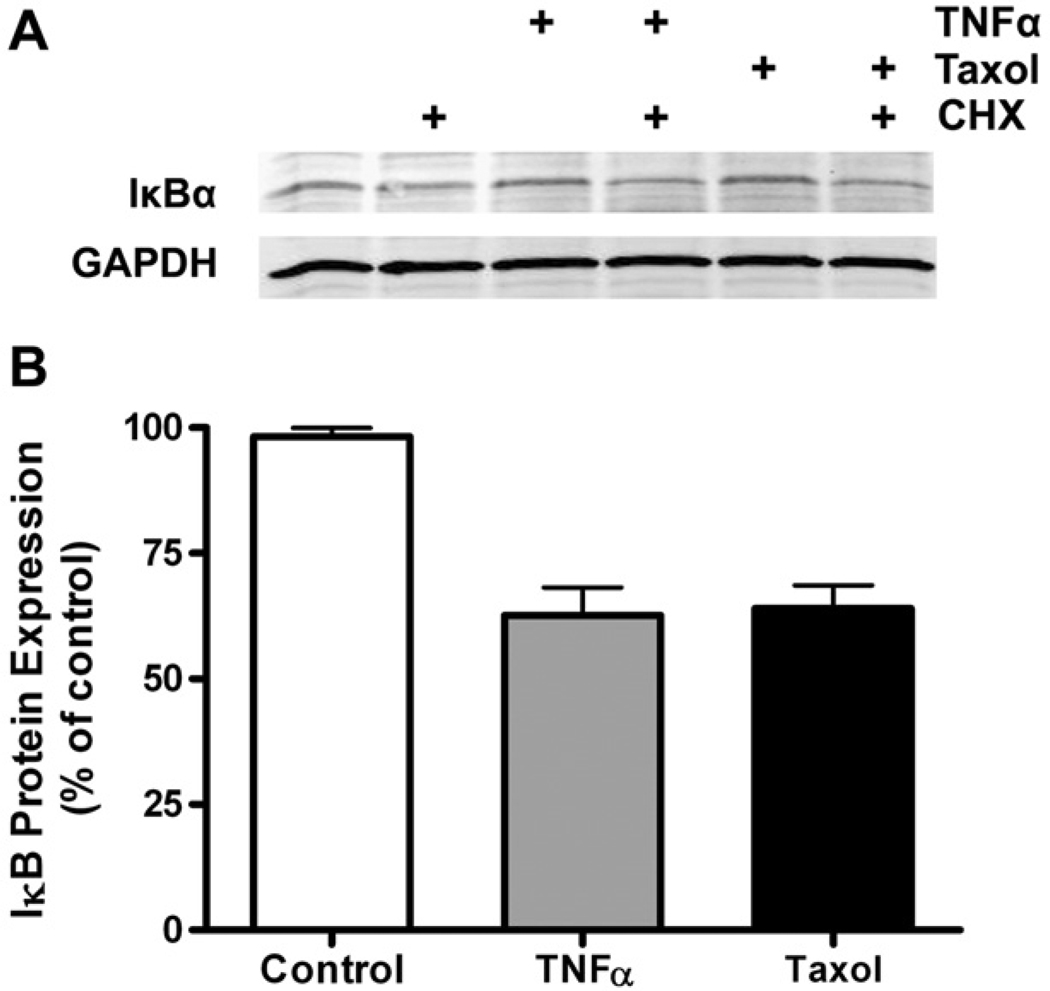
TNF-α is thought to activate NF-κB through a series of phosphorylations that ultimately induce ubiquitination and degradation of IκBα which retains Rel heterodimers (e.g., p65:p50) in the cytoplasm. We measured expression of endogenous IκBα in C2C12 cells by inducing with TNF-α or taxol in medium to which 100 µg/ml cycloheximide (CHX) had been added in order to prevent synthesis of new IκBα (Fig. 4A). When compared to the background loss of IκBα in a 4-h period, TNF-α or taxol caused the degradation of IκBα by ~40% (Fig. 4B). Although well known for TNF-α, this suggests that taxol also activates upstream NF-κB signaling rather than having its effect solely on basal cytoplasmic-nuclear cycling of Rel proteins.
Fig. 4.
IκBα protein expression decreases in response to 4 h of TNF-α or taxol treatment. (A) Immunoblot of IκBα from C2C12 cells treated with 5 ng/ml TNF-α or 20 µM taxol in the presence or absence of 355 µM CHX (100 µg/ml). (B) Quantitation of bands shows the loss of IκBα by 40% from treatment with either NF-κB inducer (P<0.05).
