SUMMARY
Purpose
The absence of validated methods to identify hepatic decompensation in cohort studies has prevented a full understanding of the natural history of chronic liver diseases and impact of medications on this outcome. We determined the ability of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events within the Veterans Aging Cohort Study (VACS).
Methods
Medical records of patients with hepatic decompensation codes and/or laboratory abnormalities of liver dysfunction (total bilirubin ≥5.0 gm/dL, albumin ≤2.0 gm/dL, international normalized ratio ≥1.7) recorded one year before through six months after VACS entry were reviewed to identify decompensation events (i.e., ascites, spontaneous bacterial peritonitis, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma) at VACS enrollment. Positive predictive values (PPVs) of diagnostic codes, laboratory abnormalities, and their combinations for confirmed outcomes were determined.
Results
Among 137 patients with a hepatic decompensation code and 197 with a laboratory abnormality, the diagnosis was confirmed in 57 (PPV, 42%; 95% CI, 33% – 50%) and 56 (PPV, 28%; 95% CI, 22% – 35%), respectively. The combination of any code plus laboratory abnormality increased PPV (64%; 95% CI, 47% - 79%). One inpatient or ≥2 outpatient diagnostic codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage had high PPV (91%; 95% CI, 77% – 98%) for confirmed hepatic decompensation events.
Conclusion
An algorithm of 1 inpatient or ≥2 outpatient codes for ascites, peritonitis, or variceal hemorrhage has sufficiently high PPV for hepatic decompensation to enable its use for epidemiologic research in VACS. This algorithm may be applicable to other cohorts.
Keywords: hepatic decompensation, end-stage liver disease, epidemiologic methods, outcomes, validation studies
INTRODUCTION
Hepatic decompensation represents the principal endpoint of chronic liver disease, particularly in patients with chronic viral hepatitis infection. Despite the clinical importance of this event, epidemiologic studies among chronic liver disease patients have largely focused on histological evidence of advanced fibrosis and/or cirrhosis.1-7 These studies are, however, only able to include patients who have undergone a liver biopsy, but certain groups, such as patients coinfected with human immunodeficiency virus (HIV) and viral hepatitis, undergo liver biopsy only rarely due to high rates of contraindications to the procedure (e.g., active alcohol use; coagulopathy) and patient refusal.8 Moreover, observational studies which focus exclusively on histological endpoints may be subject to selection bias if those who undergo liver biopsy are systematically different with respect to risk factors for advanced fibrosis.
The ability to identify hepatic decompensation within large cohorts would enable a fuller understanding of the epidemiology and natural history of chronic liver diseases and permit evaluation of the impact of medications on this outcome. However, there are very few data evaluating methods to identify hepatic decompensation events within observational cohort studies. To address this issue, we determined the ability of diagnostic codes, laboratory abnormalities, and their combinations to identify cases of hepatic decompensation at enrollment in the Veterans Aging Cohort Study.
METHODS
Study Design and Data Source
We conducted a cross-sectional study among patients enrolled in the Veterans Aging Cohort Study (VACS), an ongoing, prospective cohort study begun in June 2002 that follows HIV-infected and demographically similar HIV-uninfected US veterans receiving medical care at eight Veterans Health Administration (VA) facilities (Atlanta, GA; Bronx, NY; Houston, TX; Los Angeles, CA; Manhattan/Brooklyn, NY; Baltimore, MD; Washington, DC; Pittsburgh, PA).9 After informed consent is obtained, VACS patients complete a standardized questionnaire, at enrollment and then annually, that collects information on medical and psychiatric comorbidities, drug and alcohol use, quality of life, and medication adherence. VACS also collects electronic data from the VA's Centralized Patient Record System (CPRS),10 including demographic information, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes, laboratory values, progress notes, dispensed medications, microbiology results, pathology reports, radiology reports, and hospital discharge summaries. The electronic medical record (CPRS) is the primary source of clinical information in the VA system, and there are no paper-based records of inpatient or outpatient care.
Study Subjects
We identified patients who had inpatient and outpatient ICD-9-CM codes (Table 1) suggestive of hepatic decompensation recorded at VACS enrollment (i.e., one year before through six months after entry into the cohort) to identify possible prevalent cases. We also identified patients who had severe abnormalities in liver synthetic function suggesting hepatic decompensation, including total bilirubin ≥5.0 gm/dL, albumin ≤2.0 gm/dL, and/or an international normalized ratio (INR) ≥1.7 in the absence of warfarin use. All patients enrolled in VACS through August 15, 2005 were eligible for inclusion.
Table 1.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes used to identify hepatic decompensation events.
| Diagnosis | ICD-9-CM Code and Description |
|---|---|
| Ascites | 789.5 Ascites 572.3 Portal hypertension |
| Spontaneous bacterial peritonitis | 567.23 Spontaneous bacterial peritonitis 567.0 Peritonitis in infectious diseases classified elsewhere 567.2 Other suppurative peritonitis 567.8Other peritonitis 567.9Unspecified peritonitis |
| Variceal hemorrhage | 456.0Esophageal varices with bleeding 456.1Esophageal varices without mention of bleeding 456.2Esophageal varices in diseases classified elsewhere 456.20Esophageal varices in diseases classified elsewhere with bleeding 456.21Esophageal varices in diseases classified elsewhere without bleeding |
| Hepatic encephalopathy | 572.2 Hepatic encephalopathy 070.0 Viral hepatitis A with hepatic coma 070.2x Viral hepatitis B with hepatic coma 070.22Viral hepatitis B with hepatic coma – chronic, without mention of hepatitis delta 070.23Viral hepatitis B with hepatic coma – chronic, with hepatitis delta 070.4 Other specified viral hepatitis with hepatic coma 070.41 Acute or unspecified hepatitis C with hepatic coma 070.44 Chronic hepatitis C with hepatic coma 070.49 Other specified viral hepatitis with hepatic coma 070.6 Unspecified viral hepatitis with hepatic coma |
| Hepatocellular carcinoma | 155.0 Hepatocellular carcinoma |
| Other Conditions Potentially Suggestive of Hepatic Decompensation | 570 Acute and subacute necrosis of liver 571.2 Alcoholic cirrhosis of liver 571.5Cirrhosis of liver no alcohol 571.6Biliary cirrhosis 571.8Other chronic nonalcoholic liver disease 571.9Unspecified chronic liver disease without mention of alcohol 572.4 Hepatorenal syndrome 572.8 Other sequelae of chronic liver disease 782.4 Jaundice |
Definitions of Hepatic Decompensation Events
We defined hepatic decompensation by the presence of any one of the following five major complications of cirrhosis: ascites, spontaneous bacterial peritonitis, esophageal/gastric variceal hemorrhage, hepatic encephalopathy, and/or hepatocellular carcinoma.11,12 Definitions for each hepatic decompensation event were based on guidelines by the American Association for the Study of Liver Diseases and European Association for the Study of the Liver. Events were further classified as definite or possible based on medical record data. These definitions are listed in Table 2 and are discussed in detail in the Appendix.
