Summary
Cell rearrangements shape the Drosophila embryo through spatially regulated changes in cell shape and adhesion. We show that Bazooka/Par-3 (Baz) is required for the planar polarized distribution of myosin II and adherens junction proteins and polarized intercalary behavior is disrupted in baz mutants. The myosin II activator Rho-kinase is asymmetrically enriched at anterior and posterior borders of intercalating cells in a pattern complementary to Baz. Loss of Rho-kinase results in expansion of the Baz domain and activated Rho-kinase is sufficient to exclude Baz from the cortex. The planar polarized distribution of Baz requires its C-terminal domain. Rho-kinase can phosphorylate this domain and inhibit its interaction with phosphoinositide membrane lipids, suggesting a mechanism by which Rho-kinase could regulate Baz association with the cell cortex. These results demonstrate that Rho-kinase plays an instructive role in planar polarity by targeting Baz/Par-3 and myosin II to complementary cortical domains.
Introduction
During axis elongation in Drosophila, the embryonic epithelium more than doubles in length along the anterior-posterior (AP) axis and simultaneously narrows in width along the dorsalventral (DV) axis. This structural transformation is characterized by a striking directionality in which cell movements are oriented perpendicular to the direction of tissue elongation, a process known as cell intercalation. Cell intercalation is essential for axis elongation in frogs, fish, chicks, flies, and ascidians (Keller et al., 2000; Solnica-Krezel, 2005). Intercalation in Xenopus and zebrafish requires Wnt-dependent activation of the Frizzled planar cell polarity pathway (Zallen, 2007; Rosko et al., 2009), while cell rearrangement in Drosophila involves proteins that mediate contractility and adhesion (Bertet et al., 2004; Zallen and Wieschaus, 2004; Blankenship et al., 2006). Adherens junction proteins and the contractile machinery are enriched in mutually exclusive domains at the cell cortex where they may participate directly in polarized cell movement. An asymmetrically localized contractile actomyosin network provides the driving force for cell rearrangement (Rauzi et al, 2008; Fernandez-Gonzalez et al., 2009) and differential adhesion could influence local interactions between cells (Blankenship et al., 2006). Patterned gene expression along the anterior-posterior axis provides the spatial information that directs planar cell polarity and cell rearrangement in Drosophila and Xenopus (Irvine and Wieschaus, 1994; Ninomiya et al., 2004; Zallen and Wieschaus, 2004; Blankenship et al., 2006). However, the mechanisms that translate patterns of gene expression into asymmetric protein localization are not known.
Interactions between spatially localized protein kinases and their substrates are a common strategy for generating polarity in many cell types. In epithelial cells, the basolateral kinase PAR-1 restricts its substrate Bazooka/Par-3 to the apical domain (Benton and St. Johnston, 2003) and the apical kinase atypical protein kinase C (aPKC) restricts its substrates Lgl and PAR-1 to the basolateral domain (Hutterer et al., 2004; Suzuki et al., 2004). During asymmetric cell division in Drosophila neuroblasts, aPKC phosphorylates Numb and Miranda, restricting their localization to the basal cortex (Wirtz-Peitz et al., 2008; Atwood and Prehoda, 2009). In each case, the localized activity of protein kinases inhibits the association of their substrates with the cell cortex, providing a dynamic mechanism for establishing and maintaining cellular asymmetry.
Rho-kinase is an activator of actomyosin contractility and a conserved regulator of cell shape and behavior (Winter et al., 2001; Marlow et al., 2002; Dawes-Hoang et al., 2005; Verdier et al., 2006; Wang and Riechmann, 2007). Rho-kinase activates myosin directly by phosphorylating the myosin regulatory light chain, as well as indirectly by inhibiting the myosin light chain phosphatase (Amano et al., 1996; Kimura et al., 1996). Rho-kinase has several substrates involved in cytoskeletal regulation, including LIM kinase and ERM proteins (Amano et al., 2000; Riento and Ridley, 2003). Mammalian Rho-kinase phosphorylates the polarity protein Par-3, disrupting its association with its binding partners Par-6 and aPKC in cultured cells (Nakayama et al., 2008). However, it is not known if regulation of Par-3 by Rho-kinase is important for cell behavior in vivo. Moreover, the residue on mammalian Par-3 that is phosphorylated by Rho-kinase is not present in all Par-3 homologs, raising the question of whether this is a conserved mechanism of Par-3 regulation.
Here we show that Rho-kinase is asymmetrically localized during axis elongation in the Drosophila embryo and that Rho-kinase activity is required for the planar polarized distribution of Bazooka/Par-3 (Baz). Rho-kinase localizes to boundaries between anterior and posterior cells complementary to Baz. Loss of Rho-kinase leads to ectopic Baz localization and activated Rho-kinase is sufficient to exclude Baz from the cortex in a mechanism that requires the Baz C-terminal domain. The Baz C-terminus has been shown to bind to phophoinositide membrane lipids (Krahn et al., 2010). We show that this domain can be phosphorylated by Rho-kinase and is required for Baz planar polarity. Baz, in turn, regulates the planar polarized distribution of myosin II and adherens junction proteins and cell intercalation is disrupted in baz mutants. These results demonstrate that Rho-kinase plays an instructive role in planar cell polarity by targeting Baz/Par-3 and myosin II to complementary cortical domains.
Results
Baz/Par-3 is required for intercalary behavior during axis elongation
Axis elongation in the Drosophila embryo is characterized by the planar polarized enrichment of myosin II and F-actin at interfaces between anterior and posterior cells, and E-cadherin, β-catenin, and Baz/Par-3 at interfaces between dorsal and ventral cells (Bertet et al., 2004; Zallen and Wieschaus, 2004; Blankenship et al., 2006). While polarized actomyosin contractility has been shown to play an essential role in orienting cell behavior (Rauzi et al., 2008; Fernandez-Gonzalez et al., 2009), the role of Baz and adherens junctions is less clear. Elongation is reduced in embryos zygotically mutant for baz, but these embryos contain maternal baz mRNA and protein (Zallen and Wieschaus, 2004). To completely eliminate Baz activity, we generated embryos maternally and zygotically mutant for two null alleles, bazGD21 and bazFA50 (Experimental Procedures) (Denef et al., 2008). The zygotic lethality of bazGD21 and bazFA50 homozygotes was rescued by the Dp(1;Y)W73 duplication that contains the baz open reading frame, indicating that each contains a single lethal mutation at the baz locus. Both alleles display no detectable Baz staining in embryos maternally and zygotically mutant for baz, referred to here as baz mutant embryos.