Effect of TNF-α and taxol induction of NF-κB by TNFR1 blocking or activating antibodies
The NF-κB activity assay was also used to compare C2C12 cells treated with a TNFR1 blocking antibody and then TNF-α or taxol treatment (Fig. 5A). Although the antibody had no effect on the NF-κB activity of untreated cells, it was highly antagonistic to the NF-κB induction due to TNF-α. In striking contrast, the antibody failed to block the NF-κB activating effect of taxol, suggesting that full TNF-α activity is dependent on it acting as a ligand for TNFR1 whereas taxol is not. Further indication that taxol and TNF-α NF-κB induction is different comes from an experiment in which a TNFR1 agonist antibody was added to cells with and without these activators. The activating antibody, while producing full NF-κB reporter activation on its own, adds little to TNF-α activation (Fig. 5B). The approximately 60-fold activation of reporter activity due to addition of the activating TNFR1 antibody was evident in cells with no treatment, with TNF-α treatment, or with taxol treatment but taxol treatment alone showed less than half this level of activation again suggesting that taxol acts differently than as a ligand of the TNF receptors.
Fig. 5.
Effect of TNF-α or taxol-induced NF-κB reporter activity by the presence of either an inhibiting or activating antibody to TNFR1. (A) Pairs of histogram bars are results for untreated versus treated cells with 20 µg/ml TNFR1 inhibiting antibody. TNF-α-treated cells (0.1 or 1.0 ng/ml) plus the TNFR1 antibody showed inhibition (P<0.05) of NF-κB activity while the antibody did not affect the taxol (20 µM)-induced NF-κB activity. (B) An activating TNFR1 antibody did not further increase TNF-α-induced (10 ng/ml) NF-κB activity. (P<0.05). Taxol's activation of NF-κB activity was significantly less than the antibody alone or the antibody plus taxol.
Genes that respond to TNF-α and taxol
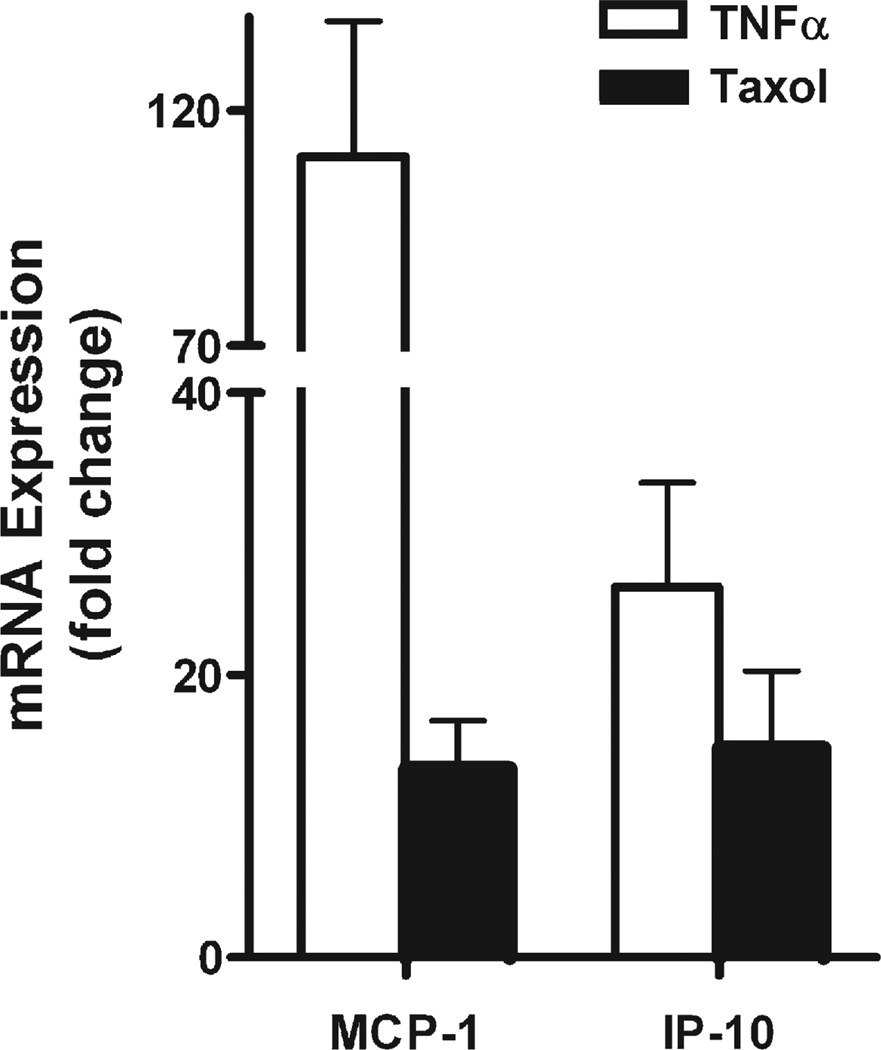
In order to determine if the changes in NF-κB reporter which we observed with taxol treatment were reflected in changes of NF-κB responsive genes, we studied two genes that have previously been shown to contain NF-κB response elements and which have shown responses to TNF-α. These genes were Mcp1 and Ip10 [14]. We performed qPCR on RNA from C2C12 cells that were treated with TNF-α or taxol. TNF-α caused a 26-fold upregulation of Ip10 and a 110-fold upregulation of Mcp1 mRNA (Fig. 6). Taxol treatment showed a 15-fold upregulation of Ip10 and a 13-fold upregulation of Mcp1.