Table 2.
Criteria for defining definite and possible hepatic decompensation events.
| DEFINITE EVENT: A definitive hepatic decompensation event occurred if at least one of the following criteria was met: | |
|---|---|
| Clinical Finding | Diagnosis |
| Any ascites documented in a radiographic report from an abdominal ultrasound, CT, and/or MRI in a patient with chronic liver disease28 | Ascites |
| Abdominal paracentesis (indicated by presence of results of peritoneal fluid analysis) performed in a patient with underlying liver disease28 | Ascites |
| Ascitic fluid absolute polymorphonuclear leukocyte count ≥250 cells/mL28,29 | Spontaneous bacterial peritonitis |
| Bacterial growth from ascitic fluid bacterial culture28,29 | Spontaneous bacterial peritonitis |
| Active or recent variceal bleeding reported on esophagogastroduodenoscopy report30,31 | Variceal hemorrhage |
| Non-bleeding varices on esophagogastroduodenoscopy in setting of acute gastrointestinal bleeding, without evidence of alternate cause for bleeding identified30,31 | Variceal hemorrhage |
| Mental confusion consistent with hepatic encephalopathy documented in a gastroenterologist's or hepatologist's progress note, in a patient with known chronic liver disease in absence of non-hepatic causes32 | Hepatic encephalopathy |
| Diagnosis of hepatocellular carcinoma documented on tissue biopsy report33,34 | Hepatocellular carcinoma |
| Arterial-enhancing liver mass >2 cm in diameter with a delayed venous wash out seen on two imaging studies (e.g., abdominal CT or MR) in a patient with biopsy-proven cirrhosis33 | Hepatocellular carcinoma* |
| Arterial-enhancing liver mass >2 cm in diameter with a delayed venous wash out seen on a single abdominal CT or MRI with a serum alpha-fetoprotein >200 ng/mL34 | Hepatocellular carcinoma* |
| POSSIBLE EVENT: A possible hepatic decompensation event occurred if at least one of the following criteria was met: | |
|---|---|
| Clinical Finding | Diagnosis |
| Variceal hemorrhage documented in a progress note; no endoscopy report results available | Variceal hemorrhage |
| Asterixis (liver flap) documented on physical examination by a gastroenterologist or hepatologist (with no concomittant documentation of hepatic encephalopathy) with a test for serum ammonia ordered within 30 days of date asterixis was reported32,35 | Hepatic encephalopathy |
| Liver mass 1-2 cm in diameter seen on a single abdominal imaging study (e.g., ultrasound, CT, or MRI) in a patient with biopsy-proven cirrhosis33,34 | Hepatocellular carcinoma* |
Among hepatitis B virus-infected patients, the presence of either another complication of cirrhosis or a biopsy demonstrating cirrhosis was required to be classified as having hepatic decompensation.
We included hepatocellular carcinoma as a decompensation event because it typically develops in the setting of cirrhosis and indicates end-stage liver disease.13,14 However, among chronic hepatitis B patients, this malignancy can develop without cirrhosis.15 Thus, among hepatitis B patients, we required the presence of either another complication of cirrhosis or a biopsy demonstrating cirrhosis to confirm hepatic decompensation.
Confirmation of Hepatic Decompensation
A single trained abstractor reviewed the medical records of patients identified with relevant ICD-9-CM codes and laboratory abnormalities beginning one year before through six months after VACS enrollment. Data were abstracted onto structured forms that collected information from: 1) abdominal ultrasound, CT, and MRI reports (presence and quantity of ascites; presence and dimensions of liver masses); 2) laboratory results (alpha-fetoprotein; ammonia; peritoneal fluid cell count, differential, culture); 3) liver biopsy results (fibrosis stage; hepatocellular carcinoma diagnosis); 4) endoscopic reports (reason for endoscopy; presence of gastritis and/or peptic ulcers; presence of varices; signs of active variceal bleeding; banding of varices); and 5) progress notes (chronic liver disease etiology; diagnosis of variceal bleeding; reports of diagnostic and/or therapeutic paracentesis; reports of hepatic encephalopathy, asterixis). Hepatic encephalopathy was identified if there was a report of altered mentation and a definite diagnosis was recorded in a gastroenterologist's or hepatologist's note. Reports of a non-hepatic etiology of altered mentation negated a hepatic encephalopathy diagnosis. Asterixis was confirmed only if documented in the physical examination of a gastroenterologist or hepatologist.
After medical record review, data forms were independently reviewed by two arbitrators with expertise in chronic liver diseases (V.L.R. and J.K.L.). Each determined if hepatic decompensation was present and recorded the date each condition was first identified. They then decided whether the event was definite, possible, or had not occurred. Hepatic decompensation was considered to have occurred at the earliest date any diagnosis consistent with the event was documented. Disagreement on classification or date resulted in review by a third arbitrator (M.B.G.) to adjudicate the event.