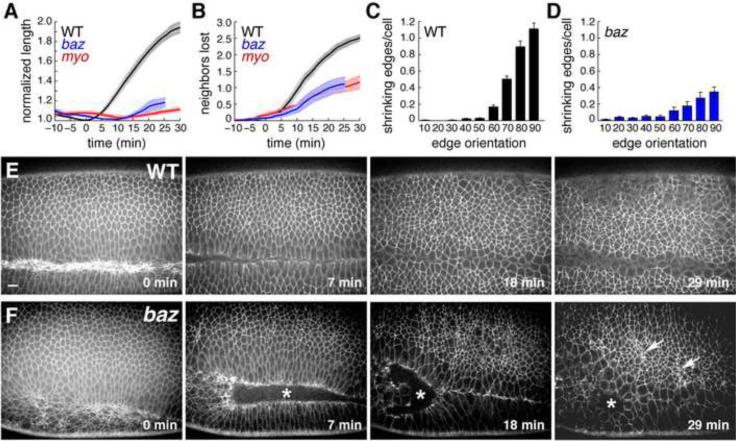
Axis elongation was severely defective in time-lapse movies of baz mutants (Figure 1A). Mutant cells engaged in fewer rearrangements (Figure 1B) and displayed a reduction in both neighbor exchange and rosette formation, two behaviors that require polarized actomyosin contractility (Bertet et al., 2004; Blankenship et al., 2006). Cell movements that occurred in baz mutants showed a moderate loss of directionality (Figure 1C,D). Some cells began to apically constrict in late stage 7, leading to the formation of ectopic grooves in stage 8 (Figure 1E,F; Movies S1, S2) (Muller and Wieschaus, 1996; Wodarz et al., 1999). These defects were not due to apoptosis, which was not present in wild-type or baz mutant embryos at these stages (Figure S1). These results demonstrate that Baz is required for cell rearrangement and spatially regulated contractile behavior during axis elongation.
Figure 1. baz mutants have defects in cell intercalation.
(A–D) Cell behavior in time-lapse movies of wild-type and mutant embryos. (A) Germband elongation is strongly reduced in bazGD21 and sqh1 mutants. (B) Fewer neighbors are lost per cell in baz and sqh mutants. An average value was obtained for each embryo, error bars indicate s.e.m. across embryos (n=10 WT, 5 bazGD21, and 2 sqh1 embryos). WT embryos were imaged with GFP:Resille (4), GFP:Spider (3), and E-cadherin:GFP (3), bazGD21 embryos were imaged with GFP:Spider, sqh1 embryos were imaged with E-cadherin:GFP. t=0 is the onset of elongation in stage 7. (C) Contractile behavior in wild type is biased toward edges perpendicular to the AP axis (angles in degrees, 0 for edges parallel to the AP axis). Bars indicate 10° bins starting at 0–10°. (D) Contractile behavior in baz mutants is reduced and partially spatially deregulated: 66% of shrinking edges were oriented at ≥60° in baz mutants compared to 88% in wild type, 16% were oriented at ≤30° in baz mutants compared to <1% in wild type (n = 10 wild-type and 5 bazGD21 embryos, 33–161 cells/embryo). (E,F) Stills from movies of wild-type (E) and bazGD21 (F) embryos expressing Spider:GFP. Asterisks indicate defects in ventral furrow closure in baz mutants and arrows point to clusters of apically constricting cells. Scale bar = 10 μm. See Figure S1, Movies S1,2.
Baz/Par-3 is required for myosin II and adherens junction planar polarity
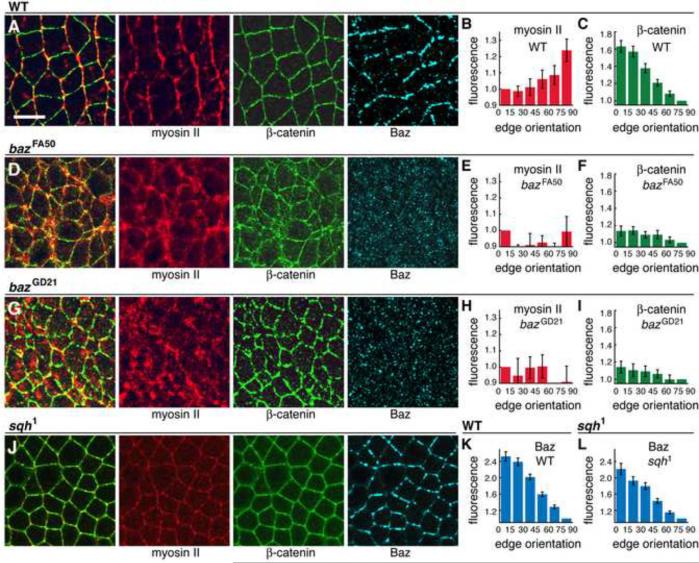
To investigate the basis of the defects in baz mutants, we analyzed protein localization in intercalating cells. In wild type, myosin II is apically localized and concentrated at boundaries between anterior and posterior cells (AP edges) (Figure 2A,B) (Zallen and Wieschaus, 2004). Myosin apical localization occurred normally in baz mutants (Figure S2; 5/5 bazGD21 and 6/6 bazFA50 embryos displayed apical myosin localization). By contrast, myosin planar polarity was strongly disrupted and myosin accumulated in an irregular fashion at edges of all orientations and at the medial apical cortex (Figure 2D–I) (0/10 bazGD21 and 0/3 bazFA50 embryos displayed myosin planar polarity). Baz was also required for the planar polarized localization of adherens junction proteins (0/3 bazGD21 and 0/7 bazFA50 embryos displayed Arm/β-catenin planar polarity). Myosin planar polarity occurred normally in embryos where Arm/β-catenin was disrupted by RNAi and in embryos maternally and zygotically mutant for arm043A06, even in cells that were visibly detached from their neighbors (Figure S2), indicating that the role of Baz in myosin regulation is separate from its role in adhesion.
Figure 2. Baz/Par-3 is required for the planar polarized localization of myosin II and β-catenin.
(A,D,G,J) Localization of myosin II (Zip heavy chain, red), β-catenin (green), and Baz (blue) in stage 7 wild-type (A), bazFA50 (D), bazGD21 (G), and sqh1 (J) embryos. Left panels show overlays of myosin (red) and β-catenin (green). Anterior left, ventral down. Scale bar = 10 μm. (B,E,H) Myosin planar polarity was disrupted in bazFA50 (P<0.001) (E) and bazGD21 (P<0.001) (H) compared to wild type (B) (n=5 wild-type, 3 bazFA50, 4 bazGD21 embryos). (C,F,I) β-catenin planar polarity was disrupted in bazFA50 (P<0.001) (F) and bazGD21 (P<0.001) (I) compared to wild type (C) (n=11 wild-type, 3 bazFA50, 4 bazGD21 embryos). (K,L) Baz planar polarity was retained in sqh1 mutants (L) compared to wild type (K) (n=11 wild-type, 14 sqh1 embryos). See Figure S2.
Baz/Par-3 is downregulated at AP edges and upregulated at DV edges, establishing planar polarity
These results demonstrate that the asymmetrically localized Baz protein regulates the localization of myosin II and β-catenin in intercalating cells. We were therefore interested in identifying the upstream mechanisms responsible for Baz localization. The accumulation of Baz at DV edges could occur through a recruitment of Baz to DV edges, an inhibition of Baz association with AP edges, or a combination of both. To distinguish between these possibilities, we analyzed Baz localization in time-lapse movies of embryos expressing Baz:GFP and Myo:mCherry. Both fusion proteins are functional and correctly localized (Benton and St. Johnston, 2003; Martin et al., 2009; McGill et al., 2009).