Fig. 6.
Expression of endogenous NF-κB responsive genes (Mcp1 and Ip10) due to TNF-α or taxol treatment measured by qPCR. Fold change of mRNA due to 4 h of treatment with 10 ng/ml TNF-α treatment or 20 µM taxol. Both MCP-1 and IP-10 were significantly upregulated due to the two treatments (P<0.05).
Colchicine inhibition of NF-κB induction
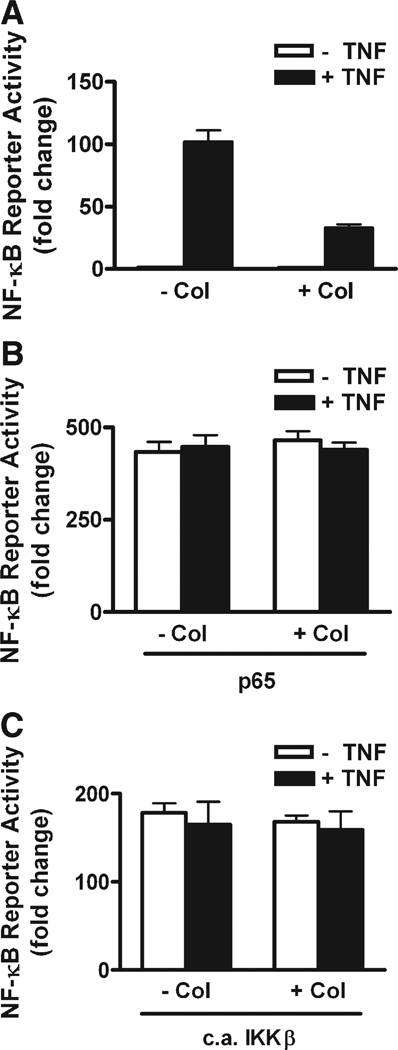
The inhibition of NF-κB activity by colchicine is minimal on normal or background levels of NF-κB while it causes a 70% inhibition of activity in cells treated with 10 ng/ml TNF-α (Fig. 1D). We also found colchicine to inhibit NF-κB activity in HeLa and A9 cells (Supplementary Fig. 3). To determine if the colchicine effect was related to the absolute level of NF-κB activity, or if it required an active induction process to be inhibitory, we performed over-expression experiments. If colchicine was inhibiting NF-κB reporter activity by blocking the transport of Rel proteins from cytoplasm to nucleus, then it would be more effective at higher levels of Rel release and that could be what was observed with TNF-α treatment. Therefore, in addition to the reporter, we transfected C2C12 cells with plasmids coding for the expression of either wild type p65 or constitutively active (c.a.) IKKβ.We then treated these cultures with or without TNF-α and with and without colchicine. Colchicine had the prototypical inhibitory effect on TNF-α-treated cells (Fig. 7A) but it did not inhibit RelA-induced NF-κB activity with or without TNF-α (Fig. 7B). Induction of NF-κB activity by c.a. IKKβ was also not inhibited by colchicine (Fig. 7C).
Fig. 7.