Collection of Clinical Data
Age, sex, race, hazardous alcohol use (defined by an Alcohol Use Disorders Identification Test-C score >4 in men or >2 in women at VACS entry and/or an ICD-9-CM code for hazardous drinking any time prior to enrollment 16,17), HIV infection (positive HIV antibody and/or RNA), hepatitis B (positive hepatitis B surface antigen), and hepatitis C (positive hepatitis C antibody and/or RNA) were collected from the VACS database at enrollment.
Data Analysis
We determined the positive predictive values (PPVs) of ICD-9-CM codes (grouped by condition indicative of hepatic decompensation [Table 1]), laboratory abnormalities, and their combinations for hepatic decompensation events (definite or possible). We also evaluated the PPVs of only 1 inpatient or ≥2 outpatient codes, as has previously been used to identify psychiatric diseases 18-20 and HIV infection.21,22 Our focus was on PPV because if this parameter is sufficiently high, researchers will have confidence in the coding- and/or laboratory-based algorithm to warrant its use as a tool to identify cases of hepatic decompensation within observational cohorts.
Although our analyses focused on subjects with diagnostic codes or laboratory abnormalities suggestive of hepatic decompensation, a random sample of 100 patients without a code or laboratory abnormality was selected to evaluate for the absence of hepatic decompensation outcomes. This sample provided an upper bound of the 95% confidence interval (CI) of 3.6% if no cases of hepatic decompensation were identified. Based on our finding that no cases of hepatic decompensation were identified among this random sample, we estimated negative predictive value, specificity, percent agreement, and kappa statistic for the diagnostic codes, laboratory abnormalities, and their combinations.
The prevalence of hepatic decompensation among VACS subjects was determined by dividing the number of cases confirmed at the time of enrollment by the total cohort size. Results were stratified by HIV status, viral hepatitis status, and hazardous alcohol use.
We estimated that 75 patients would allow determination of the PPV of the diagnostic codes and laboratory abnormalities with a maximum 95% CI of ±0.12, assuming a PPV of 80%. All data were analyzed using Stata 11.0 (Stata Corp, College Station, TX).
Ethics Board Approval
The study protocol was approved by the Institutional Review Boards of the VA Connecticut Healthcare System, Yale University, Philadelphia VA Medical Center, and University of Pennsylvania.
RESULTS
Subject Characteristics
Among 6,280 patients enrolled in VACS through August 15, 2005, 137 (2.2%) patients were identified with a hepatic decompensation code, and 197 (3.1%) had a laboratory abnormality indicating liver dysfunction at enrollment. Ninety-eight (1.6%) patients had an ICD-9-CM code alone, 158 (2.5%) had a laboratory abnormality only, and 39 (0.6%) had both an ICD-9-CM code and laboratory abnormality. Subjects identified with either a code or laboratory abnormality were older and more commonly had a history of hazardous alcohol use, HIV, hepatitis B, and hepatitis C than those without (Table 3). Patients coinfected with HIV and hepatitis C (147 [50%]) comprised the largest proportion of these individuals.
Table 3.
Characteristics of subjects at enrollment in the Veterans Aging Cohort Study. The table presents characteristics of: 1) all subjects in the cohort at enrollment; 2) a random sample of 100 subjects without a diagnostic code or laboratory abnormalities suggestive of hepatic decompensation and whose medical records were reviewed to confirm the absence of this outcome; 3) all subjects without a diagnostic code or laboratory abnormalities indicative of hepatic decompensation; and 4) subjects identified with either diagnostic codes or laboratory abnormalities suggestive of hepatic decompensation.
| Characteristic |
All Subjects (n=6,280) |
Random Sample of Screen– (n=100) |
Screen– (n=5,985) |
Screen+ (n=295) |
P-Value* |
|---|---|---|---|---|---|
|
Median age at enrollment (yrs, IQR)
|
50 (44 – 55) |
48 (41 – 55) |
50 (44 – 55) |
51 (47 – 56) |
0.001 |
|
Male sex (no., %)
|
5,953 (95%) |
100 (100%) |
5,665 (95%) |
288 (98%) |
0.03 |
| Race (no., %) | 0.8 | ||||
| African-American | 4,035 (64%) | 59 (59%) | 3,845 (64%) | 190 (64%) | |
|
White
|
1,399 (22%) |
35 (35%) |
1,338 (22%) |
61 (21%) |
|
|
Hazardous alcohol use (no., %)†
|
2,350 (37%) |
37 (37%) |
2,219 (37%) |
131 (44%) |
0.01 |
|
HIV infection (no., %)
|
3,152 (50%) |
51 (51%) |
2,940 (49%) |
212 (72%) |
<0.001 |
|
Hepatitis B virus infection (no., %)
|
372 (6%) |
13 (13%) |
318 (5%) |
54 (18%) |
<0.001 |
|
Hepatitis C virus infection (no., %)
|
2,331 (37%) |
24 (24%) |
2,136 (36%) |
195 (66%) |
<0.001 |
| HIV, viral hepatitis status (no., %) | <0.001 | ||||
| Uninfected | 2,294 (37%) | 32 (32%) | 2,261 (38%) | 33 (11%) | |
| HIV only | 1,475 (23%) | 33 (33%) | 1,425 (24%) | 50 (17%) | |
| Hepatitis B only | 30 (0.5%) | 3 (3%) | 28 (0.5%) | 2 (1%) | |
| Hepatitis C only | 769 (12%) | 12 (12%) | 726 (12%) | 43 (14%) | |
| Hepatitis B + hepatitis C | 35 (0.5%) | 2 (2%) | 30 (0.5%) | 5 (2%) | |
| HIV + hepatitis B | 150 (2%) | 8 (8%) | 135 (2%) | 15 (5%) | |
| HIV + hepatitis C | 1,370 (22%) | 10 (10%) | 1,255 (21%) | 115 (39%) | |
| HIV + hepatitis B + hepatitis C | 157 (3%) | 0 (0%) | 125 (2%) | 32 (11%) |
Hazardous alcohol use defined by: 1) Alcohol Use Disorders Identification Test-C score >4 in men or >2 in women at enrollment and/or an International Classification of Diseases, Ninth Revision, Clinical Modification code for hazardous drinking recorded at any time prior to enrollment
P-value for comparison of characteristics between patients who screened positive versus negative for possible hepatic decompensation
IQR=interquartile range; HIV=human immunodeficiency virus
Positive Predictive Values of Diagnostic Codes and Laboratory Abnormalities
The PPVs of ICD-9-CM codes, laboratory abnormalities, and their combinations for confirmed hepatic decompensation events are listed in Table 4. Among 137 patients with a hepatic decompensation code and 197 with a laboratory abnormality, the diagnosis was confirmed in 57 (PPV, 42%; 95% CI, 33% – 50%) and 56 (PPV, 28%; 95% CI, 22% – 35%), respectively. PPVs of individual hepatic decompensation events were low (Table 4), with the exception of ascites (PPV, 86%; 95% CI, 67% – 96%).