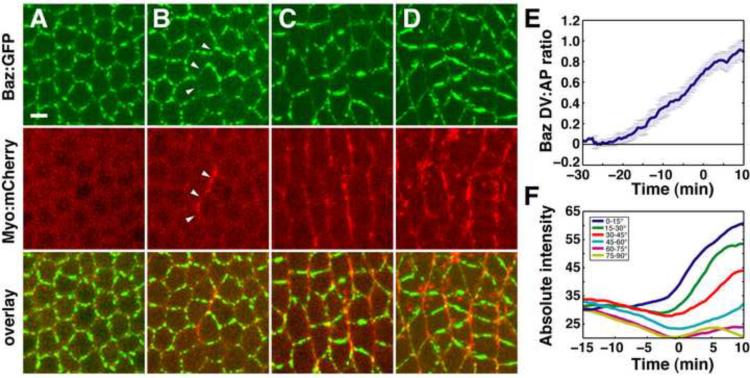
Baz was apically localized in stage 5, while myosin apical localization was first detected in stage 6 (Figure 3A,B) (Movie S3). Baz was initially present in a punctate unpolarized distribution at the apical cortex and became restricted to DV edges in stage 6, prior to the onset of intercalation (Figure 3A,B). By contrast, myosin was enriched at AP edges as soon as it was detected apically (Figure 3B). Myosin recruitment to AP edges corresponded to sites where Baz was depleted. Planar polarity became more prominent at the onset of intercalation in stage 7, when Baz was strongly localized to DV edges and myosin was predominantly AP (Figure 3C–E). Quantitation of Baz levels in time-lapse movies revealed that Baz levels initially decrease at AP edges and later increase at DV edges (Figure 3F, Figure S3). These results indicate that Baz planar polarity is generated from a uniform distribution through a dual mechanism in which Baz is first downregulated at AP edges, followed by an increase in Baz apical localization that is restricted to DV edges.
Figure 3. Baz/Par-3 is downregulated at AP edges and upregulated at DV edges, establishing planar polarity.
(A–D) Stills from a movie of intercalating cells in an embryo expressing Myo:mCherry (red) and Baz:GFP under the control of a Gal4 driver (green). t = 0 is the onset of elongation in stage 7. A −11 min, B −4 min, C 3 min, D 9 min. (B) In stage 6, Baz:GFP is displaced from and Myo:mCherry accumulates at AP edges (arrowheads). (C,D) In stage 7, Myo:mCherry and Baz:GFP occupy AP and DV edges, respectively. Scale bar = 5 μm.
(E,F) Quantitation of Baz:GFP fluorescence intensity. (E) The log2 ratio of Baz:GFP intensity at DV edges (oriented at ≤30° relative to the AP axis) relative to AP edges (oriented at ≥60° relative to the AP axis) was averaged across all cells (n = 5 embryos, 764–2142 edges/embryo). Error bars indicate s.e.m. across embryos. t = 0 is the onset of elongation in stage 7. (F) Absolute edge intensities grouped by edge orientation in a single embryo. Baz is first downregulated at AP edges (yellow, purple) and then upregulated at DV edges (dark blue, green). See Figure S3, Movie S3.
Rho-kinase is required for Baz/Par-3 planar polarity
Since the loss of Baz from AP edges correlates with the recruitment of myosin, we asked if Baz planar polarity requires myosin activity. Although it is not possible to generate embryos lacking myosin, embryos maternally mutant for a partial mutation in the myosin regulatory light chain (sqh1) (Karess et al., 1991) are severely defective for axis elongation and cell intercalation (Figure 1A,B). The myosin heavy chain was present at reduced cortical levels in these mutants and was no longer planar polarized (Figure 2J; 1/11 sqh1 embryos displayed myosin planar polarity). Despite this mislocalization of the myosin heavy chain, Baz was concentrated in DV edges as in wild type (Figure 2J–L) (9/11 sqh1 mutant embryos displayed Baz planar polarity). Therefore while Baz is necessary for myosin localization, myosin planar polarity is dispensable for Baz localization.
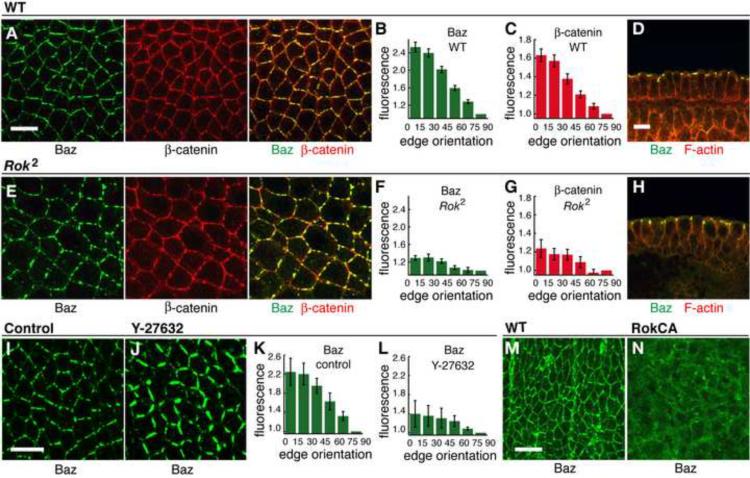
To identify alternative Baz regulators, we turned to Rho-kinase, which has been shown to phosphorylate Par-3 in cultured mammalian cells (Nakayama et al., 2008). Rho-kinase is required for myosin cortical localization in Drosophila (Figure S4), consistent with previous results in the embryo (Bertet et al., 2004; Dawes-Hoang et al., 2005) and other cell types (Winter et al., 2001; Dean and Spudich, 2006; Wang and Riechmann, 2007). To ask if Rho-kinase is required for Baz localization, we analyzed embryos maternally mutant for a null Rho-kinase mutation (DRok2 mutants). Baz association with apical adherens junctions occurred normally in DRok2 mutants, but Baz planar polarity was strongly defective (Figure 4A–H) (0/12 DRok2 mutant embryos displayed wild-type Baz planar polarity). Arm/β-catenin planar polarity was also defective in DRok2 mutants (0/12 DRok2 mutant embryos displayed wild-type β-catenin planar polarity). Similar defects were observed in embryos maternally mutant for the independent DRok1 allele (Figure S4). Two mutant classes with defects of varying severity were observed, consistent with a weak paternal contribution (Figure S4). These results demonstrate that Rho-kinase is required for the planar polarized localization of Baz and its effector proteins in intercalating cells.
Figure 4. Rho-kinase is required for Baz/Par-3 planar polarity.
