Abstract
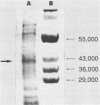
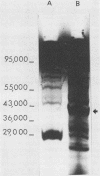
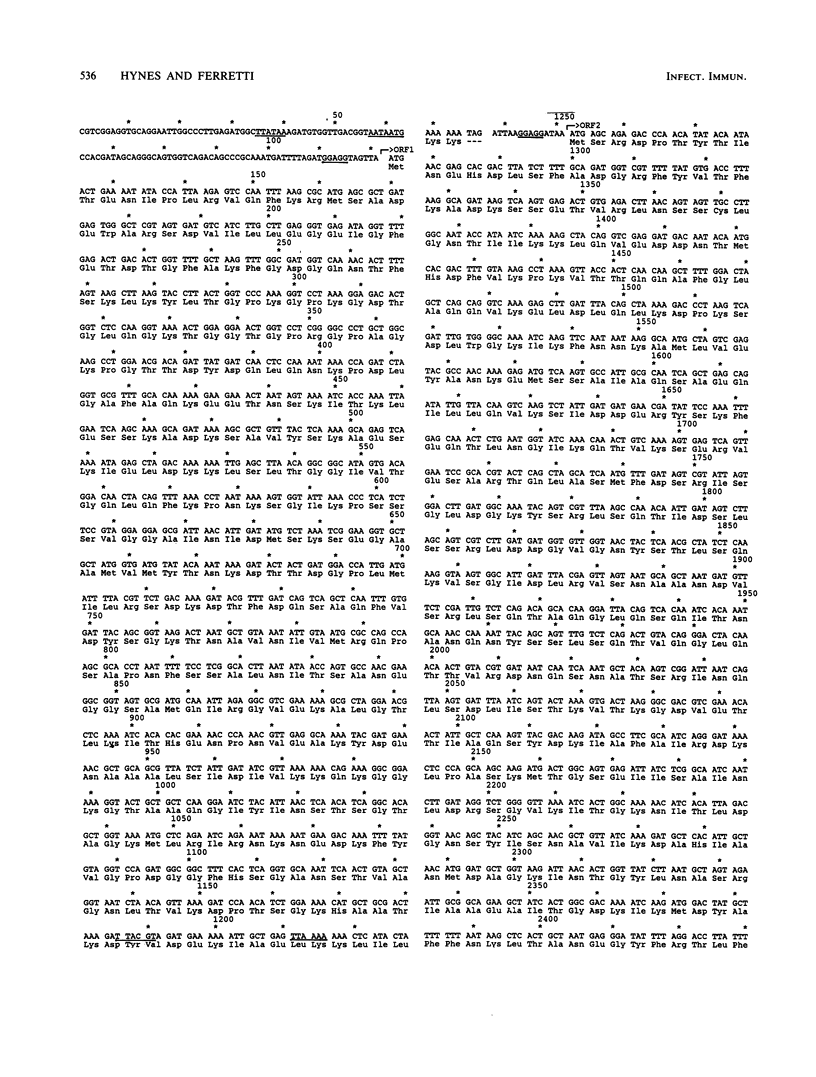
The hyaluronidase gene (hylP) from Streptococcus pyogenes bacteriophage H4489A was previously cloned into Escherichia coli plasmid pUC8 as a 3.1-kilobase ThaI fragment. Southern hybridization experiments confirmed the origin of this fragment in bacteriophage H4489A before determination of the nucleotide sequence of the entire fragment. Two open reading frames (ORFs) were found, the first of which specified a 39,515-molecular-weight protein identified as the bacteriophage hyaluronidase. The second ORF encoded a 65,159-molecular-weight protein of unknown function. Putative transcription and translation control sequences for each ORF were identified by using a plasmid containing a promoterless chloramphenicol acetyltransferase gene. Controlled exclusive expression of the hylP gene via the T7 polymerase-promoter system in E. coli resulted in a 40,000-dalton protein, a result consistent with the coding capacity of the hylP gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benchetrit L. C., Gray E. D., Edstrom R. D., Wannamaker L. W. Purification and characterization of a hyaluronidase associated with a temperate bacteriophage of group A, type 49 streptococci. J Bacteriol. 1978 Apr;134(1):221–228. doi: 10.1128/jb.134.1.221-228.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchetrit L. C., Gray E. D., Wannamaker L. W. Hyaluronidase activity of bacteriophages of group A streptococci. Infect Immun. 1977 Feb;15(2):527–532. doi: 10.1128/iai.15.2.527-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchetrit L. C., Pahuja S. L., Gray E. D., Edstrom R. D. A sensitive method for the assay of hyaluronidase activity. Anal Biochem. 1977 May 1;79(1-2):431–437. doi: 10.1016/0003-2697(77)90418-3. [DOI] [PubMed] [Google Scholar]
- Benchetrit L. C., Wannamaker L. W., Gray E. D. Immunological properties of hyaluronidases associated with temperate bacteriophages of group A streptococci. J Exp Med. 1979 Jan 1;149(1):73–83. doi: 10.1084/jem.149.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Fiszer-Szafarz B. Hyaluronidase polymorphism detected by polyacrylamide gel electrophoresis. Application to hyaluronidases from bacteria, slime molds, bee and snake venoms, bovine testes, rat liver lysosomes, and human serum. Anal Biochem. 1984 Nov 15;143(1):76–81. doi: 10.1016/0003-2697(84)90560-8. [DOI] [PubMed] [Google Scholar]
- Halperin S. A., Ferrieri P., Gray E. D., Kaplan E. L., Wannamaker L. W. Antibody response to bacteriophage hyaluronidase in acute glomerulonephritis after group A streptococcal infection. J Infect Dis. 1987 Feb;155(2):253–261. doi: 10.1093/infdis/155.2.253. [DOI] [PubMed] [Google Scholar]
- Hill J. Purification and properties of streptococcal hyaluronate lyase. Infect Immun. 1976 Sep;14(3):726–735. doi: 10.1128/iai.14.3.726-735.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJEMS E. Studies on streptococcal bacteriophages. 2. Adsorption, lysogenization, and one-step growth experiments. Acta Pathol Microbiol Scand. 1958;42(1):56–66. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MAXTED W. R. Enhancement of streptococcal bacteriophage lysis by hyaluronidase. Nature. 1952 Dec 13;170(4337):1020–1021. doi: 10.1038/1701020b0. [DOI] [PubMed] [Google Scholar]
- Malke H., Roe B., Ferretti J. J. Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene. 1985;34(2-3):357–362. doi: 10.1016/0378-1119(85)90145-3. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W., Skjold S., Maxted W. R. Characterization of bacteriophages from nephritogenic group A streptococci. J Infect Dis. 1970 Apr;121(4):407–418. doi: 10.1093/infdis/121.4.407. [DOI] [PubMed] [Google Scholar]