Table 4.
Performance characteristics of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, laboratory abnormalities, and their combinations for determining hepatic decompensation events.
| ICD-9-CM Codes or Laboratory Abnormalities |
No. with Parameter |
No. with Hepatic Decompensation |
Positive Predictive Value |
Estimated Negative Predictive Value* |
Estimated Sensitivity* |
Estimated Specificity* |
Percent Agreement |
Kappa StatisticΨ |
|---|---|---|---|---|---|---|---|---|
| ICD-9-CM Code† | ||||||||
| Ascites | 28 | 24 | 86% | 99% | 27% | 99% | 99% | 0.41 |
| Spontaneous bacterial peritonitis | 6 | 4 | 67% | 99% | 5% | 99% | 99% | 0.08 |
| Variceal hemorrhage | 15 | 8 | 53% | 99% | 9% | 99% | 99% | 0.15 |
| Hepatic encephalopathy | 9 | 1 | 11% | 99% | 1% | 99% | 99% | 0.02 |
| Hepatocellular carcinoma | 6 | 2 | 33% | 99% | 2% | 99% | 99% | 0.04 |
| Other conditions potentially suggestive of hepatic decompensation‡ | 109 | 48 | 44% | 99% | 55% | 99% | 99% | 0.48 |
| Any ICD-9 code |
137 |
57 |
42% |
99% |
65% |
99% |
98% |
0.50 |
| Laboratory Abnormalities | ||||||||
| Total bilirubin ≥5.0 mg/dL | 44 | 20 | 45% | 99% | 23% | 99% | 99% | 0.30 |
| Albumin ≤2.0 gm/dL | 144 | 46 | 32% | 99% | 52% | 98% | 98% | 0.39 |
| International normalized ratio ≥1.7 | 59 | 26 | 44% | 99% | 30% | 99% | 99% | 0.35 |
| Any laboratory abnormality |
197 |
56 |
28% |
99% |
64% |
98% |
97% |
0.38 |
| Combinations of ICD-9-CM Codes and/or Laboratory Abnormalities | ||||||||
| Any ICD-9 code or laboratory abnormality | 295 | 88 | 30% | 100% | 100% | 97% | 97% | 0.45 |
| Any ICD-9 code + laboratory abnormality | 39 | 25 | 64% | 99% | 28% | 99% | 99% | 0.39 |
| Any ICD-9 code for ascites + laboratory abnormality | 15 | 14 | 93% | 99% | 16% | 99% | 99% | 0.27 |
| Any 1 inpatient or 2 outpatient ICD-9 codes for ascites + laboratory abnormality | 14 | 14 | 100% | 99% | 16% | 100% | 99% | 0.27 |
| Any ICD-9 code for ascites or spontaneous bacterial peritonitis | 33 | 27 | 82% | 99% | 31% | 99% | 99% | 0.44 |
| Any 1 inpatient or 2 outpatient ICD-9 codes for ascites or spontaneous bacterial peritonitis | 29 | 26 | 90% | 99% | 30% | 99% | 99% | 0.44 |
| Any ICD-9 code for ascites or spontaneous bacterial peritonitis + laboratory abnormality | 17 | 16 | 94% | 99% | 18% | 99% | 99% | 0.30 |
| Any 1 inpatient or 2 outpatient ICD-9 codes for ascites or spontaneous bacterial peritonitis + laboratory abnormality | 16 | 16 | 100% | 99% | 18% | 100% | 99% | 0.30 |
| Any ICD-9 code for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage | 42 | 30 | 71% | 99% | 34% | 99% | 99% | 0.45 |
| Any 1 inpatient or 2 outpatient ICD-9 codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage | 32 | 29 | 91% | 99% | 33% | 99% | 99% | 0.48 |
| Any ICD-9 code for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage + laboratory abnormality | 20 | 18 | 90% | 99% | 20% | 99% | 99% | 0.33 |
| Any 1 inpatient or 2 outpatient ICD-9 codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage + laboratory abnormality | 18 | 18 | 100% | 99% | 20% | 100% | 99% | 0.33 |
Negative predictive value and specificity were calculated based on the assumption that all cases of hepatic decompensation in cohort were identified and confirmed.
ICD-9-CM codes for conditions indicative of hepatic decompensation are listed in Table 2a.
These conditions included: acute and subacute necrosis of the liver; alcoholic cirrhosis of the liver; cirrhosis of the liver with no alcohol; biliary cirrhosis; other chronic non-alcoholic liver diseases; unspecified chronic liver disease without mention of alcohol; hepatorenal syndrome; other sequelae of chronic liver disease; and jaundice.
Kappa statistic measured agreement between ICD-9-CM codes and/or laboratory abnormalities and confirmed hepatic decompensation events, taking into account chance agreement.