(A–H) Localization of Baz (green) and β-catenin (red) in stage 7 wild-type (A–D) and DRok2 mutant (E–H) embryos. Cross sections in D,H. Quantitation of Baz and Arm/β-catenin planar polarity in wild type (B,C) and DRok2 (F,G). Baz and β-catenin planar polarity were strongly reduced in DRok2 mutant embryos compared to wild type (P<0.001 for each result) (n=11 wild-type, 8 DRok2 embryos). Two classes of defects were observed, with the more defective class shown here. See Figure S4.
(I–L) Localization of Baz (green) in stage 7 embryos injected with water (I) or Y-27632 (J). Baz planar polarity was reduced in Y-27632-injected embryos (K) compared to wild type (L) (P=0.002) (n=5 control injected, 4 Y-27632-injected embryos).
(M,N) Localization of Baz (green) in the dorsolateral epidermis of a stage 14 control embryo (M) or an embryo expressing activated Rho-kinase (Rok-CA, N). Scale bars = 10 μm.
To ask if Rho-kinase activity is required for its role in Baz localization, we injected embryos with the Rho-kinase inhibitor Y-27632 at stages 7/8 after the onset of intercalation. Y-27632 led to a loss of cortical myosin (data not shown) and a rapid redistribution of Baz within 5–10 min of injection (Figure 4I–L) (0/41 Y-27632 injected embryos displayed Baz planar polarity vs. 10/10 control injected embryos), indicating an ongoing requirement for Rho-kinase during intercalation. Y-27632 has also been shown to inhibit aPKC (Atwood and Prehoda, 2009), but aPKC is unlikely to be responsible for these defects as planar polarity is retained in aPKC and Par6 mutants (Blankenship et al., 2006; Harris and Peifer, 2007).
Rho-kinase is asymmetrically localized to AP cell borders complementary to Baz/Par-3
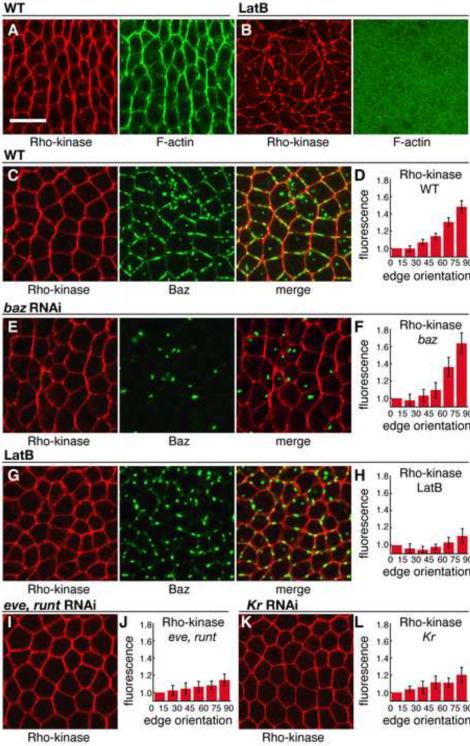
To investigate the mechanism by which Rho-kinase regulates Baz localization, we first analyzed the distribution of Rho-kinase in intercalating cells. Wild-type Rho-kinase tagged with HA was selectively enriched at AP boundaries of intercalating cells, colocalizing with F-actin (Figure 5A, 7/7 embryos). Since high levels of Rho-kinase often disrupted embryo morphology, we generated a Venus-tagged Rho-kinase variant mutated for a single conserved residue in the kinase domain that disrupts catalytic activity (Winter et al., 2001). Rho-kinaseK116A localized to the cellularization front in stage 5 and to the apical cortex in stage 6, in a region that overlaps with the adherens junctions (data not shown). At stage 6 and during cell intercalation in stages 7/8, Rho-kinaseK116A was present in a planar polarized distribution at AP edges (Figure 5C,D), similar to the wild-type protein (Figure 5A).
Figure 5. Rho-kinase is planar polarized in intercalating cells.
(A,B) Localization of Rho-kinase (HA:Rok, red) and F-actin (phalloidin, green) in stage 7 wild-type (A) or LatB-injected (B) embryos.
(C–L) Localization of Rho-kinase (Venus:RokK116A, red) and Baz (green). Stage 7 wild-type embryo (C) or embryos injected with baz dsRNA (E), LatB (G), eve and runt dsRNA (I), or Kr dsRNA (K). Quantitation of planar polarity in wild-type (D) and injected embryos (F,H,J,L). Rho-kinase planar polarity was not affected by baz RNAi (F) (n=6 wild-type Venus:RokK116A expressing embryos, 7 baz dsRNA-injected embryos, P=0.29). Rho-kinase planar polarity was reduced by injection of LatB (H) (5 embryos, P<0.001), eve and runt dsRNA (J) (7 embryos, P<0.001), or Kr dsRNA (L) (4 embryos, P=0.01). Scale bar = 10 μm.
Rho-kinase planar polarity occurred normally in embryos depleted for Baz (Figure 5E,F) (16/16 baz dsRNA-injected embryos displayed Rho-kinase planar polarity vs. 9/9 control injected embryos), which may explain why the residual myosin contractility in baz mutants is correctly oriented (Figure 1D). To ask if F-actin is required for Rho-kinase planar polarity, we injected embryos with Latrunculin B (LatB), a toxin that inhibits actin polymerization. LatB injection during intercalation in stages 7/8 resulted in a rapid loss of Rho-kinase planar polarity and a reduction of Baz cortical localization within 5–10 min of injection (Figure 5B,G,H) (0/13 LatB-injected embryos displayed Rho-kinase planar polarity vs. 11/11 control injected embryos). These results indicate that an actin-dependent asymmetry is required for Rho-kinase planar polarity, but not for its cortical localization.
Spatial cues from the AP patterning system are necessary and sufficient for planar cell polarity (Zallen and Wieschaus, 2004). RNAi-mediated disruption of the pair rule genes eve and runt or the gap gene Kruppel resulted in a loss of Rho-kinase planar polarity (Figure 5I–L) (0/10 eve and runt dsRNA-injected embryos and 0/4 embryos Kruppel dsRNA-injected embryos displayed Rho-kinase planar polarity), indicating that asymmetric Rho-kinase localization requires the AP patterning system.
