Abstract
In human cells, hMLH1, hMLH3, hPMS1 and hPMS2 are four recognised and distinctive homologues of MutL, an essential component of the bacterial DNA mismatch repair (MMR) system. The hMLH1 protein forms three different heterodimers with one of the other MutL homologues. As a first step towards functional analysis of these molecules, we determined the interacting domains of each heterodimer and tried to understand their common features. Using a yeast two-hybrid assay, we show that these MutL homologues can form heterodimers by interacting with the same amino acid residues of hMLH1, residues 492–742. In contrast, three hMLH1 partners, hMLH3, hPMS1 and hPMS2 contain the 36 homologous amino acid residues that interact strongly with hMLH1. Contrary to the previous studies, these homologous residues reside at the N-terminal regions of three subdomains conserved in MutL homologues in many species. Interestingly, these residues in hPMS2 and hMLH3 may form coiled-coil structures as predicted by the MULTICOIL program. Furthermore, we show that there is competition for the interacting domain in hMLH1 among the three other MutL homologues. Therefore, the quantitative balance of these three MutL heterodimers may be important in their functions.
INTRODUCTION
The DNA mismatch repair (MMR) system ensures genomic stability in almost all living organisms (1,2). The primary role of MMR is to correct base–base mismatches and insertion-deletion loops (IDLs) resulting from DNA replication and recombination events. A lessened ability to correct such mismatches results in an increased mutation rate and instability of genomic DNA, such as microsatellite instability and postmeiotic segregation, predisposing a normal cell to malignancy. For example, germline mutations in several MMR genes cause hereditary non-polyposis colorectal cancer (HNPCC) in humans (3). In addition, some sporadic cancers are associated with MMR deficiency (4).
In Escherichia coli, a homodimer of MutS binds to the mismatched DNA and forms an ATP-binding-dependent tertiary complex with a homodimer of MutL. This complex then activates a MutH, which incises the unmethylated DNA strand at the nearest hemimethylated GATC sequence. After incision, UvrD helicase and one of three exonucleases (ExoI, ExoVII, or RecJ) excise the nascent strand for some distance past the mismatch. The resulting single-stranded gap is filled by DNA polymerase III holoenzyme in the presence of single-strand DNA-binding protein, and the nick is sealed by DNA ligase to complete repair (5).
In the yeast Saccharomyces cerevisiae, the MMR system comprises multiple MutS and MutL heterodimers with partially overlapping functions (6,7). For example, MutSα, a heterodimer of Msh2 and Msh6, is involved primarily in correcting various mismatches and +1 IDL heterologies, whereas MutSβ, a heterodimer of Msh2 and Msh3, functions in correction of IDLs with 1–14 bases (8–10). In the case of MutL homologues, MutLα, a heterodimer of Mlh1 and Pms1, plays a major role in the yeast MMR system (11,12), whereas a heterodimer of Mlh1 and Mlh3 appears to act in concert with MutSβ to avoid frameshift mutations (13). The function of the third MutL heterodimer, Mlh1–Mlh2, is less clear, but a role in processing some forms of DNA damage is implied by the finding that mlh2 mutants are resistant to cisplatin and other related anticancer drugs (14). Recently, Wang et al. (15) extensively analysed the functions of these MutL heterodimers during meiosis. It was suggested that Mlh1–Mlh3 promotes meiotic crossing-over and that Mlh1–Pms1 and Mlh1–Mlh2 play important roles in mismatch correction during meiosis.
In human, five genes homologous to MutS (hMSH2 to hMSH6) and several genes homologous to MutL (hMLH1, hMLH3, hPMS1 and hPMS2, as well as a cluster of PMS2-like genes on chromosome 7) have been identified (16–20). Human MutSα (a heterodimer of hMSH2 and hMSH6) and MutSβ (a heterodimer of hMSH2 and hMSH3) appear to behave in a similar manner to the yeast MutSα and MutSβ described above (21). On the other hand, hMLH1 forms three different MutL heterodimers with hMLH3, hPMS1 and hPMS2. The heterodimer of hMLH1 and hPMS2, hMutLα, plays an essential role in the MMR system, as suggested by a complementation study using a human hMLH1-deficient cell line, HCT116 (22). A heterodimer of hMLH1 and hMLH3 has been suggested to play an important role in the repair of a subset of IDLs by the functional analysis of the Mlh1–Mlh3 heterodimer in yeast. However, more detailed analyses are necessary to clarify their precise functions (23). In addition, although extensive work by Räschle et al. (24) has examined the function of hMLH1–hPMS1 heterodimer, the involvement of this heterodimer in the human MMR system remains to be demonstrated.
In this paper, we analysed the interacting domains of three MutL heterodimers in humans and found that these domains are composed of 36 homologous amino acid residues. Furthermore, the interacting domains in hPMS2 and hMLH3 carry the heptad repeats characteristic of leucine zipper proteins.
