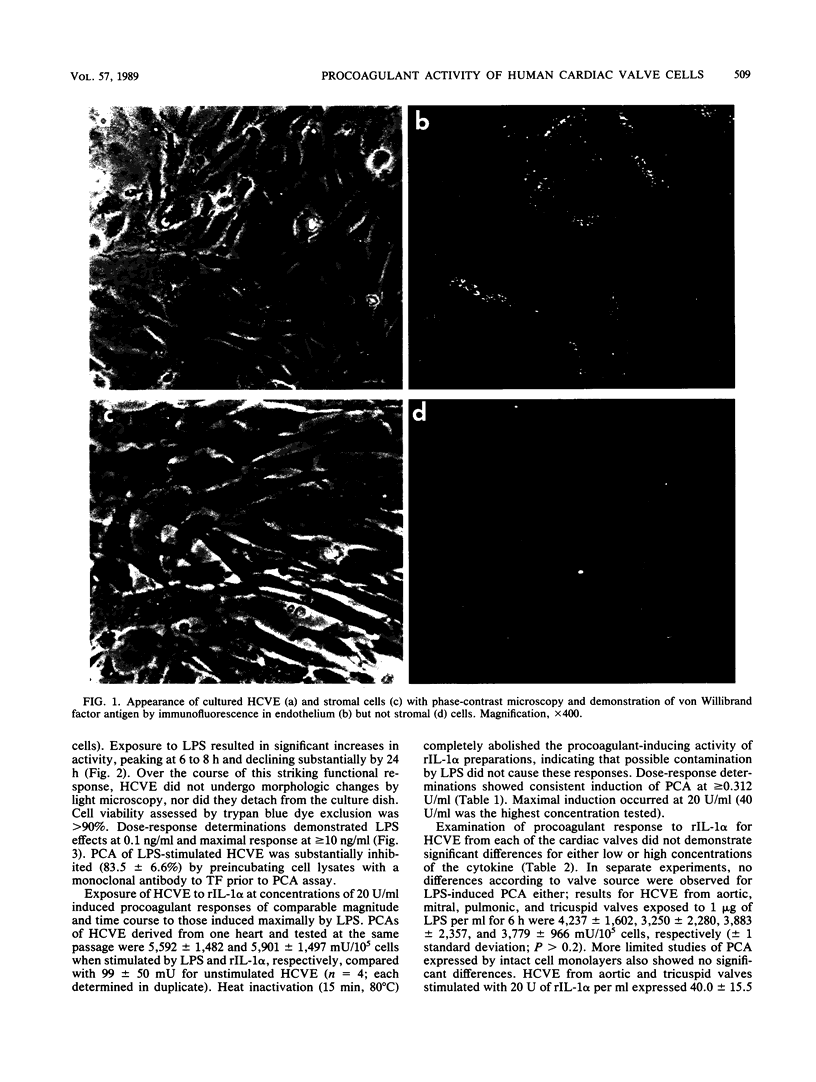
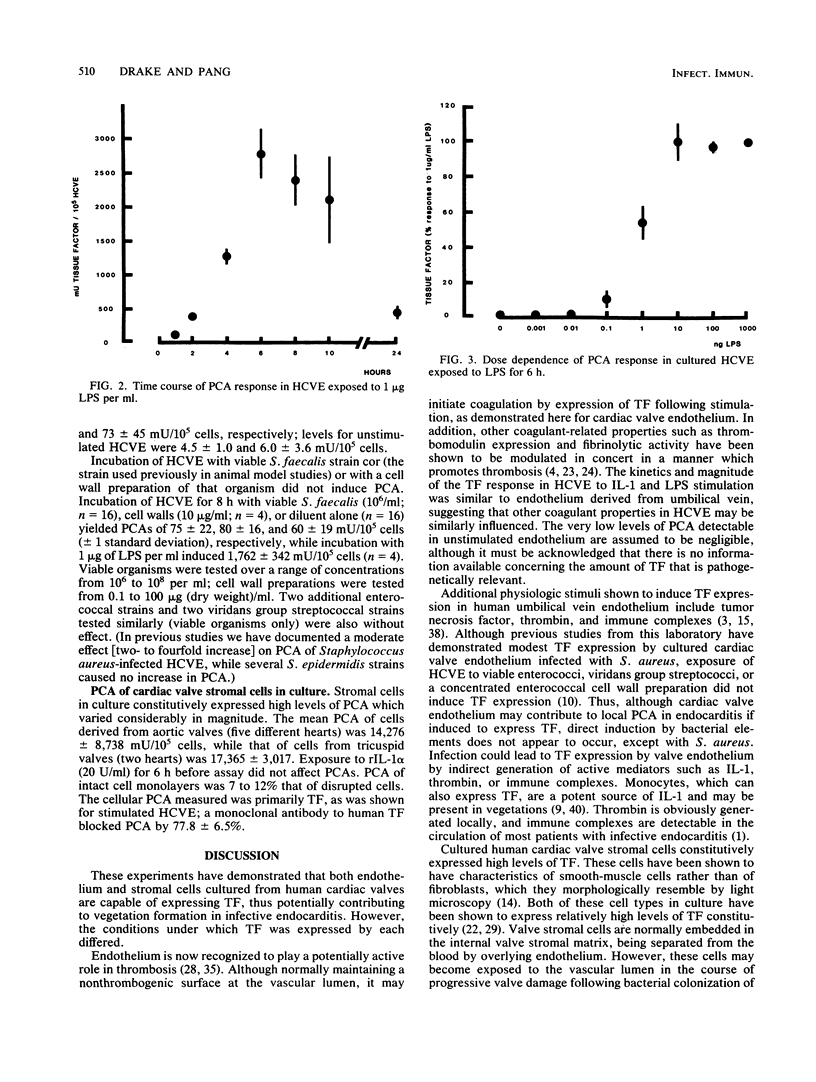
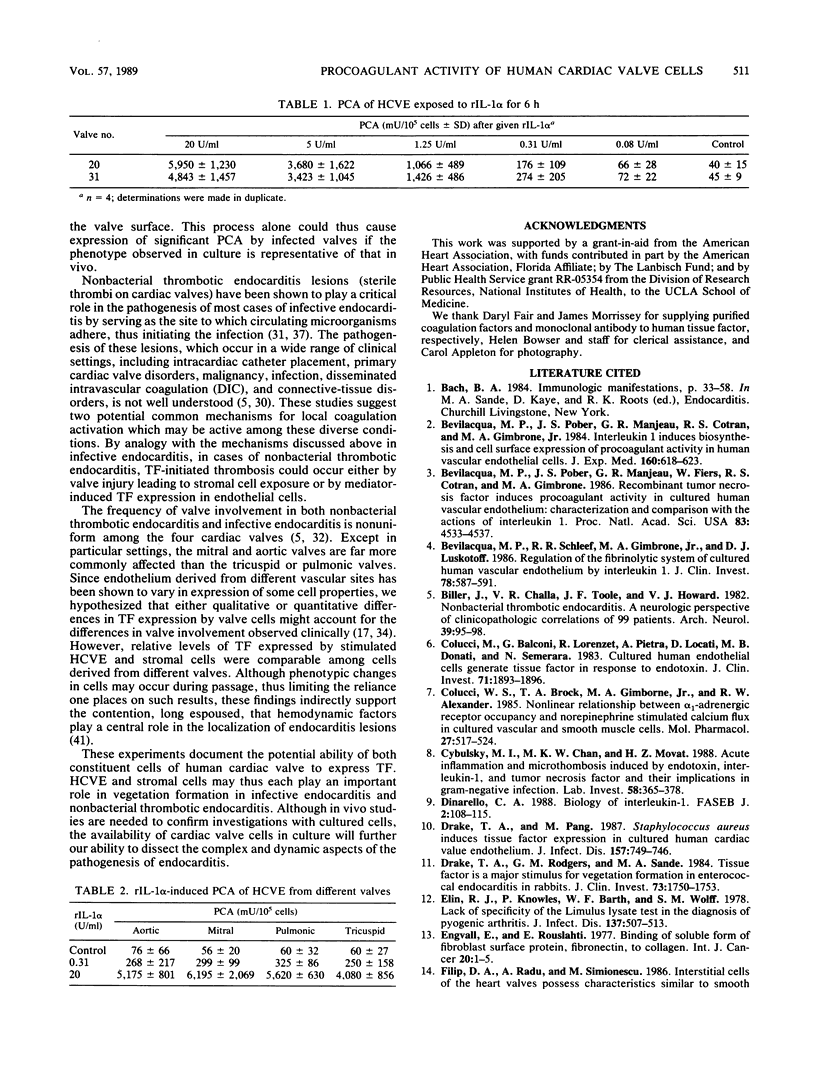
Abstract
Fibrin is the primary constituent of the vegetation in infective endocarditis, and tissue factor expression is a major mechanism of coagulation activation on infected valves. To determine which cells may participate in coagulation activation in this setting, expression of procoagulant activity (PCA; shown to be tissue factor) was studied in cultured endothelial and stromal cells derived from human cardiac valves. Endothelial cells had negligible PCA (99 +/- 50 mU/10(5) cells, mean +/- 1 standard deviation) unless stimulated by lipopolysaccharide or interleukin-1, which increased PCA to 5,592 +/- 1,482 and 5,901 +/- 1,497 mU/10(5) cells, respectively, in 6 h. Incubation of cells with viable enterococci or viridans streptococci or with an enterococcal cell wall preparation did not induce PCA. Cultured valve stromal cells constitutively expressed high levels of PCA (14,276 +/- 8,738 mU/10(5) cells) which was not changed with exposure to interleukin-1. PCAs of stromal or stimulated endothelial cells from valves of both right and left sides of the heart were comparable. The results suggest that endothelial cells may contribute to fibrin deposition during infection if stimulated, but PCA is not directly induced by bacteria. Stromal cells could contribute PCA if exposed to blood in the course of valve injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Schleef R. R., Gimbrone M. A., Jr, Loskutoff D. J. Regulation of the fibrinolytic system of cultured human vascular endothelium by interleukin 1. J Clin Invest. 1986 Aug;78(2):587–591. doi: 10.1172/JCI112613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller J., Challa V. R., Toole J. F., Howard V. J. Nonbacterial thrombotic endocarditis. A neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol. 1982 Feb;39(2):95–98. doi: 10.1001/archneur.1982.00510140029007. [DOI] [PubMed] [Google Scholar]