Rho-kinase is sufficient to displace Baz/Par-3 from the cortex
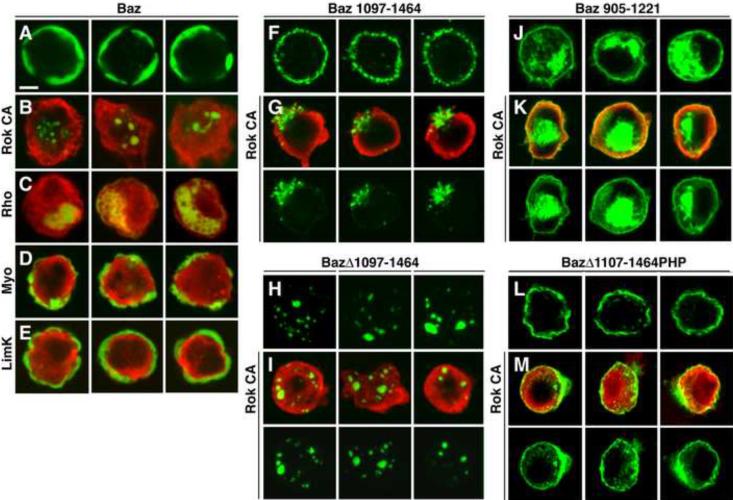
Rho-kinase accumulates in cortical domains where Baz levels are reduced, suggesting that Rho-kinase could inhibit Baz association with the cortex. To test this model, we generated a variant of Rho-kinase that terminates after 553 of 1390 amino acids before the Rho-binding and pleckstrin homology domains, resulting in constitutive kinase activity (Amano et al., 1996; Winter et al., 2001). Although we were unable to obtain high levels of activated Rho-kinase in the early embryo, presumably due to toxicity of the transgene, expression of activated Rho-kinase (Rok CA) in late embryos resulted in loss of Baz from the cortex (Figure 4M,N) (1/6 embryos expressing Rok CA had cortical Baz localization vs. 7/7 wild-type embryos). Rho-kinase also displaced Baz from the cortex in cultured cells (Figure 6A,B). Similar effects were observed when Baz was coexpressed with activated Rho1 GTPase (RhoV14), an upstream activator of Rho-kinase (Figure 6C). Together, these results indicate that Rho-kinase is necessary and sufficient to exclude Baz from the cortex, consistent with an instructive role in regulating Baz localization.
Figure 6. Rho-kinase regulates the association of the Baz/Par-3 C-terminal domain with the cortex.
(A–E) Drosophila S2R+ cells expressing Baz:Venus alone (green, A) or with activated versions of Rho-kinase (Rok CA red, B), Rho1 GTPase (RhoV14 red, C), myosin regulatory light chain (sqhE20,E21 red, D) or Lim kinase (limKE591 isoform C red, E). Baz:Venus was cortical in 78% of cells without Rok CA (n=216) vs. 5% with Rok CA (n=284). Expression of RhoV14, but not sqhE20,E21 or limKE591, led to a loss of Baz from the cortex.
(F–K) Cells expressing Baz:Venus variants alone (green, F,H,J) or with Rok CA (red, G,I,K). (F,G) The Baz C-terminus is sufficient for cortical localization (F) and is displaced from the cortex by Rok CA (G). Baz 1097-1464 was cortical in 99% of cells without Rok CA (n=190) vs. 2% with Rok CA (n=178). (H,I) Deletion of the Baz C-terminus (BazΔ1097–1464) abolished Baz cortical localization in the absence or presence of Rok CA. (J,K) An adjacent region of the Baz C-terminus (Baz 905–1221) was not affected by Rok CA. Baz 905–1221 was cortical in 91% of cells without Rok CA (n=137) vs. 97% with Rok CA (n=208).
(L,M) Cells expressing a GFP:Baz variant where the C-terminus was replaced with a heterologous PH domain. BazΔ1107–1464PHP localizes to the cortex (L) but was not efficiently displaced by Rok CA (M). GFP:Baz cortical localization was reduced 5.5-fold from 94% to 17% by Rok CA. GFP:BazΔ1107–1464PHP cortical localization was reduced 1.4-fold from 74% to 54% by Rok CA (n=183–257 cells/condition). Scale bar = 5 μm. See Figures S5,6.
The regulation of Baz by Rho-kinase could be direct or it could occur indirectly through alternative Rho-kinase effectors. To distinguish between these possibilities, we asked if Baz localization is affected by two known substrates of Rho-kinase, myosin II and Lim kinase. Expression of a phosphomimetic form of the myosin regulatory light chain can bypass the requirement for Rho-kinase in some cell types (Winter et al., 2001; Dean and Spudich, 2006; Monier et al., 2010). However, activated myosin regulatory light chain (sqhE20,E21) did not disrupt Baz cortical localization in cultured cells (Figure 6D) and did not rescue the effect of Y-27632 injection on Baz localization in vivo (data not shown). Similarly, activated Lim kinase (limKE591 isoform C) did not disrupt Baz cortical localization in cultured cells, despite effects on the actin cytoskeleton indicating that the protein was active (Figure 6E and data not shown). These results indicate that the regulation of Baz by Rho-kinase is unlikely to occur through myosin II or Lim kinase, although these or other Rho-kinase substrates could contribute to Baz localization in vivo.
Baz/Par-3 planar polarity requires the C-terminal domain, which is phosphorylated by Rho-kinase in vitro
Rho-kinase can phosphorylate mammalian Par-3 at Thr833 in the aPKC binding domain, disrupting its association with aPKC (Nakayama et al., 2008). This residue is not conserved in Drosophila or C. elegans, raising the question of whether this is a conserved mechanism of Par-3 regulation. To ask if Rho-kinase regulates the interaction of Baz with aPKC in Drosophila, we cotransfected S2R+ cells with Baz and aPKC with or without activated Rho-kinase. We found that Baz coimmunoprecipitated with aPKC in the presence or absence of activated Rho-kinase (Figure S5), suggesting that Rho-kinase is not sufficient to disrupt the Baz-aPKC interaction.
A conserved region in the Baz C-terminal domain has been shown to mediate Baz cortical association through a direct interaction with phosphoinositide membrane lipids (Krahn et al., 2010). Baz lacking its C-terminal domain fails to localize to the cortex while the C-terminal domain alone is sufficient for cortical association (Figure 6F,H) (Krahn et al., 2010). We found that the C-terminal domain of Baz (amino acids 1097–1464) was efficiently excluded from the cortex by Rho-kinase, with residual accumulation in actin-rich membrane blebs (Figure 6G). This effect was specific, as Rho-kinase did not interfere with the cortical localization of an overlapping Baz fragment that contains the aPKC-binding motif and part of the C-terminal domain (amino acids 905–1221) (Figure 6J,K). These results indicate that Rho-kinase acts on the Baz C-terminal domain to inhibit its association with the cell cortex.
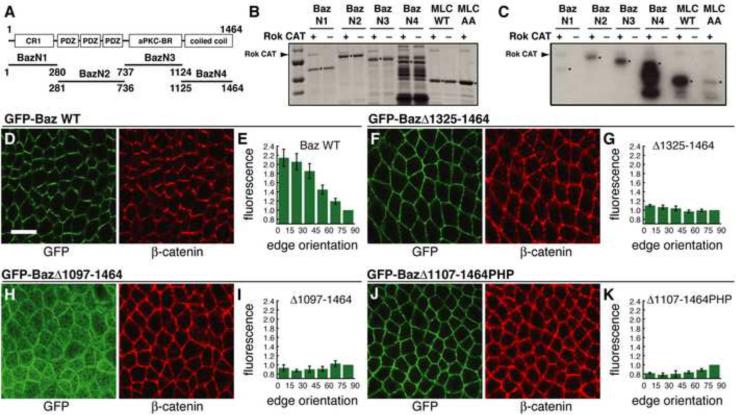
Since mammalian Par-3 is a substrate for Rho-kinase (Nakayama et al., 2008), we asked if this interaction is conserved in Drosophila. We tested the in vitro kinase activity of human Rho-kinase 2, which is 75% identical to Drosophila Rho-kinase in the kinase domain, on Baz fragments purified from E. coli. The C-terminal region of Baz (amino acids 1125–1464), which has homology to coiled-coil domains (Nishimura et al., 2005), was efficiently phosphorylated by Rho-kinase (Baz N4, Figure 7A–C). The Baz N2 fragment (amino acids 281–736), which contains the PDZ domains, and the Baz N3 fragment (amino acids 737–1124), which contains the aPKC binding region, were also weakly phosphorylated in this assay (Figure 7A–C).