MATERIALS AND METHODS
Strains and plasmid constructions
All plasmids were constructed and prepared by standard techniques (25). Escherichia coli strain DH5αF’ was used to propagate all plasmids. Isolation of hMLH1 and hPMS2 cDNA clones was described previously (20). Two cDNA clones of hMLH3 and hPMS1 were isolated by DNA amplification from the normal colon cDNA library (Stratagene, La Jolla, CA) using KOD DNA polymerase (Toyobo, Osaka, Japan). The yeast two-hybrid vectors, pBTM116 and pVP16, and the reporter strain of S.cerevisiae, L40, were kindly provided by Dr S.M.Hollenberg (Oregon Health Sciences University, Portland, OR) (26). Deletion mutants of hMLH1, hMLH3, hPMS1 and hPMS2 cDNAs in both pBTMd and pVPd vectors (20) were constructed by the use of unique restriction endonucleases or DNA amplifications using KOD DNA polymerase. The glutathione S-transferase (GST) fusion protein of hMLH1 was made using a pGEX-2TK vector (Amersham Pharmacia Biotech, Buckinghamshire, UK). Deletion constructs of hPMS2 containing various regions were also subcloned into pcDNA3.1/V5-His vector (Invitrogen, Carlsbad, CA) for the GST-IVTT assay or a pFLAG-CMV-2 vector (Scientific Imaging Systems, Eastman Kodak, New Haven, CT) for the immunoprecipitation study. For the competition study, the cDNA clones of hMLH1, hMLH3 and hPMS1 were also subcloned into pcDNA3.1/V5-His vector.
Yeast two-hybrid assay
Yeast transformation was performed by the polyethylene glycol–lithium acetate method (27). For β-gal assay in liquid culture using O-nitrophenyl-1-thio-β-d-galactopyranoside (ONPG) as the substrate, three independent colonies from the same transformation were cultured overnight and refreshed in YPD medium at 30°C until the OD600 = 1.0–1.5 (28). Approximately 1.5 ml of each yeast isolate was precipitated at 12 000 g for 30 s, washed once with Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4 pH 7.0), and resuspended in 0.3 ml of Z buffer. To measure the β-gal activity, 0.1 ml of the cell suspension was used. The cells were disrupted with 0.1 mg of glass beads using a vortex mixer, added to 0.7 ml of Z buffer containing 1.9 µl of 2-mercaptoethanol, combined with 160 µl of 4 mg/ml ONPG in Z buffer and incubated at 30°C. Reactions were then stopped by addition of 1 M Na2CO3 when a medium-yellow colour had developed. The reaction tubes were centrifuged at 12 000 g for 5 min and the supernatant was collected to measure the OD420 of each sample. The β-gal activity was calculated by the following formula:
β-gal units = 1000 × OD420/(t × V × OD600),
where t is the time of reaction (min) and V is the volume of culture used in the assay (ml). The protein concentration was measured by the DC protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
GST-in vitro transcription and translation (IVTT) assay
GST-fused hMLH1 protein-associated glutathione beads were prepared as described previously (29). IVTT reactions with [35S]methionine (Promega, Madison, WI) were performed with hPMS2 cDNAs containing various regions. Each IVTT-hPMS2 deletion mutant was added to a tube that contained the GST-fused hMLH1 protein-associated glutathione beads. They were then incubated for at least 1 h at 4°C on a rocker. The beads were washed three times with the binding buffer (20 mM Tris–HCl pH 7.5, 10% glycerol, 150 mM NaCl, 5 mM EDTA, 1 mM DTT, 0.1% Tween-20, 0.75 mg/ml BSA, 0.5 mM PMSF, 0.8 µg/ml leupeptin and 0.8 µg/ml pepstatin) and resuspended in 50 µl of SDS loading buffer (62.5 M Tris–HCl pH 6.8, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol and 0.005% bromophenol blue). The samples were resolved on 8, 15 or 10–20% SDS–PAGE and then imaged using a FUJIFILM BAS-1500. The relative interaction (Intrel) of each hPMS2 deletion mutant with GST-hMLH1 was determined as the fraction of the deletion mutant interaction ratio (IRm) divided by the full-length interaction ratio (IRfl), as described previously (29). The IRm was determined by quantifying the amount of IVTT-hPMS2 deletion mutant that interacted with GST-hMLH1 and dividing this number by the original amount of IVTT-hPMS2 deletion mutant added to the reaction. This quantification was determined for each experiment that contained a series of mutant proteins as well as the full-length protein control on a single SDS–PAGE. The IRfl was calculated similarly by quantifying the amount of full-length IVTT protein precipitated in an interaction experiment and dividing it by the IVTT control. Results are presented as the mean and SD of three separate experiments.
Immunoprecipitation studies using HEC-1-A cell line
Each pFLAG-CMV-2 construct containing a hPMS2 deletion mutant was transfected into a hPMS2-deficient endometrial cancer cell line, HEC-1-A (ATCC, Rockville, MD). Cells were maintained in McCoy’s 5a medium supplemented with 10% fetal bovine serum. Transfection of 4 µg plasmid DNA into cells grown in 10 cm dishes using Lipofectamine Plus Reagent (Life Technologies, Gaithersburg, MD) was performed according to the supplier’s recommendation. At 48 h post-transfection, the cells were recovered by scraping, collected by centrifugation at 600 g for 5 min at 4°C, and lysed in 500 µl of Brij 97 cell extraction buffer (20 mM Tris–HCl pH 7.4, 75 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1% Brij 97, 1 mM phenylmethyl sulfonyl fluoride, 20 µg/ml aprotinin) (30). Expression of each hPMS2 deletion mutant protein was detected by western blotting, and analyses of immunoprecipitation were performed as described previously (20).
Coiled-coil prediction in hMutL homologues
To estimate positions and probabilities of coiled-coil domains, we ran the MULTICOIL program (http://nightingale.lcs.mit.edu/cgi-bin/multicoil) (31) using a probability cut-off of 0.2 and a window of 21 residues on hMutL homologues.