The combination of any diagnostic code plus laboratory abnormality increased PPV (64%; 95% CI, 47% - 79%). Several combinations of codes and/or laboratory abnormalities had PPVs exceeding 80% (Table 4). Among these algorithms, the presence of 1 inpatient or ≥2 outpatient diagnostic codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage had high PPV for confirmed hepatic decompensation (PPV, 91%; 95% CI, 77% – 98%) and captured the most events. Results were similar when stratified by HIV or hepatitis C status (data not shown).
Of the 100 patients randomly selected from among those without a diagnostic code or laboratory abnormality indicative of hepatic decompensation, no events were detected by either arbitrator (0%; 95% CI, 0 – 3.6%).
Characteristics of Subjects with Hepatic Decompensation
After endpoints arbitration, 88 (30%) of the 295 patients identified with either a diagnostic code or laboratory abnormality were confirmed to have hepatic decompensation (84 definite; 4 possible). The overall percent agreement in events between the two arbitrators was 99% (293/295), and the kappa score was 0.98. Ascites was identified in 67 (23%) patients, spontaneous bacterial peritonitis in 8 (3%), variceal hemorrhage in 21 (7%; 13 definite; 8 possible), hepatic encephalopathy in 39 (13%; 38 definite; 1 possible), and hepatocellular carcinoma in 6 (2%; 5 definite; 1 possible). The characteristics of these patients are described in Table 5. Eighty percent were infected with hepatitis C, 70% were HIV-infected, and 55% had a history of hazardous alcohol use.
Table 5.
Characteristics of Veterans Aging Cohort Study patients with confirmed hepatic decompensation at enrollment (n=88), overall and by human immunodeficiency virus status.
| Characteristic |
All Subjects (n=88) |
HIV– (n=26) |
HIV+ (n=62) |
P-Value |
|---|---|---|---|---|
|
Median age at enrollment (yrs, IQR)
|
51 (46 – 56) |
54 (49 – 59) |
50 (46-56) |
0.05 |
|
Male sex (no., %)
|
86 (98%) |
26 (100%) |
60 (97%) |
>0.5 |
| Race (no., %) | 0.1 | |||
| African-American | 49 (56%) | 11 (42%) | 38 (61%) | |
|
White
|
22 (25%) |
10 (38%) |
12 (19%) |
|
|
Hazardous alcohol use (no., %)†
|
48 (55%) |
17 (65%) |
31 (50%) |
0.2 |
|
Hepatitis B virus infection (no., %)
|
18 (20%) |
3 (12%) |
15 (24%) |
0.3* |
|
Hepatitis C virus infection (no., %)
|
70 (80%) |
16 (62%) |
54 (87%) |
0.007 |
| Prior liver biopsy (no., %) | 19 (22%) | 4 (15%) | 15 (24%) | 0.4* |
P-values for differences between subjects with and without HIV determined by Fisher's exact test
Hazardous alcohol use defined by: 1) Alcohol Use Disorders Identification Test-C score >4 in men or >2 in women at enrollment and/or an International Classification of Diseases, Ninth Revision, Clinical Modification code for hazardous drinking recorded at any time prior to enrollment
IQR=interquartile range; HIV=human immunodeficiency virus
Prevalence of Hepatic Decompensation in VACS
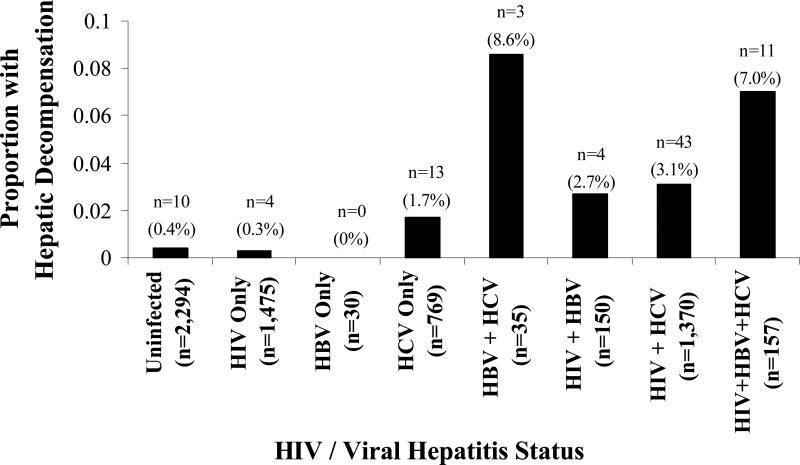
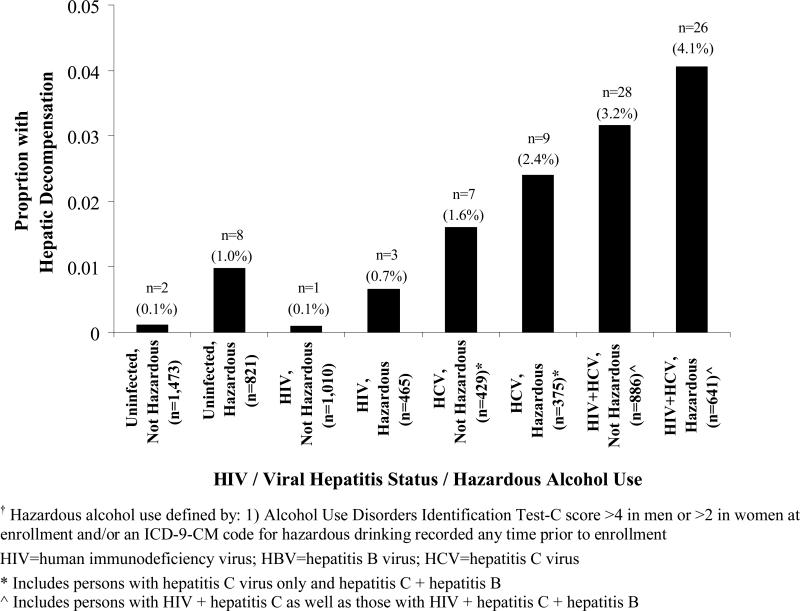
Among 6,280 VACS subjects, 88 cases of hepatic decompensation were confirmed (prevalence, 1.4%; 95% CI, 1.1% – 1.7%). Figure 1 shows the prevalence of hepatic decompensation among the 6,280 VACS patients by HIV and viral hepatitis status (Figure 1a) and additionally by hazardous alcohol use (Figure 1b).