Figure 7. The Baz/Par-3 C-terminal domain is necessary for Baz planar polarity.
(A–C) GST-Baz fragments were incubated with activated Rho-kinase (Rok-CA) and γ-32P-ATP. (A) Domains in each fragment. (B) Total protein quantified by Coomassie staining. Molecular weight markers in kD (top to bottom): 100, 75, 50 and 37. (C) Phosphorylated proteins were detected by autoradiography. Arrowheads indicate Rok-CA, which shows weak autophosphorylation. Asterisks indicate GST-Baz fragments, wild-type chick myosin regulatory light chain (MRLC) (positive control), and unphosphorylatable chick MRLC T19A,S20A (negative control).
(D–K) Localization of GFP-Baz transgenes in stage 7 embryos. Endogenous baz was knocked down by dsRNA injection and embryos were labeled with antibodies to GFP (green) and β-catenin (red). (D,E) Full-length GFP-Baz displays wild-type planar polarity (5 embryos). (F,G) GFP-BazΔ1325–1464 localizes to the cortex but fails to localize asymmetrically (4 embryos, P<0.001). (H,I) GFP-BazΔ1097–1464 lacking a larger region of the C-terminus shows reduced cortical association and fails to localize asymmetrically (3 embryos, P<0.001). (J,K) GFP-BazΔ1107–1464:PHP where the C-terminal 358 amino acids of Baz were replaced with a heterologous PH domain restores cortical localization but fails to localize asymmetrically (6 embryos, P<0.001). Scale bar = 10 μm. See Figure S6.
To test the role of the Baz C-terminal domain in vivo, we expressed truncated Baz proteins in intercalating cells. Since Baz can oligomerize through its N-terminal domain (Benton and St. Johnston, 2003; Mizuno et al., 2003), endogenous Baz levels were reduced by injecting dsRNA homologous to the baz 5' untranslated region, which was not present in the transgenes. Full-length Baz localized correctly to DV edges (Figure 7D,E) (11/11 embryos displayed GFP-Baz planar polarity). By contrast, a deletion construct lacking the C-terminal 140 amino acids abolished Baz planar polarity (Figure 7F,G) (0/11 embryos displayed GFP-BazΔ1325–1464 planar polarity) but retained cortical localization as previously reported (Krahn et al., 2010). Similar results were obtained for a construct that removes a larger region of the Baz C-terminal domain (1/7 embryos displayed GFP-BazΔ1222–1464 planar polarity). A deletion construct lacking the entire C-terminal domain showed reduced cortical localization (Krahn et al., 2010) and the residual protein that reached the cortex failed to localize asymmetrically (Figure 7H,I) (0/5 embryos displayed GFP-BazΔ1097–1464 planar polarity). These results demonstrate that the C-terminal domain is necessary for Baz planar polarity in vivo.
The Baz C-terminus binds directly to phosphoinositide membrane lipids, and replacement of this domain with a heterologous phospholipid binding pleckstrin homology (PH) domain from human phospholipase Cδ is sufficient to restore Baz cortical localization (Krahn et al., 2010). We found that this Baz variant was not displaced from the cortex by Rho-kinase in cultured cells (Figure 6L,M) and did not achieve a planar polarized distribution in vivo (Figure 7J,K) (0/7 embryos displayed GFP-BazΔ1107–1464PHP planar polarity). These results suggest a model in which Rho-kinase regulates Baz planar polarity by inhibiting the association of the Baz C-terminal domain with the cell cortex. Consistent with this, Rho-kinase inhibits the ability of the Baz C-terminal domain to bind phosphoinositide membrane lipids in vitro (Figure S6). In the absence of Rho-kinase, the Baz C-terminal fragment (amino acids 1125–1464) bound to PIP, PIP2, PIP3 and phosphatidic acid. Phosphorylation by Rho-kinase efficiently disrupted this binding, with the strongest effects for PIP2 and PIP3. This effect was reversed by the Y-27632 Rho-kinase inhibitor. Together, these results demonstrate that the C-terminal domain is necessary for Baz planar polarity and suggest that Rho-kinase regulates Baz cortical localization by reducing the affinity of the Baz C-terminal domain for phosphoinositide membrane lipids.
Discussion
The spatially regulated activity of protein kinases with multiple substrates provides an efficient strategy for the control of cell polarity in different contexts. Here we show that Rho-kinase is an asymmetrically localized protein that plays an instructive role in planar polarity in the Drosophila embryo by excluding its substrate Baz/Par-3 from the cell cortex. Rho-kinase prevents expansion of the Baz domain and Baz in turn directs the localization of contractile and adherens junction proteins that are required for axis elongation, converting a localized source of kinase activity into a robust bias in polarized cell behavior. The effect of Rho-kinase on Baz planar polarity appears to be independent of its role in regulating myosin II, as Baz localization is not affected in myosin mutants and activated myosin does not reproduce the effects of Rho-kinase in culture. Instead, Rho-kinase can directly phosphorylate the Baz C-terminal coiled-coil domain that is required for Baz association with the cortex. Deletions within the Baz C-terminal domain or replacement of the Baz C-terminus with a heterologous phospholipid binding motif abolish Baz planar polarity in vivo. These results are consistent with a model in which Rho-kinase directly inhibits the association of the Baz C-terminal domain with specific regions of the cell cortex.
Rho-kinase has been shown to phosphorylate mammalian Par-3 in cultured cells, disrupting its interaction with the Par complex proteins Par-6 and aPKC (Nakayama et al., 2008). The Par complex is necessary for some aspects of epithelial organization (Nagai-Tamai et al., 2002; Horikoshi et al., 2009; McCaffrey and Macara, 2009) but dispensable for others (Chen and Macara, 2005; Morais de Sa et al., 2010; Walther and Pichaud, 2010). Par-6 and aPKC are not required for Baz planar polarity in Drosophila (Blankenship et al., 2006; Harris and Peifer, 2007), suggesting that the role of Rho-kinase in this process is unlikely to occur through a similar mechanism. Here we provide evidence for a different mechanism of regulation by Rho-kinase involving the Baz C-terminal domain, which is phosphorylated by Rho-kinase in vitro and is necessary for Baz planar polarity in vivo. The Baz C-terminus has been shown to bind directly to phosphoinositide membrane lipids including PI(3,4,5)P3, PI(3,4)P2 and PIP (Krahn et al., 2010). We show that Rho-kinase inhibits the association of Baz with phosphoinositide membrane lipids in vitro, consistent with a model in which Rho-kinase directly regulates Baz association with the cortex. Alternatively, Rho-kinase could regulate Baz localization indirectly through other proteins that interact with the Baz C-terminal domain. Despite potential differences in the mechanism, these results demonstrate that the regulation of Par-3 localization or activity by Rho-kinase is a conserved feature of cell polarity in Drosophila and mammals.