Competition assay using IVTT proteins
For competition studies by the in vitro translated proteins, 100 µl of GST-fused hMLH1 protein-associated glutathione beads were incubated with reticulocyte lysates containing 2 µl of 35S-labeled hPMS2 in the presence of indicated amounts of unlabeled luciferase, hPMS1, or hMLH3 as competitors for 30 min at 4°C on the rocker. The beads were washed three times with the binding buffer and resuspended in 20 µl of SDS loading buffer. The samples were resolved on 10–20% SDS–PAGE.
GenBank accession numbers
hMLH3, AB039667; hPMS1, U13695; hPMS2, U13696; hMLH1, U07418.
RESULTS
Domain of hMLH1 interacting with hMLH3, hPMS1 and hPMS2
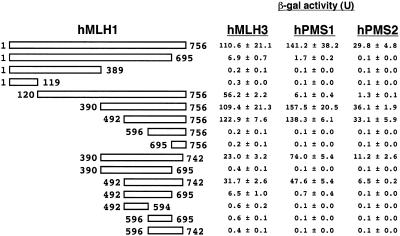
We first determined the domain of hMLH1 interacting with hMLH3, hPMS1 and hPMS2 using the yeast two-hybrid assay. Full-length hMLH3, hPMS1 and hPMS2 cDNAs were inserted into a lexA DNA binding domain (BD) plasmid, pBTM116. In addition, hMLH1 cDNAs containing a full-length or 15 deletion mutants were inserted into a VP16 transcriptional activation domain (AD) plasmid, pVP16. These plasmids were transformed in various combinations into S.cerevisiae, L40 strain. Schematic diagrams of hMLH1 deletion mutants and results of yeast two-hybrid assay are summarised in Figure 1. Our experiment indicated that the minimal region conserving the interacting activity of hMLH1 with hMLH3, hPMS1 and hPMS2 corresponded to a region between residues 492 and 742. However, their interacting activities were about one-quarter of those using full-length hMLH1. To obtain β-gal activities similar to those with full-length hMLH1, the C-terminal region seems to be essential. For example, deletion of 61 amino acids from residues 695 to 756 in hMLH1 completely abolished the interacting activity with hPMS2, whereas the same deletion mutants of hMLH1 still appeared to interact with hMLH3 and hPMS1, although their β-gal activities were extremely low.
Figure 1.
Domains of hMLH1 interacting with hMLH3, hPMS1 and hPMS2. The β-gal activities in yeast cells containing various lexA DNA BD and VP16 transcriptional AD plasmids. The left figure represents schematic diagrams of the full-length (residues 1–756) and the 15 hMLH1 deletion constructs in AD plasmids. Lane hMLH3, hPMS1 or hPMS2 indicates the β-gal activities of yeast cells that contain the full-length hMLH3, hPMS1 or hPMS2 BD plasmid and the corresponding truncated hMLH1 AD plasmid. The mean and SD of three independent transformants are shown. The mean β-gal activities in yeast cells containing only hMLH3, hPMS1 or hPMS2 BD plasmid are 0.3, 0.1 or 0.1 U, respectively.
Domain of hPMS2 interacting with hMLH1
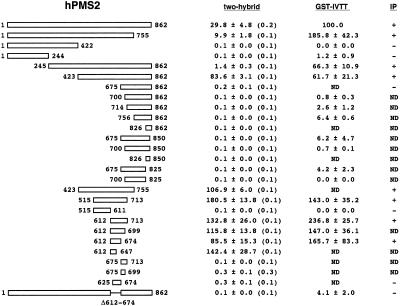
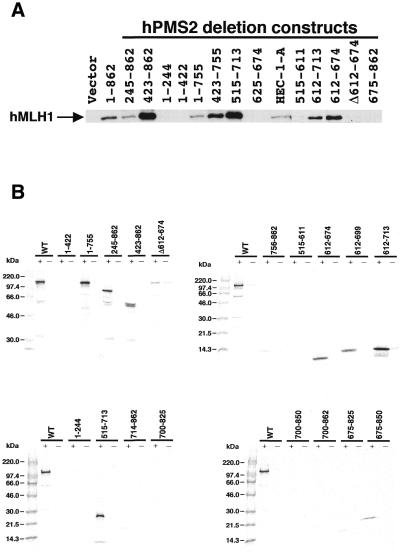
Next, we analysed the domain of hPMS2 interacting with hMLH1 by the yeast two-hybrid assay. Surprisingly, the result was completely different from that obtained from Guerrette et al. (29). They determined the domain of hPMS2 interacting with hMLH1 to be at residues 675–850 by the GST-IVTT assay, whereas we determined it at residues 612–674 by the yeast two-hybrid assay (Fig. 2). To confirm this result, we used two different methods: an immunoprecipitation study using a human hPMS2-deficient cell line HEC-1-A (Figs 2 and 3A) and the GST-IVTT assay used originally by Guerrette et al. (29) (Figs 2 and 3B). All three independent analyses clearly demonstrated that hMLH1 interacts with hPMS2 at residues 612–674. In Figure 3B, several deletion mutants of hPMS2 such as residues Δ612–674, 756–862, 675–825 and 675–850 revealed very faint bands in the presence of GST-hMLH1 in this assay. However, these bands are non-specific or imply a minor effect on the interaction with hMLH1, because the other two methods clearly deny the interaction of hMLH1 with these residues of hPMS2.
Figure 2.