Figure 1a.
Prevalence of hepatic decompensation at enrollment among 6,280 Veterans Aging Cohort Study subjects, by human immunodeficiency virus (HIV) status, viral hepatitis status, and hazardous alcohol use.
Hepatic decompensation, by HIV and viral hepatitis status.
Figure 1b.
Prevalence of hepatic decompensation at enrollment among 6,280 Veterans Aging Cohort Study subjects, by human immunodeficiency virus (HIV) status, viral hepatitis status, and hazardous alcohol use.
Prevalence of hepatic decompensation, by HIV status, hepatitis C virus status, and hazardous alcohol use†.
DISCUSSION
This study examined the ability of diagnostic codes, liver-related laboratory abnormalities, and their combinations to identify hepatic decompensation events among subjects in the VACS. The presence of 1 inpatient or ≥2 outpatient ICD-9-CM codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage had a PPV exceeding 90% for confirmed hepatic decompensation and captured the most events among the algorithms of diagnostic codes and laboratory abnormalities evaluated. Results did not vary by HIV or hepatitis C status. This algorithm could therefore be used for epidemiologic studies examining hepatic decompensation within the VA healthcare system setting.
The PPV of the ICD-9-CM diagnostic codes that we selected for medical record-confirmed hepatic decompensation was low. Our inclusion of several non-specific codes for conditions potentially suggestive of end-stage liver disease likely contributed to this result (Table 1). Subsequent analyses excluded these non-specific diagnoses and evaluated the accuracy of codes grouped by specific hepatic decompensation complications (i.e., ascites, spontaneous bacterial peritonitis, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma). However, with the exception of ascites, these codes also had low PPV. These findings may have been due to the complexities of the definitions for each of the hepatic decompensation conditions, which could lead to inaccurate coding of these diagnoses.
Because of the observed low accuracy of individual ICD-9-CM hepatic decompensation codes, we examined coding algorithms that required the presence of 1 inpatient or ≥2 outpatient codes, as has been used to identify psychiatric diseases 18-20 and HIV infection.21,22 We found that 1 inpatient or ≥2 outpatient diagnoses for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage increased the PPV for confirmed events to above 90%, though at the cost of missing some subjects that were identified by other diagnostic codes or laboratory abnormalities.
Our use of abnormal liver-related laboratory results increased our detection of additional hepatic decompensation events beyond the diagnostic codes alone by 54% (31 additional events/57 events detected using ICD-9-CM codes alone). However, the PPV of the selected abnormalities in liver-related laboratory tests for hepatic decompensation was also low. The severe abnormalities in liver synthetic dysfunction identified cases of severe liver disease without an accompanying hepatic decompensation event. Utilizing coding algorithms that required both a diagnostic code and laboratory abnormality increased the PPV for true events, but these algorithms identified only small numbers of cases and considerably less than the 1 inpatient/≥2 outpatient code-based algorithm for ascites, peritonitis, or variceal hemorrhage.
Few studies have evaluated the validity of administrative and electronic medical record data to identify hepatic decompensation events within observational cohort studies. Fleming et al.23 used the General Practice Research Database to identify patients with cirrhosis using diagnostic codes for cirrhosis, esophageal varices, and portal hypertension and then evaluated hepatic decompensation based on review of medical records. However, the accuracy of the diagnostic codes was not investigated in this study.
After confirmation of hepatic decompensation, we examined the prevalence of these events among certain subgroups in the VACS. Hepatic decompensation events were highest among patients coinfected with hepatitis B and C viruses and with viral hepatitis and HIV coinfection (Figure 1a). Further, a history of hazardous alcohol use increased the prevalence of hepatic decompensation within each hepatitis- and/or HIV-infected subgroup (Figure 1b). These findings are consistent with current evidence suggesting that the course of chronic liver disease is accelerated in HIV/viral hepatitis-coinfected patients 24-26 and that alcohol is an important cofactor for liver disease progression.27
This work has clinical implications for future epidemiologic research, particularly in the setting of chronic liver diseases. A validated method to identify cases of hepatic decompensation will enable a more thorough delineation of the natural history of a number of chronic liver diseases. This will allow observational studies to determine more accurately incidence rates of hepatic decompensation; identify risk factors (e.g., viral, immunologic, genetic, medications) for this outcome to suggest mechanisms and cofactors of disease progression; and develop clinical predictive indices to classify patients with chronic liver diseases according to their risk of developing hepatic decompensation over a clinically relevant time period, which could impact the prescription of treatment for the underlying liver disease. Moreover, a more thorough identification of the risk factors for end-stage liver disease may suggest interventions that could be undertaken in the future, after further testing, for the primary prevention of hepatic decompensation.
Our study has several potential limitations. First, there exists the possibility that the 1 inpatient/≥2 outpatient diagnostic coding algorithm for ascites, peritonitis, and variceal hemorrhage might misclassify hepatic decompensation events. However, we minimized the likelihood of misclassification by: 1) using standardized definitions for complications indicative of hepatic decompensation based on guidelines from US and European hepatology societies, 2) requiring suspected cases to undergo chart review for confirmation, and 3) employing two endpoints arbitrators to confirm events.