- Colucci M., Balconi G., Lorenzet R., Pietra A., Locati D., Donati M. B., Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983 Jun;71(6):1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci W. S., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Nonlinear relationship between alpha 1-adrenergic receptor occupancy and norepinephrine-stimulated calcium flux in cultured vascular smooth muscle cells. Mol Pharmacol. 1985 May;27(5):517–524. [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Drake T. A., Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988 Apr;157(4):749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Rodgers G. M., Sande M. A. Tissue factor is a major stimulus for vegetation formation in enterococcal endocarditis in rabbits. J Clin Invest. 1984 Jun;73(6):1750–1753. doi: 10.1172/JCI111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Knowles R., Barth W. F., Wolff S. M. Lack of specificity of the limulus lysate test in the diagnosis of pyogenic arthritis. J Infect Dis. 1978 May;137(5):507–513. doi: 10.1093/infdis/137.5.507. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Filip D. A., Radu A., Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986 Sep;59(3):310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- Galdal K. S., Lyberg T., Evensen S. A., Nilsen E., Prydz H. Thrombin induces thromboplastin synthesis in cultured vascular endothelial cells. Thromb Haemost. 1985 Aug 30;54(2):373–376. [PubMed] [Google Scholar]
- Gumkowski F., Kaminska G., Kaminski M., Morrissey L. W., Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987;24(1-2):11–23. [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell B., Levine E., Shapiro S., Williams S., Carabasi R. A., Mueller S., Thornton S. Human adult endothelial cell growth in culture. J Vasc Surg. 1984 Nov;1(6):757–764. doi: 10.1067/mva.1984.avs0010757. [DOI] [PubMed] [Google Scholar]
- Maynard J. R., Dreyer B. E., Stemerman M. B., Pitlick F. A. Tissue-factor coagulant activity of cultured human endothelial and smooth muscle cells and fibroblasts. Blood. 1977 Sep;50(3):387–396. [PubMed] [Google Scholar]
- Moore K. L., Andreoli S. P., Esmon N. L., Esmon C. T., Bang N. U. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987 Jan;79(1):124–130. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H., Revak D., Tejada P., Fair D. S., Edgington T. S. Resolution of monomeric and heterodimeric forms of tissue factor, the high-affinity cellular receptor for factor VII. Thromb Res. 1988 May 15;50(4):481–493. doi: 10.1016/0049-3848(88)90197-1. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y., Bach R. Tissue factor revisited. Prog Hemost Thromb. 1982;6:237–261. [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers G. M., Greenberg C. S., Shuman M. A. Characterization of the effects of cultured vascular cells on the activation of blood coagulation. Blood. 1983 Jun;61(6):1155–1162. [PubMed] [Google Scholar]
- Rodgers G. M. Hemostatic properties of normal and perturbed vascular cells. FASEB J. 1988 Feb;2(2):116–123. doi: 10.1096/fasebj.2.2.3277885. [DOI] [PubMed] [Google Scholar]
- Rowley K. M., Clubb K. S., Smith G. J., Cabin H. S. Right-sided infective endocarditis as a consequence of flow-directed pulmonary-artery catheterization. A clinicopathological study of 55 autopsied patients. N Engl J Med. 1984 Nov 1;311(18):1152–1156. doi: 10.1056/NEJM198411013111804. [DOI] [PubMed] [Google Scholar]