Summary of the two-hybrid assay, GST-IVTT and immunoprecipitation study between the full-length hMLH1 and various hPMS2 deletion constructs. These three assays were performed as described in Materials and Methods. The left figure represents schematic diagrams of the full-length (residues 1–862) and the twenty-six hPMS2 deletion constructs. The mean and SD of β-gal activities in three independent yeast transformants containing various hPMS2 deletion constructs in the BD plasmid and the full-length hMLH1 cDNA in the AD plasmid are indicated in the two-hybrid lane. The values in parentheses represent the mean β-gal activity in the yeast cell containing only the corresponding BD plasmid. Results of the GST-IVTT assay are presented as the mean and SD of three separate experiments and are indicated as relative values when the binding ability of the full-length hPMS2 to GST-hMLH1 is 100. Lane IP indicates the presence (+) or absence (–) of the immunoprecipitated band. ND, not detected.
Figure 3.
Determination of the hMLH1-interactive domain of hPMS2 at residues 612–674 using the immunoprecipitation study (A) and the GST-IVTT assay (B). (A) Extracts of HEC-1-A cells transfected with pFLAG plasmids containing various hPMS2 deletion constructs were subjected to immunoprecipitation analyses using the anti-FLAG M2 monoclonal antibody. Immunoprecipitated proteins were analysed by the use of anti-hMLH1 monoclonal antibody. Lane HEC-1-A illustrates 20 µg of the cell extract that was subjected to immunoblotting without prior immunoprecipitation as the control. The arrow indicates the position of the hMLH1 protein. The minimal interaction domain of hPMS2 with hMLH1 lies between residues 612 and 674. (B) 35S-labeled full-length and deletion mutant proteins of IVTT-hPMS2 were added to glutathione beads that had been pre-treated with either GST alone (–) or GST-hMLH1 (+). Samples were resolved on 8% (top left), 15% (top right) or 10–20% (bottom) SDS-PAGE and examined by BAS-1500 and LAS-1000 (Fuji Film). Again the minimal interacting domain of hPMS2 with hMLH1 resides at residues 612–674.
Domains of hMLH3 and hPMS1 interacting with hMLH1
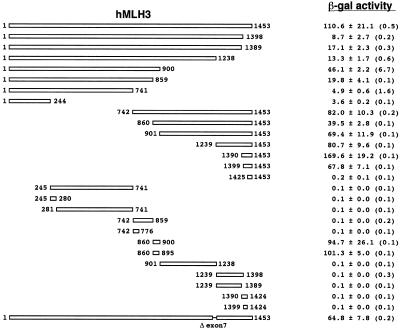
The fact that hMLH3, hPMS1 and hPMS2 have extremely similar interacting properties with hMLH1 led us to consider that these three MutL proteins might contain similar interacting residues. Therefore, we searched for amino acid residues homologous to 612–674 of hPMS2 on hMLH3 and hPMS1 by using GENETYX-MAC 9.0 (Software Development Co., Ltd, Tokyo, Japan). As shown in Figure 4, hPMS1 had only one homologous region at residues 693–728, whereas hMLH3 had four homologous regions at 245–280, 741–776, 860–895 and 1390–1424. All these regions were homologous to the 36 amino acid residues at 612–647 that corresponded to the N-terminus of residues 612–674 of hPMS2. To confirm whether residues 612–647 of hPMS2 comprise a minimal interacting domain with hMLH1, we co-transformed BD-hPMS2 (612–647) and AD-hMLH1 plasmids into the yeast L40 strain and measured the β-gal activity. As shown in Figure 2, the yeast cell containing BD-hPMS2 (612–647) and AD-hMLH1 displayed about 1.7-fold more activity than one containing BD-hPMS2 (612–674) and AD-hMLH1. We concluded that the specific 36 amino acid residues from 612 to 647 in hPMS2 is the minimal interacting domain with hMLH1. The ratios of conserved amino acid residues with hPMS2 at 612–647 were the following; 44.4% (16/36) in hPMS1 at 693–728, 25.0% (9/36) in hMLH3 (245–280), 38.9% (14/36) in hMLH3 (741–776), 55.6% (20/36) in hMLH3 (860–895) and 40.0% (14/35) in hMLH3 (1390–1424). To determine whether these candidate regions also interact with hMLH1, we used the two-hybrid assay described above. As shown in Figure 5, residues 693–728 of hPMS1 strongly interacted with hMLH1. On the other hand, as shown in Figure 6, residues 860–895 of hMLH3, which was the region most homologous with hPMS2 (612–647), interacted with hMLH1 among the four candidate regions. Interestingly, hMLH3 also interacted with two other regions; residues 1–244 and 1399–1453. Although residues 1–244 in hMLH3 interact with hMLH1, the interacting activity is extremely weak. Residues 1399–1453 in hMLH3 interact strongly with hMLH1; this region partially overlaps with residues 1390–1424, the homologous region with hPMS2 (612–647). Thus, even in the region of residues 1399–1453, the homologous amino acid residues might play an important role in the interaction with hMLH1.
Figure 4.
36 homologous amino acid residues conserved in hPMS2, hPMS1 and hMLH3. The proportions of identity or similarity with residues 612–674 of hPMS2 are presented. Lowercase letters indicate positions of the heptad repeat in the predicted coiled-coil motif. Bold letters in the consensus sequence correspond to identical amino acids. X, non-conserved amino acids. As for hMLH3, four homologous regions were identified, however, only residues 860–895, the highest homologous region, can interact with hMLH1.
Figure 5.
Domain of hPMS1 interacting with hMLH1. Results of the two-hybrid assay in yeast cells that contain the corresponding hPMS1 constructs (left figure) in the BD plasmid and the full-length hMLH1 cDNA in the AD plasmid. Parentheses indicate the mean β-gal activities in the yeast cells containing only the corresponding BD plasmid. Each β-gal activity is the mean and SD of three independent transformants. The minimal interacting domain of hPMS1 with hMLH1 resides at residues 693–728 as established in Figure 4.