It is also possible that the 1 inpatient/≥2 outpatient code-based algorithm may miss subjects with hepatic decompensation. Clinicians might not record an ICD-9-CM code for hepatic decompensation, as was evident by the additional cases ascertained using laboratory abnormalities. Cohorts with access to administrative data, laboratory results, and medical records could use both diagnostic codes and laboratory abnormalities as a screening mechanism to identify patients who might have developed hepatic decompensation. Medical records of these subjects could then be reviewed in the manner performed in this study to confirm decompensation events. Notably, no events were identified among randomly sampled patients who neither had a diagnostic code nor laboratory abnormality suggestive of hepatic decompensation.
Finally, our results will not be generalizable to cohorts that do not record ICD-9-CM codes or laboratory results as directly analyzable fields. Alternative methods to confirm hepatic decompensation events will need to be developed for these cohorts.
In conclusion, 1 inpatient or ≥2 outpatient diagnostic codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage accurately identified hepatic decompensation events in the VA healthcare system setting. This algorithm can be used in future studies to examine the epidemiology of a variety of chronic liver diseases and evaluate the impact of medications on hepatic decompensation.
KEY POINTS.
One inpatient or ≥2 outpatient diagnostic codes for ascites, spontaneous bacterial peritonitis, or variceal hemorrhage was highly predictive of hepatic decompensation.
The ability to identify validly cases of hepatic decompensation events within cohort studies can enable a more accurate determination of the incidence rates of hepatic decompensation associated with chronic liver diseases and evaluate the impact of medications on this outcome.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases (grant number K01-AI070001 to Dr. Lo Re) and the National Institute on Alcohol Abuse and Alcoholism (U10-AA13566). The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
We thank Dr. James D. Lewis for his review and thoughtful comments on the manuscript.
List of Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- CI
confidence interval
- CT
computed tomography
- EASL
European Association for the Study of the Liver
- EGD
esophagogastroduodenoscopy
- HIV
human immunodeficiency virus
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- INR
international normalized ratio
- MRI
magnetic resonance imaging
- VACS
Veterans Aging Cohort Study
APPENDIX
Hepatic decompensation was defined by the presence of any one of the following five major complications of cirrhosis: ascites, spontaneous bacterial peritonitis, esophageal/gastric variceal hemorrhage, hepatic encephalopathy, and/or hepatocellular carcinoma.11,12 Definitions for each of these conditions were based on guidelines by the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL). Hepatic decompensation events were further defined as definite or possible based on medical record data.
Ascites
Ascites may be identified by abdominal imaging (e.g., ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) or paracentesis.28 Patients with underlying chronic liver disease who had either a report of ascites on an abdominal imaging study or results of peritoneal fluid analysis available were classified as definite hepatic decompensation events.
Spontaneous bacterial peritonitis
Spontaneous bacterial peritonitis is confirmed when there is a positive ascitic fluid bacterial culture and/or an elevated ascitic fluid neutrophil count (≥250 cells/mm3).28,29 Patients who met this criterion were classified as having definite hepatic decompensation. Patients with a non-hepatic etiology of ascites (e.g., pancreatitis, tuberculosis) were not considered to have had hepatic decompensation.
Esophageal/gastric variceal hemorrhage
This diagnosis is confirmed when esophagogastroduodenoscopy (EGD) shows one of the following: active bleeding from a varix, clots overlying a varix, or varices with no other potential source of bleeding.30,31 We classified variceal hemorrhage as a definite hepatic decompensation event if: 1) active variceal bleeding was visible on endoscopy, or 2) non-bleeding varices were present on endoscopy in the setting of acute upper gastrointestinal hemorrhage with no alternative cause for bleeding. A report of variceal hemorrhage in a progress note with no endoscopy report available was considered only a possible decompensation event.
Hepatic encephalopathy
Hepatic encephalopathy is diagnosed when a change in mentation is present in the setting of chronic liver disease with no other cause for the abnormal mental status.32 Chronic liver disease patients with a documented diagnosis of hepatic encephalopathy in a progress note of a gastroenterologist or hepatologist and without evidence for a non-hepatic etiology for altered mental status were classified as definite hepatic decompensation events. Further, since determination of asterixis (liver flap) and serum ammonia concentration may suggest the diagnosis but are individually nonspecific,32,35 we classified patients who had no documentation of hepatic encephalopathy but who had both asterixis and a serum ammonia concentration measured within 30 days of report of asterixis as having possible hepatic decompensation.
Hepatocellular carcinoma
Hepatocellular carcinoma typically develops in the setting of cirrhosis and signifies hepatic decompensation.13,14 However, among chronic hepatitis B patients, this malignancy can develop without cirrhosis.15 Thus, among hepatitis B patients, we required the presence of either another complication of cirrhosis or a biopsy demonstrating cirrhosis to confirm hepatic decompensation.
Tissue biopsy provides definitive evidence of hepatocellular carcinoma. However, in the absence of a biopsy, AASLD guidelines state that the diagnosis can be made when an arterial-enhancing liver mass >2 cm in diameter with a delayed venous wash out, and a serum alpha-fetoprotein >200 ng/mL are simultaneously detected.33 In addition, EASL guidelines state that the diagnosis can be made without a confirmatory biopsy in patients with pathologically proven cirrhosis who have a >2 cm arterial-enhancing liver mass with a delayed venous wash out on two separate CT or MRI studies.34 Accordingly, we classified patients who met either definition as having definite hepatic decompensation. Hepatic masses of 1 – 2 cm in diameter in a cirrhotic liver may represent hepatocellular carcinoma, but AASLD and EASL recommend confirmatory biopsies.33,34 Therefore, patients with biopsy-proven cirrhosis who had a 1 – 2 cm hepatic mass on a single abdominal imaging study were classified as having a possible event.
Footnotes
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
POTENTIAL CONFLICTS OF INTEREST
All other authors report no potential conflicts of interest related to this manuscript.