- Smariga P. E., Maynard J. R. Platelet effects on tissue factor and fibrinolytic inhibition of cultured human fibroblasts and vascular cells. Blood. 1982 Jul;60(1):140–147. [PubMed] [Google Scholar]
- Speiser W., Anders E., Preissner K. T., Wagner O., Müller-Berghaus G. Differences in coagulant and fibrinolytic activities of cultured human endothelial cells derived from omental tissue microvessels and umbilical veins. Blood. 1987 Mar;69(3):964–967. [PubMed] [Google Scholar]
- Striker G. E., Harlan J. M., Schwartz S. M. Human endothelial cells in vitro. Methods Cell Biol. 1980;21A:135–151. doi: 10.1016/s0091-679x(08)60763-3. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Drake T. A., Sande M. A. Pathogenesis of endocarditis. Am J Med. 1985 Jun 28;78(6B):110–115. doi: 10.1016/0002-9343(85)90373-0. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. H., Finko R., Cines D. B. Antibody and immune complexes induce tissue factor production by human endothelial cells. J Immunol. 1986 Sep 1;137(5):1532–1537. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Weinstein L., Schlesinger J. J. Pathoanatomic, pathophysiologic and clinical correlations in endocarditis (first of two parts). N Engl J Med. 1974 Oct 17;291(16):832–837. doi: 10.1056/NEJM197410172911609. [DOI] [PubMed] [Google Scholar]
- van Ginkel C. J., Thörig L., Thompson J., Oh J. I., van Aken W. G. Enhancement of generation of monocyte tissue thromboplastin by bacterial phagocytosis: possible pathway for fibrin formation on infected vegetations in bacterial endocarditis. Infect Immun. 1979 Jul;25(1):388–395. doi: 10.1128/iai.25.1.388-395.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