Figure 6.
Domains of hMLH3 interacting with hMLH1. Results of the two-hybrid assay in yeast cells that contain the corresponding hMLH3 constructs (left figure) in the BD plasmid and the full-length hMLH1 cDNA in the AD plasmid. Parentheses indicate the mean β-gal activities in the yeast cells containing only the corresponding BD plasmid. Each β-gal activity is the mean and SD of three independent transformants. The domain of hMLH3 interacting with hMLH1 resides at residues 860–895, as established in Figure 4. There are two additional domains of hMLH3 interacting with hMLH1 at residues 1–244 and 1399–1453.
Predicted coiled-coil structures in the domains interacting with hMLH1
We attempted to search for features of amino acid residues that influence heterodimer formation. Coiled-coils were first identified as a sequence motif in eukaryotic transcription factors and shown to form dimers that consisted of a pair of parallel α-helices. Sequences capable of forming coiled-coils are characterised by a heptad repeats (abcdefg)n (32). The hydrophobic core of the dimer interface is formed by residues at the a and d positions. The solvent-accessible e and g positions are predominantly occupied by charged or polar amino acids (33). As shown in Figure 4, the hMLH1-interactive domains appear to contain 21 amino acids (three heptad repeats) necessary for the formation of a coiled-coil motif. In the interacting domain of hPMS2, 11 of 12 positions in the coiled-coil motif fit the above definition; the exception is A at position e2. In hMLH3, 10 of 12 positions fit the above definition; the exceptions are S at a2 and A at e2. In hPMS1, 9 of 12 positions fit the definition; the exceptions are P at e1, Q at d3, and V at g3. Furthermore, the amino acid residues of the full-length hMLH3, hPMS1 and hPMS2 were analysed by using the MULTICOIL program to predict coiled-coil structures (31). MULTICOIL compares the pairwise amino acid frequencies in protein sequences to frequencies in known coiled-coils and predicts the probability that these sequences will form two-stranded (dimeric) or three-stranded (trimeric) coiled-coils. The coiled-coil probabilities of these interacting domains in hPMS2 and hMLH3 are 0.54 and 0.46, respectively, strongly suggesting the formation of such structures. On the other hand, we could not find any predicted coiled-coil structures in the homologous interacting domain of hPMS1.
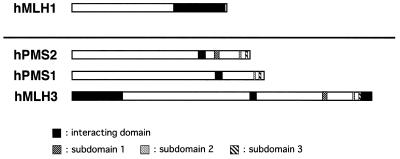
Relationship between each interacting domain and MutL subdomains
Figure 7 summarises the relationship between the locations of interacting domains in each MutL homologue and those of MutL subdomains described previously. The interacting domains of hMLH3 (860–895), hPMS1 (693–728) and hPMS2 (612–647) reside at the N-terminal regions of MutL subdomains. Two other interacting domains of hMLH1 in hMLH3, residues 1–244 and 1399–1453, are also absent in the three subdomains.
Figure 7.
The structural relationship between each interacting domain and three subdomains in four MutL homologues: hMLH1, hPMS2, hPMS1 and hMLH3. The 36 homologous amino acid residues showing strong interacting activities were all located in the N-terminal regions of three MutL subdomains.
Competitive inhibition of hPMS2 binding to GST-hMLH1 by hMLH3 or hPMS1
To examine the effectiveness of hMLH3 or hPMS1 in competing for the binding of hPMS2 to hMLH1, we have performed a competition experiment using the IVTT proteins. For this study, GST-hMLH1 and 35S-labeled hPMS2 proteins were mixed with various amounts of unlabeled competitors, luciferase, hPMS1 or hMLH3 and incubated for 30 min to allow heterodimer formation. Then the amounts of hPMS2 complexed with GST-hMLH1 were analysed by 10–20% SDS–PAGE. As shown in Figure 8, 50-fold excess amounts of hPMS1 or hMLH3 extensively reduced the interaction of hPMS2 with hMLH1. On the other hand, 50-fold excess amounts of luciferase (a negative control) did not influence the binding ability of hPMS2 to hMLH1. This experiment was further confirmed by the displacement of labeled hPMS2 by hPMS1 or hMLH3 (data not shown). Therefore, hMLH3, hPMS1 and hPMS2 in fact compete for the same interacting target of hMLH1.
Figure 8.
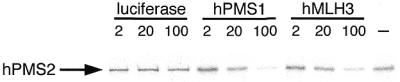
Competitive inhibition of hPMS2 binding by hPMS1 or hMLH3 when complexed with GST-hMLH1. The GST-hMLH1 and 2 µl of 35S-labeled IVTT-hPMS2 were incubated with indicated amounts of unlabeled IVTT-luciferase, IVTT-hPMS1 or IVTT-hMLH3. Then hPMS2 complexed with GST-hMLH1 was resolved by 10–20% SDS–PAGE and examined by BAS-1500. The arrow indicates the position of hPMS2 protein. Both hPMS1 and hMLH3 compete with hPMS2 for the binding to hMLH1.