The results presented here demonstrate that Rho-kinase is an asymmetrically localized protein that initiates a cascade of events required for the planar polarized distribution of contractile and adherens junction proteins in intercalating cells. The upstream signals that generate localized Rho-kinase activity are not known. Differences between cells conferred by striped or graded patterns of gene expression orient cell movement during axis elongation (Irvine and Wieschaus, 1994; Ninomiya et al., 2004; Zallen and Wieschaus, 2004), and AP patterning genes expressed in stripes are necessary for the asymmetric localization of Rho-kinase (this work). These findings raise the possibility that planar cell polarity may be generated by the local activation of a Rho GTPase signaling pathway. The Drosophila genome contains 21 RhoGEFs and 19 RhoGAPs that are candidate upstream regulators in this process (Hu et al., 2004). Rho GTPase pathways are activated by a number of upstream signals including G protein-coupled receptors, receptor tyrosine kinases, cytokine receptors, and cell-cell and cell-substrate adhesion. Identification of the signals upstream of Rho-kinase will help to elucidate the spatial cues that initiate planar polarity in the Drosophila embryo.
The role of Rho-kinase in planar cell polarity is reinforced by the effect of Baz on the localization of contractile and adherens junction proteins. The relationship between Baz and myosin II is complex. In the C. elegans zygote, a contractile myosin network carries PAR-3 to the anterior cell cortex (Munro et al., 2004), suggesting a positive relationship between these proteins. In other cell types myosin appears to be dispensable for Baz localization (Simone and DiNardo, 2010 and this work). PAR-3 is required to sustain myosin contractility in C. elegans and Drosophila (Munro et al., 2004; David et al., 2010), and Baz promotes myosin apical localization during C. elegans gastrulation (Nance et al., 2003) and in the Drosophila follicular epithelium (Wang and Riechmann, 2007). The ectopic association of myosin with DV cell boundaries in baz mutants, and the complementary distributions of Baz and myosin in several contexts (Zallen and Wieschaus, 2004; Major and Irvine, 2006; Simone and DiNardo, 2010), raise the possibility of inhibitory effects of Baz on myosin. This regulation could also occur indirectly through effects of Baz on apical-basal polarity (Goldstein and Macara, 2007) or the actin cytoskeleton (Chen and Macara, 2005; Ramachandran et al., 2009).
Differential adhesion is sufficient to drive cell sorting in culture (Steinberg and Takeichi, 1994) and has been proposed to influence tissue morphogenesis in vivo (Irvine and Wieschaus, 1994; Zallen and Blankenship, 2008). We show that Rho-kinase and Baz regulate the planar polarized localization of the adherens junction protein β-catenin. Rho-kinase has been shown to downregulate adhesion in culture (Sahai and Marshall, 2002, Samarin et al., 2007), an activity that is thought to occur through myosin II, which can play positive and negative roles in junctional stabilization. The ability of Rho-kinase to exclude the Baz/Par-3 junctional regulator from the cortex suggests an alternative mechanism for the regulation of adherens junctions by Rho GTPases. These results suggest that Rho-kinase can both promote contractility and inhibit adhesion, providing a single molecular mechanism linking cortical contraction with adherens junction disassembly during tissue morphogenesis.
Experimental Procedures
Fly stocks and genetics
Embryos were generated at 20°C. Wild type was y,w unless otherwise indicated. Alleles were bazFA50, bazGD21 (gifts of N. Denef and T. Schüpbach), arm043A06 (Tolwinski and Wieschaus, 2001), DRok1, DRok2 (Winter et al., 2001), and sqh1 (Karess et al., 1991). Embryos expressing UASp-Venus:RokK116A (this work), UASp-HA:Rok (Wang and Riechmann, 2007), UASp-Baz:GFP (Benton and St. Johnston, 2003), or Baz transgenes (Krahn et al., 2010) were the F2 progeny of UASp males x matαtub67;15 females (gift of D. St. Johnston). Germline clones were generated with the FLP-DFS system and ovoD1 FRT101 (Chou and Perrimon, 1996), ovoD2 FRT19A (gift of N. Tolwinski) or ovoD2 FRT18D.
The baz alleles were sequenced by PCR amplification of all exons and splice junctions from GFP negative larval progeny of FM7,Kr-Gal4,UAS-GFP heterozygous females and FM7,Kr-Gal4,UAS-GFP males and confirmed in independent PCR reactions from different larvae. The bazGD21 allele contains a 4-nt deletion of nt 793–796 in the open reading frame, introducing a frameshift predicted to truncate the protein after 264 of 1464 amino acids, removing the PDZ domains, aPKC-binding region and C-terminal domain. The bazFA50 allele contains an 8-nt deletion of nt 2463–2470, introducing a frameshift predicted to truncate the protein after 821 of 1461 amino acids, removing the aPKC-binding region and C-terminal domain.
Transgenic lines
UASp-Venus:RokK116A was generated by cloning full-length DRok cDNA into the pENTR/D TOPO cloning vector and cloning Venus into the AscI site followed by site-directed mutagenesis (Stratagene Quik-Change system). Clones were recombined into pUASp-w-attB (gift of Mike Buszczak) with the Gateway system (Invitrogen). pUASp-HA:Rok-CA was generated by amplifying the first 553 codons of the DRok cDNA and HA from pUASp-HA:Rok (Wang and Riechmann, 2007) and subcloned into pUASp. Transgenes were inserted in the attP40 site on chromosome II (Genetic Services).
Immunohistochemistry
For antibodies to Arm/β-catenin, Baz, HA and myosin, embryos were boiled 10 s in 0.03% Triton X-100/0.4% NaCl, cooled on ice and devitellinized in heptane:methanol. For antibodies to GFP, embryos were fixed 20 min in 4% PFA (EMS) in PBS/heptane and devitellinized in heptane:methanol. For phalloidin, embryos were fixed 1 hr in 4% PFA in PBS/heptane and manually devitellinized. Antibodies were used at the following concentrations: mouse anti-Arm (1:50, Developmental Studies Hybridoma Bank, DSHB), guinea pig anti-Baz (1:500, made by JAZ as in Wodarz et al., 2000), rat anti-DE-cadherin (1:100, DSHB), rabbit anti-GFP (1:100, Torrey Pines), rat anti-HA (1:500, Roche) and rabbit anti-myosin II heavy chain (Zipper, 1:1250, gift of C. Field). Secondary antibodies conjugated to Alexa-488, Alexa-568, or Alexa-647 (Molecular Probes) were used at 1:500. Rhodamine-conjugated phalloidin (Molecular Probes) was used at 1:1000. Embryos were mounted in Prolong Gold (Molecular Probes) and imaged on a Zeiss LSM510 META confocal with a PlanNeo 40×/1.3NA objective. 1.0 μm Z slices were acquired at 0.5 μm steps. Maximum intensity projections of 2–3 μm in the apical junctional domain were analyzed.