REFERENCES
- 1.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 2.Di Martino V, Rufat P, Boyer N, Renard P, Degos F, Martinot-Peignoux M, Matheron S, Le Moing V, Vachon F, Degott C, Valla D, Marcellin P. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34(6):1193–9. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 3.Fuster D, Planas R, Muga R, Ballesteros AL, Santos J, Tor J, Sirera G, Guardiola H, Salas A, Cabre E, Ojanguren I, Barluenga E, Rey-Joly C, Clotet B, Tural C. Advanced liver fibrosis in HIV/HCV-coinfected patients on antiretroviral therapy. AIDS Res Hum Retroviruses. 2004;20(12):1293–7. doi: 10.1089/aid.2004.20.1293. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Samaniego J, Soriano V, Castilla J, Bravo R, Moreno A, Carbo J, Iniguez A, Gonzalez J, Munoz F. Influence of hepatitis C virus genotypes and HIV infection on histological severity of chronic hepatitis C. The Hepatitis/HIV Spanish Study Group. Am J Gastroenterol. 1997;92(7):1130–4. [PubMed] [Google Scholar]
- 5.Pol S, Lamorthe B, Thi NT, Thiers V, Carnot F, Zylberberg H, Berthelot P, Brechot C, Nalpas B. Retrospective analysis of the impact of HIV infection and alcohol use on chronic hepatitis C in a large cohort of drug users. J Hepatol. 1998;28(6):945–50. doi: 10.1016/s0168-8278(98)80341-3. [DOI] [PubMed] [Google Scholar]
- 6.Romeo R, Rumi MG, Donato MF, Cargnel MA, Vigano P, Mondelli M, Cesana B, Colombo M. Hepatitis C is more severe in drug users with human immunodeficiency virus infection. J Viral Hepat. 2000;7(4):297–301. doi: 10.1046/j.1365-2893.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 7.Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, Hernandez-Quero J, Rey C, Abad MA, Rodriguez M, Sales Gilabert M, Gonzalez F, Miron P, Caruz A, Relimpio F, Torronteras R, Leal M, Lissen E. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 8.Fultz SL, Justice AC, Butt AA, Rabeneck L, Weissman S, Rodriguez-Barradas M. Testing, referral, and treatment patterns for hepatitis C virus coinfection in a cohort of veterans with human immunodeficiency virus infection. Clin Infect Dis. 2003;36(8):1039–46. doi: 10.1086/374049. [DOI] [PubMed] [Google Scholar]
- 9.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, Bryant K. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamoreaux J. The organizational structure for medical information management in the Department of Veterans Affairs. An overview of major health care databases. Med Care. 1996;34(3 Suppl):MS31–44. doi: 10.1097/00005650-199603001-00004. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31(5):468–75. doi: 10.1007/BF01320309. [DOI] [PubMed] [Google Scholar]
- 12.Gines P, Quintero E, Arroyo V, Teres J, Bruguera M, Rimola A, Caballeria J, Rodes J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7(1):122–8. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 13.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325(10):675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 14.Tradati F, Colombo M, Mannucci PM, Rumi MG, De Fazio C, Gamba G, Ciavarella N, Rocino A, Morfini M, Scaraggi A, Taioli E. A prospective multicenter study of hepatocellular carcinoma in italian hemophiliacs with chronic hepatitis C. The Study Group of the Association of Italian Hemophilia Centers. Blood. 1998;91(4):1173–7. [PubMed] [Google Scholar]
- 15.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2(8256):1129–33. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 16.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AJ, Maisto SA, McNeil M, Kraemer KL, Conigliaro RL, Kelley ME, Conigliaro J. Three questions can detect hazardous drinkers. J Fam Pract. 2001;50(4):313–20. [PubMed] [Google Scholar]
- 18.Bagchi A, Sambamoorthi U, McSpiritt E, Yanos P, Walkup J, Crystal S. Use of antipsychotic medications among HIV-infected individuals with schizophrenia. Schizophr Res. 2004;71(2-3):435–44. doi: 10.1016/j.schres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Lurie N, Popkin M, Dysken M, Moscovice I, Finch M. Accuracy of diagnoses of schizophrenia in Medicaid claims. Hosp Community Psychiatry. 1992;43(1):69–71. doi: 10.1176/ps.43.1.69. [DOI] [PubMed] [Google Scholar]
- 20.Walkup J, Sambamoorthi U, Crystal S. Incidence and consistency of antiretroviral use among HIV-infected medicaid beneficiaries with schizophrenia. J Clin Psychiatry. 2001;62(3):174–8. doi: 10.4088/jcp.v62n0307. [DOI] [PubMed] [Google Scholar]
- 21.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 22.Walkup JT, Wei W, Sambamoorthi U, Crystal S. Sensitivity of an AIDS case-finding algorithm: who are we missing? Med Care. 2004;42(8):756–63. doi: 10.1097/01.mlr.0000132749.20897.46. [DOI] [PubMed] [Google Scholar]
- 23.Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 32(11-12):1343–50. doi: 10.1111/j.1365-2036.2010.04473.x. [DOI] [PubMed] [Google Scholar]
- 24.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992-2001. Arch Intern Med. 2004;164(21):2349–54. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 25.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 26.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, Moore RD, Afdhal NH, Thomas DL. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21(16):2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian S, Kowdley KV. Effect of alcohol on viral hepatitis and other forms of liver dysfunction. Clin Liver Dis. 2005;9(1):83–101. doi: 10.1016/j.cld.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 29.Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology. 1982;2(4):399–407. doi: 10.1002/hep.1840020402. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 31.Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW, Bosch J, Stiegmann GV, Henderson JM, de Franchis R, Wagner JL, Conn HO, Rodes J. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28(3):868–80. doi: 10.1002/hep.510280339. [DOI] [PubMed] [Google Scholar]
- 32.Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337(7):473–9. doi: 10.1056/NEJM199708143370707. [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 34.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 35.Lockwood AH. Blood ammonia levels and hepatic encephalopathy. Metab Brain Dis. 2004;19(3-4):345–9. doi: 10.1023/b:mebr.0000043980.74574.eb. [DOI] [PubMed] [Google Scholar]