DISCUSSION
To date, three MutL heterodimers, hMLH1–hMLH3, hMLH1–hPMS1 and hMLH1–hPMS2, have been identified. In this study, we aimed to determine the interacting domains of each heterodimer and to understand their properties. The domain of hMLH1 interacting with hPMS2 resides at residues 492–742, as indicated by the yeast two-hybrid assay (Fig. 1). This result is consistent with that described by Guerrette et al. (29), who used the GST-IVTT assay and determined the domain of hMLH1 interacting with hPMS2 to be at residues 506–756. Our results establish that the other two hMLH1 partners, hMLH3 and hPMS1, also interact with residues 492–742 of hMLH1, sharing the same interacting target with hPMS2. Because these three proteins compete for the same target on hMLH1 (Fig. 8), they may be involved in a subunit exchange process that generates three different heterodimers with shared domains. This leads to the possibility that the balance of these three hMLH1 partners might be important in expressing specific functions. If the function of a specific MutL heterodimer was necessary for the cell, it could be achieved by quantitative regulation of the three hMLH1 partners. We still have not determined the detailed quantification ratios of MutL homologues in various tissues during the process of development. Recently, Räschle et al. (24) reported that hPMS2 is expressed in protein amounts ∼10-fold higher than hPMS1 in HeLa cell nuclear extracts. On the other hand, there is still no information about the relationships in protein amounts between hPMS2 and hMLH3. At the mRNA level, the serial analysis of gene expression (SAGE) database SAGEMAP (http://www.ncbi.nlm.nih.gov/SAGE) can be used; however, it is difficult to compare the mRNA expression level because of the extremely low number of SAGE tags counted in each cell source (mostly 0, 1 or 2). It remains necessary to examine this aspect to clarify the role of each heterodimer.
This paper suggested the 36 homologous amino acid residues necessary for the interaction with hMLH1 on hMLH3, hPMS1 and hPMS2. These small fragments were all located at the C-terminal regions of each hMLH1 partner. In yeast, Pang et al. (34) defined three subdomains (1, 2 and 3) that are conserved between the Mlh1-interactive domain of Pms1 (residues 692–904) and the C-terminal portion of Mlh3; these subdomains were suggested to play a role in heterodimer formation with Mlh1. In man, Guerrette et al. (29) determined the domain of hPMS2 interacting with hMLH1 to be at residues 675–850, which contains three MutL subdomains. In addition, Lipkin et al. (23) indicated that the deletion mutant of hMLH3 exon 7, hMLH3Δexon7, could not interact with hMLH1 by the two-hybrid assay. Exon 7 of hMLH3 spans residues 1215–1238 and contains most of subdomain 1. Therefore, they suggested that subdomains 1 and 3 of MutL homologues are the critical regions for the interaction with hMLH1. In our study, however, these MutL subdomains were not present in the 36 homologous amino acid residues that interact with hMLH1. Our two-hybrid data clearly indicated that the N-terminal region of subdomain 1 (residues 612–674) in hPMS2 contributes to the interaction with hMLH1. This result was further confirmed by two other methods. First, the immunoprecipitation study using a human hPMS2-deficient cell, HEC-1-A, determined that residues 612–674 of hPMS2 in fact comprise the domain interacting with hMLH1 in the mammalian cell (Figs 2 and 3A). Secondly, we examined the domain of hPMS2 interacting with hMLH1 using the GST-IVTT assay, the same method that was used by Guerrette et al. (29) (Figs 2 and 3B). Although we tested a number of constructs in the GST-IVTT assay, we could not repeat their conclusion. Therefore, we concluded that the residues 612–674 of hPMS2 comprise the actual interacting domain with hMLH1. This conclusion was further supported by the discovery of the 36 homologous amino acid residues on hMLH3, hPMS1 and hPMS2 interacting with hMLH1. Furthermore, as shown in Figure 6, exon 7 of hMLH3 was not responsible for the interaction with hMLH1. We analysed the interaction between hMLH1 and hMLH3Δexon7 by two methods, the yeast two-hybrid assay (Fig. 6) and the GST-IVTT assay (data not shown), and found that hMLH3Δexon7 has a strong interacting activity with hMLH1. This conclusion is also supported by the analysis of Mlh1–Mlh2 interaction in yeast. Wang et al. (15) found that subdomains 1 and 3 are not present in the 98 amino acid residues of Mlh2 that interact with Mlh1 (15). Furthermore, subdomains 2 and 3 are not conserved in Mlh2. Therefore, subdomains 1, 2 and 3 do not seem to be relevant for the Mlh1–Mlh2 interaction. Taken together, subdomains 1, 2 and 3 are not likely to be responsible for the formation of MutL heterodimers in either man or yeast.
The fact that three hMLH1 partners interact with the same region of hMLH1 encouraged us to search for amino acid residues homologous to hPMS2 (612–674) within hMLH3 and hPMS1. There is one candidate located at residues 693–728 in hPMS1, and this region in fact interacted with hMLH1 as shown in Figure 5. As for hMLH3, it was more complicated, because there were four candidate regions, residues 245–280, 741–776, 860–895 and 1390–1424. Among these regions, only residues 860–895, which were highly homologous to hPMS2 (612–647), interacted with hMLH1 (Fig. 6). The hMLH3 has two additional interacting domains, residues 1–244 and 1399–1453. Residues 1–244 of hMLH3 have very slight interacting activity with hMLH1, but residues 1399–1453 of hMLH3 show strong interacting activity. Because residues 1399–1453 overlap with the candidate interacting domain (1390–1424), the amino acid residues of the candidate region might contribute greatly to the interaction with hMLH1. If this is the case then two regions, residues 860–895 and 1399–1453, may share the same interacting domain of hMLH1. Amino acid residues homologous to hPMS2 (612–647) were found in mouse PMS2 but not in yeast Pms1 and Mlh3 or in E.coli MutL. The analysis of sequence homology between man and yeast indicates that the region responsible for the interaction of yeast Pms1 with Mlh1 (residues 692–904) is located more C-terminally than that in human (residues 612–647). These findings might imply that the regions responsible for the formation of MutL heterodimer are slightly different between the yeast Pms1 and hPMS2. The region in which the coiled-coil structures are formed in Pms1 may be the primary target for the domain interacting with Mlh1. Further analysis would clarify this point. After we found the critical amino acid residues interacting with hMLH1, we searched for other hMLH1 partners from the protein database search program (PSI-BLAST 2.1) using residues 612–647 of hPMS2. However, we have not yet found any candidates. To analyse the roles of MutL heterodimers in the cell, it would be interesting to know whether or not the addition of these 36 amino acid residues inhibits the MMR system or other cellular functions.