Time-lapse imaging
Time-lapse imaging was done with the following markers: GFP:Spider and GFP:Resille (gifts of Alain Debec), ubi-DE-cadherin:GFP (Oda and Tsukita, 2001), sqh-Sqh:GFP (Royou et al., 2004), UASp-Baz:GFP (Benton and St. Johnston, 2003), sqh-Sqh:mCherry (Martin et al., 2009) and a GFP insertion in the endogenous baz gene (Fly Trap). Embryos were dechorionated 1 min in 50% bleach, washed in water, mounted on a YSI membrane with halocarbon oil 27 (Sigma) and imaged on a Perkin Elmer RS5 spinning disk confocal with a Zeiss PlanNeo 40×/1.3NA objective. Z stacks were acquired at 1 μm steps and 15 s intervals. Maximum intensity projections of 2–3 μm in the apical junctional domain were analyzed.
Polarity measurements
User-drawn 3-pixel wide lines for all edges in a 50 μm × 50 μm region were used to calculate the mean pixel intensity and orientation of each edge. Intensities were averaged for all edges in a 15° angular bin after subtracting background (defined as the average value of cytoplasmic pixels ≥ 1 μm from the plasma membrane) with custom Matlab software. Mean intensity values in each bin were normalized to the mean intensity of edges parallel (0–15°) or perpendicular (75–90°) to the AP axis. A representative subset of images was quantified for each experiment. A mean value was obtained for each embryo and error bars indicate s.e.m. in all figures. P values were calculated by comparing normalized mean intensities of edges in the 0–30° or 60–90° angular range in mutant and control embryos with the F test followed by the appropriate t test.
Drug injection
Stage 7/8 embryos were dechorionated, glued to a cover slip, dehydrated 5 min, covered in 1:1 halocarbon oil 27/700 and injected in the ventral perivitelline space with 100–200 pl of 10–100 mM Rho-kinase inhibitor Y-27632 (TOCRIS) in water or 10 mM Latrunculin B (Sigma) in DMSO. Control embryos were injected with water or DMSO. Drugs are diluted ~50-fold in the embryo. For immunostaining, embryos were washed off the cover slip with heptane 5–10 min after injection, fixed 1 h in 4% PFA in PBS/heptane and manually devitellinized.
Supplementary Material
Acknowledgments
We are grateful to Andreas Wodarz for sharing information and reagents prior to publication, Natalie Denef and Trudi Schüpbach for the baz alleles, Nick Tolwinski for the ovoD2 FRT19A stock, and Mike Buszczak for the pUASp-w-attB plasmid. We thank Justina Sanny and Leah Greenspan for excellent technical assistance and Emily Marcinkevicius, Athea Vichas, Frederik Wirtz-Peitz and Richard Zallen for helpful discussions and comments on the manuscript. This work was supported by a postdoctoral fellowship from Fundação para a Ciência e Tecnologia, Portugal to SS and a Burroughs Wellcome Fund Career Award in the Biomedical Sciences, March of Dimes Basil O'Connor Starter Scholar Award, Searle Scholar Award, W. M. Keck Foundation Distinguished Young Scholar in Medical Research Award, and NIH/NIGMS R01 grant GM079340 to JAZ. JAZ is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Atwood SX, Prehoda KE. aPKC phosphorylates Miranda to polarize fate determinants during neuroblast asymmetric cell division. Curr Biol. 2009;19:723–729. doi: 10.1016/j.cub.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, St Johnston D. A conserved oligomerization domain in Drosophila Bazooka/PAR-3 is important for apical localization and epithelial polarity. Curr Biol. 2003;13:1330–1334. doi: 10.1016/s0960-9822(03)00508-6. [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- David DJ, Tishkina A, Harris TJ. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development. 2010;137:1645–1655. doi: 10.1242/dev.044107. [DOI] [PubMed] [Google Scholar]
- Dean SO, Spudich JA. Rho kinase's role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS One. 2006;1:e131. doi: 10.1371/journal.pone.0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schüpbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes S, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–738. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci. 2009;122:1595–1606. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- Hu H, Li M, Labrador JP, McEwen J, Lai EC, Goodman CS, Bashaw G. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc Natl Acad Sci. 2005;102:4613–4618. doi: 10.1073/pnas.0409325102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Irvine KD, Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994;120:827–841. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- Karess RE, Chang XJ, Edwards KA, Kulkarni S, Aguilera I, Kiehart DP. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell. 1991;65:1177–1189. doi: 10.1016/0092-8674(91)90013-o. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Krahn MP, Klopfenstein DR, Fischer N, Wodarz A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr Biol. 2010;20:1–7. doi: 10.1016/j.cub.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsalventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. The Par-3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009;223:1450–1460. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, McKinley RFA, Harris TJC. Independent cadherin-catenin and Bazooka clusters interact to assemble adherens junctions. J Cell Biol. 2009;185:787–796. doi: 10.1083/jcb.200812146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Suzuki A, Hirose T, Kitmura K, Kutsuzawa K, Futaki M, Amano Y, Ohno S. Self-association of PAR-3 mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J Biol Chem. 2003;278:31240–31250. doi: 10.1074/jbc.M303593200. [DOI] [PubMed] [Google Scholar]
- Monier B, Pelissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–65. doi: 10.1038/ncb2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais de Sa E, Mirouse V, St. Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7:1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130:5339–5350. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2001;114:493–501. doi: 10.1242/jcs.114.3.493. [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Barria R, Ashley J, Budnik V. A critical step for postsynaptic F-actin organization: regulation of Baz/Par-3 localization by aPKC and PTEN. Dev Neurobiol. 2009;69:583–602. doi: 10.1002/dneu.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Rosko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–997. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou A, Field C, Sisson JC, Sullivan W, Karess R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol Biol Cell. 2004;15:838–850. doi: 10.1091/mbc.E03-06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007;18:3429–3439. doi: 10.1091/mbc.E07-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone RP, DiNardo S. Actomyosin contractility and Discs large contribute to junctional conversion in guiding cell alignment within the Drosophila embryonic epithelium. Development. 2010;137:1385–1394. doi: 10.1242/dev.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammlian epithelial polarity. Curr Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development. 2001;128:2107–2117. doi: 10.1242/dev.128.11.2107. [DOI] [PubMed] [Google Scholar]
- Verdier V, Johndrow JE, Betson M, Chen GC, Hughes DA, Parkhurst SM, Settleman J. Drosophila Rho-kinase (DRok) is required for tissue morphogenesis in diverse compartments of the egg chamber during oogenesis. Dev Biol. 2006;297:417–432. doi: 10.1016/j.ydbio.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol. 2010;20:1065–1074. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–1355. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161–173. doi: 10.1016/j.cell.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Semin Cell Dev Biol. 2008;19:263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.