Colchicine inhibits TNF-α-induced NF-κB activity but not activity induced by overexpression of p65 or c.a. IKKβ in C2C12 cells. Cells were transfected with reporter alone or with plasmids encoding p65 or c.a.IKKβ. (A) Colchicine (100 µM) inhibition of 4 h of TNF-α-induced NF-κB activity (P<0.05). (B) Overexpression of p65 for 2 days strongly induced NF-κB reporter activity and TNF-α addition for 4 h did not further activate the reporter. Colchicine did not inhibit the elevated NF-κB activity due to p65 overexpression in the presence or absence of TNF-α. (C) Overexpression of c.a. IKKβ for 2 days induced NF-κB reporter activity and TNF addition for 4 h did not further activate the reporter. Colchicine did not inhibit the elevated NF-κB activity due to c.a. IKKβ overexpression in the presence or absence of TNF-α.
Discussion
The NF-κB signaling system is important in many dynamic cellular responses especially in the action of agents promoting inflammation and regulating apoptosis [15]. In cancer, NF-κB signaling is often associated with oncogenesis most likely due to its anti-apoptotic and thus pro-survival effects on cancer cells [16]. In skeletal muscle, this system is thought to be important in the atrophy of cachexia [9,17,18] and has been shown to be required for atrophy due to disuse [19]. Although much has been written about the regulatory and effecting component parts of the NF-κB pathway, the involvement of the cytoskeleton has been mostly considered as providing a framework in which the active molecules reside. In this paper, we present data showing a requirement for the microtubule component of the cytoskeleton in NF-κB activation. Microtubules do create structure but more than that, in the case of NF-κB they appear to be able to regulate the progress of the entire signaling pathway. We have found that microtubules are required for TNF-α induction of NF-κB. Colchicine is inhibitory to both TNF-α and taxol activation of NF-κB. Taxol, a microtubule stabilizer, is capable of induction of NF-κB activity in the absence of other inducers. Taxol plus TNF-α do not show an additive effect on reporter activity which obviates the notion that separate mechanisms are involved. We observed that taxol does not induce the production of TNF-α in the cells, and so we have focused most of our discussion on the comparison between taxol and TNF-α induction of NF-κB.
TNF-α induction of NF-κB
The current understanding for the pathway of NF-κB induction from TNF-α is the following series of events [15,20,21]. TNF-α exists as a trimer in its active form which binds to the 55-kDa (TNFR1) or 75-kDa (TNFR2) receptors, causing the receptors to form trimers in an active configuration [22]. The active multimers of TNFR1 recruit TRADD at the “death domain” and this is followed by recruitment of TRAF2 [20]. TNFR2 lacks a death domain and so active TNFR2 recruits TRAF2. Under the differential regulation by other factors, depending on the tissue type, TRAF2 interacts with RIP and these activated intracellular domain receptor-associated proteins bind to the IKK complex via interaction with NEMO (IKKγ) [15]. The induced IKK then activates the NF-κB cascade. The TRAF2 pathway, therefore, activates NF-κB, downstream of both TNF receptors.
TNF-α versus taxol activation of NF-κB activity
Not only does taxol activate NF-κB reporter activity but it activates endogenous NF-κB signaling since three inhibitors acting at different places in the NF-κB signaling pathway all blocked taxol-induced reporter activity to the same extent as they did with TNF-α. Consistent with endogenous activation of NF-κB signaling by taxol we showed that, similar to TNF-α, taxol treatment in the presence of cycloheximide significantly reduced IκBα suggesting that both molecules induce proteasomal degradation of IκBα. Further, we showed that at least two endogenous chemokine genes that are known NF-κB targets (MCP-1, IP-10) were activated by both TNF-α and taxol further supporting taxol's effect as an endogenous NF-κB signaling inducer.
Differences in the mechanism of TNF-α and taxol in the induction of NF-κB activation were revealed in experiments showing that an inhibitory TNFR1 antibody does not affect the taxol induction of NF-κB, but this antibody significantly inhibits activation by TNF-α, which is known to act as a ligand for both TNF receptors [21]. A TNFR1 activating antibody strongly induced the NF-κB reporter, similar to that seen with TNF-α treatment alone. In comparison, taxol-induced NF-κB activity was less than half the induction of the antibody plus taxol or the antibody alone. This suggests that taxol's activation of NF-κB is not via the extracellular domain of the TNF receptor, and that taxol and TNF-α have different but overlapping mechanisms of action. It should be noted that the TNFR1-activating antibody used may also activate TNFR2 since this polyclonal antibody recognized the extracellular domain regions of both receptors in immunoblots of C2C12 cells (data not shown).