Our analysis clearly indicates that there are common features in the domains interacting with hMLH1. Newman et al. (35) recently predicted coiled-coils from the yeast genome by using the MULTICOIL program and determined the associations between coiled-coils by using the yeast two-hybrid assay. Using this program, they identified 213 unique interactions between 162 putative coiled-coil sequences with ≥0.2 probability. We found by MULTICOIL that the 36 homologous residues in hPMS2 and hMLH3 necessary for the interaction with hMLH1 are predicted to include coiled-coil structures. Because the interacting domain of hMLH1 spans ∼250 amino acid residues (492–742), as suggested by our present study and Guerrette et al. (29), the higher order of conformation in hMLH1 would be necessary for the heterodimer formation. Therefore, it would be of great interest to determine the structural features of these interacting domains by X-ray crystallographic or NMR studies.
Our findings also help in understanding the implications of some mutations around these interacting domains. Two germline mutations of hPMS2 have been so far reported in HNPCC patients and both lose the hMLH1-interactive domain, residues 612–647. On the other hand, as reported by Guerrette et al. (29), most missense germline mutations located in the C-terminal region of hMLH1 showed extensive reduction in binding to hPMS2. For example, four missense alterations (L574P, K616Δ, R659P and A681T) displayed nearly undetectable interaction with hPMS2 (>99%), whereas two alterations (K618A and K618T) displayed a significant loss of interaction (>85%). Both the V506A and E578G hMLH1 missense alterations appeared to display consistent reduced binding to hPMS2 (25–65%). Furthermore, we observed that all examined hMLH1 alterations caused by frameshift or nonsense mutations in HNPCC greatly reduced the physical interaction with hPMS2 (data not shown). In these types of mutations, the inhibition of hMLH1–hPMS2 interaction would be one of the primary causes of MMR defect. Interestingly, although an extension type of C-terminus caused by a 4 bp insertion at the stop codon (756 Ins TGTT at 2270) in hMLH1 contained the hPMS2-interacting domain, residues 492–742, this alteration displayed extensive reduction in binding to hPMS2. Further analysis would clarify the relationship between each pathogenic mutation in HNPCC and the influence on various functions of MMR proteins.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Drs Toshikazu Takeshita (Shinshu University School of Medicine, Matsumoto, Japan) for valuable advice and discussion and to Barbara Lee Smith Pierce (Life Science Coordinator for the University of Maryland Asian Division) for editorial work in the preparation of this manuscript. This work was supported by the Ministry of Education, Science, Sports and Culture of Japan.
DDBJ/EMBL/GenBank accession no. AB039667
References
- 1.Modrich P. (1991) Mechanisms and biological effects of mismatch repair. Annu. Rev. Genet., 25, 229–253. [DOI] [PubMed] [Google Scholar]
- 2.Kolodner R. (1996) Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev., 10, 1433–1442. [DOI] [PubMed] [Google Scholar]
- 3.Peltomäki P. and Vasen,H.F.A. (1997) Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. Gastroenterology, 113, 1146–1158. [DOI] [PubMed] [Google Scholar]
- 4.Eshleman J.R. and Markowitz,S.D. (1995) Microsatellite instability in inherited and sporadic neoplasms. Curr. Opin. Oncol., 7, 83–89. [PubMed] [Google Scholar]
- 5.Modrich P. and Lahue,R. (1996) Mismatch repair in replication fidelity, genetic recombination and cancer biology. Annu. Rev. Biochem., 65, 101–133. [DOI] [PubMed] [Google Scholar]
- 6.Jiricny J. (1998) Eukaryotic mismatch repair: an update. Mutat. Res., 409, 107–121. [DOI] [PubMed] [Google Scholar]
- 7.Kolodner R.D. and Marsischky,G.T. (1999) Eukaryotic DNA mismatch repair. Curr. Opin. Genet. Dev., 9, 89–96. [DOI] [PubMed] [Google Scholar]
- 8.Marsischky G.T., Filosi,M., Kane,M.F. and Kolodner,R.D. (1996) Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev., 10, 407–420. [DOI] [PubMed] [Google Scholar]
- 9.Johnson R.E., Kovvali,G.K., Prakash,L. and Prakash,S. (1996) Requirement of the yeast MSH3 and MSH6 genes for MSH2-dependent genomic stability. J. Biol. Chem., 271, 7285–7288. [DOI] [PubMed] [Google Scholar]
- 10.Genschel J., Littman,S.J., Drummond,J.T. and Modrich,P. (1998) Isolation of MutSβ from human cells and comparison of the mismatch repair specificities of MutSβ and MutSα. J. Biol. Chem., 273, 19895–19901. [DOI] [PubMed] [Google Scholar]
- 11.Prolla T.A., Christie,D.-M. and Liskay,R.M. (1994) Dual requirement in yeast DNA mismatch repair for MLH1 and PMS1, two homologs of the bacterial mutL gene. Mol. Cell. Biol., 14, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prolla T.A., Pang,Q., Alani,E., Kolodner,R.D. and Liskay,R.M. (1994) MLH1, PMS1 and MSH2 interactions during the initiation of DNA mismatch repair in yeast. Science, 265, 1091–1093. [DOI] [PubMed] [Google Scholar]
- 13.Flores-Rozas H. and Kolodner,R.D. (1998) The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc. Natl Acad. Sci. USA, 95, 12404–12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durant S.T., Morris,M.M., Illand,M., Mckay,H.J., McCormick,C., Hirst,G.L., Borts,R.H. and Brown,R. (1999) Dependence on RAD52 and RAD1 for anticancer drug resistance mediated by inactivation of mismatch repair genes. Curr. Biol., 9, 51–54. [DOI] [PubMed] [Google Scholar]
- 15.Wang T.-F., Kleckner,N. and Hunter,N. (1999) Functional specificity of MutL homologs in yeast: evidence for three Mlh1-based heterocomplexes with distinct roles during meiosis in recombination and mismatch correction. Proc. Natl Acad. Sci. USA, 96, 13914–13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiricny J. and Nyström-Lahti,M. (2000) Mismatch repair defects in cancer. Curr. Opin. Genet. Dev., 10, 157–161. [DOI] [PubMed] [Google Scholar]
- 17.Paquis-Flucklinger V., Santucci-Darmanin,S., Paul,R., Saunières,A., Turc-Carel,C. and Desnuelle,C. (1997) Cloning and expression analysis of a meiosis-specific MutS homolog: The human MSH4 gene. Genomics, 44, 188–194. [DOI] [PubMed] [Google Scholar]
- 18.Winand N.J., Panzer,J.A. and Kolodner,R.D. (1998) Cloning and characterization of the human and Caenorhabditis elegans homologs of the Saccharomyces cerevisiae MSH5 gene. Genomics, 53, 69–80. [DOI] [PubMed] [Google Scholar]
- 19.Horii A., Han,H.-J., Sasaki,S., Shimada,M. and Nakamura,Y. (1994) Cloning, characterization and chromosomal assignment of the human genes homologous to yeast PMS1, a member of mismatch repair genes. Biochem. Biophys. Res. Commun., 204, 1257–1264. [DOI] [PubMed] [Google Scholar]
- 20.Kondo E., Horii,A. and Fukushige,S. (1999) The human PMS2L proteins do not interact with hMLH1, a major DNA mismatch repair protein. J. Biochem., 125, 818–825. [DOI] [PubMed] [Google Scholar]
- 21.Palombo F., Iaccarino,I., Nakajima,E., Ikejima,M., Shimada,T. and Jiricny,J. (1996) hMutSβ, a heterodimer of hMSH2 and hMSH3, binds to insertion/deletion loops in DNA. Curr. Biol., 6, 1181–1184. [DOI] [PubMed] [Google Scholar]
- 22.Li G.-M. and Modrich,P. (1995) Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc. Natl Acad. Sci. USA, 92, 1950–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipkin S.M., Wang,V., Jacoby,R., Banerjee-Basu,S., Baxevanis,A.D., Lynch,H.T., Elliott,R.M. and Collins,F.S. (2000) MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat. Genet., 24, 27–35. [DOI] [PubMed] [Google Scholar]
- 24.Räschle M., Marra,G., Nyström-Lahti,M., Schär,P. and Jiricny,J. (1999) Identification of hMutLβ, a heterodimer of hMLH1 and hPMS1. J. Biol. Chem., 274, 32368–32375. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 26.Vojtek A.B., Hollenberg,S.M. and Cooper,J.A. (1993) Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell, 74, 205–214. [DOI] [PubMed] [Google Scholar]
- 27.Gietz D., Jean,A.S., Woods,R.A. and Schiestl,R.H. (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res., 20, 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staudinger J., Perry,M., Elledge,S.J. and Olson,E.N. (1993) Interaction among vertebrate helix–loop–helix proteins in yeast two-hybrid system. J. Biol. Chem., 268, 4608–4611. [PubMed] [Google Scholar]
- 29.Guerrette S., Acharya,S. and Fishel,R. (1999) The interaction of the human MutL homologues in hereditary nonpolyposis colon cancer. J. Biol. Chem., 274, 6336–6341. [DOI] [PubMed] [Google Scholar]
- 30.Higuchi M., Asao,H., Tanaka,N., Oda,K., Takeshita,T., Nakamura,M., Van Snick,J. and Sugamura,K. (1996) Dispensability of Jak1 tyrosine kinase for interleukin-2-induced cell growth signaling in a human T cell line. Eur. J. Immunol., 26, 1322–1327. [DOI] [PubMed] [Google Scholar]
- 31.Wolf E., Kim,P.S. and Berger,B. (1997) MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci., 6, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodges R., Sodak,J., Smillie,L. and Jurasek,L. (1972) Tropomyosin: amino acid sequence and coiled-coil structure. Cold Spring Harb. Symp. Quant. Biol., 37, 299–310. [Google Scholar]
- 33.Cohen C. and Parry,D. (1990) α-helical coiled coils and bundles: how to design an α-helical protein. Proteins, 7, 1–14. [DOI] [PubMed] [Google Scholar]
- 34.Pang Q., Prolla,T.A. and Liskay,R.M. (1997) Functional domains of the Saccharomyces cerevisiae Mlh1p and Pms1p DNA mismatch repair proteins and their relevance to human hereditary nonpolyposis colorectal cancer-associated mutations. Mol. Cell. Biol., 17, 4465–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman J.R.S., Wolf,E. and Kim,P.S. (2000) A computationally directed screen identifying interacting coiled coils from Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 97, 13203–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]