Colchicine inhibits TNF-α and taxol induction of NF-κB
There is an inhibition by colchicine on TNF-α or taxol-induced NF-κB signaling. We have shown that both activators induce degradation of IκBα. Therefore, the logical site for colchicine to be inhibitory is at or before this degradation of IκBα. Even with that argument, we were concerned that colchicine might be acting at least partially on the transport of Rel proteins from cytoplasm to nucleus.
It is known that Rel proteins are constantly shuttling from cytoplasm to nucleus [23] and so we reasoned that if we greatly increased the rate of shuttling then if colchicine was regulating that transport, it would produce a measurable depression of the new levels of constitutive activation. In fact, when we increased the levels of NF-κB activity by overexpressing either more Rel protein (p65) or by increasing the activation of the IκBα by c.a. IKKβ, colchicine had no effect on NF-κB activity. In spite of the fact that we had increased activity and therefore transport of Rel to the nucleus to levels at or above those created by TNF-α, colchicine had no effect, arguing that its effect is more likely on the activation by TNF-α prior to the release of IκBα, perhaps even before activation of IKK. This is consistent with the data of Mikenberg et al. [24] who showed that microtubules do not participate in NF-κB nuclear transport in cells other than neurons.
A model for microtubule-based TNF receptor modulation in NF-κB signaling
We present the following model for the action of taxol, colchicine, and therefore microtubules in the functioning of TNF receptor activation of NF-κB. Our primary interpretation of these data is that microtubules are involved in the activation of TNF receptors or the intracellular domain receptor-associated complex including IKK. Stabilization of microtubules by taxol is capable of activating this mechanism. Colchicine is capable of inhibiting signaling from activation of TNF-α or taxol. Data in this study show that the cytoskeleton plays much more than a passive organizational role in cell signaling. Our results show microtubules to be important in the signal transduction of the TNFα-NF-κB system, and the relevance may be higher in cells with significant NF-κB signaling and a well-developed cytoskeleton. These results are therefore important for therapies containing microtubule modifying drugs in which TNF-α is involved such as in inflammatory settings or cancer therapeutics.
Supplementary Material
Acknowledgments
HeLa cells were a gift from Dr. Sarah Appel. We thank Dr. Denis Guttridge for the p65 plasmid and Dr. Dongsheng Cai for the c.a.IKKβ plasmid. We thank Chia-Ling Wu for excellent help with the figures. This work was supported by awards (AR041705, AR054446) from the NIH.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yexcr.2009.08.020.
REFERENCES
- 1.Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 2.Gardner MK, Hunt AJ, Goodson HV, Odde DJ. Microtubule assembly dynamics: new insights at the nanoscale. Curr. Opin. Cell Biol. 2008;20:64–70. doi: 10.1016/j.ceb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 5.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J. Biol. Chem. 2003;278:7445–7452. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- 6.Cvetkovic I, Miljkovic D, Vuckovic O, Harhaji L, Nikolic Z, Trajkovic V, Mostarica Stojkovic M. Taxol activates inducible nitric oxide synthase in rat astrocytes: the role of MAP kinases and NF-kappaB. Cell Mol. Life Sci. 2004;61:1167–1175. doi: 10.1007/s00018-004-3408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Baldwin AS., Jr Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 9.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Rosette C, Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J. Cell Biol. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia YF, Ye BQ, Li YD, Wang JG, He XJ, Lin X, Yao X, Ma D, Slungaard A, Hebbel RP, Key NS, Geng JG. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J. Immunol. 2004;173:4207–4217. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- 12.Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 13.Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- 14.Leung TH, Hoffmann A, Baltimore D. One nucleotide in a kappaB site can determine cofactor specificity for NF-kappaB dimers. Cell. 2004;118:453–464. doi: 10.1016/j.cell.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 17.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 18.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J. Biol. Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. Electronic publication 2002 Nov 12. [DOI] [PubMed] [Google Scholar]
- 19.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J. Clin. Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 21.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends. Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 22.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Birbach A, Gold P, Binder BR, Hofer E, de Martin R, Schmid JA. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J. Biol. Chem. 2002;277:10842–10851. doi: 10.1074/jbc.M112475200. [DOI] [PubMed] [Google Scholar]
- 24.Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C. TNF-alpha mediated transport of NF-kappaB to the nucleus is independent of the cytoskeleton-based transport system in non-neuronal cells. Eur. J. Cell Biol. 2006;85:529–536. doi: 10.1016/j.ejcb.2006.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.