Abstract
Anoxic insults cause hyperexcitability and cell death in mammalian neurons. Conversely, in anoxia-tolerant turtle brain, spontaneous electrical activity is suppressed by anoxia (i.e., spike arrest; SA) and cell death does not occur. The mechanism(s) of SA is unknown but likely involves GABAergic synaptic transmission, because GABA concentration increases dramatically in anoxic turtle brain. We investigated this possibility in turtle cortical neurons exposed to anoxia and/or GABAA/B receptor (GABAR) modulators. Anoxia increased endogenous slow phasic GABAergic activity, and both anoxia and GABA reversibly induced SA by increasing GABAAR-mediated postsynaptic activity and Cl− conductance, which eliminated the Cl− driving force by depolarizing membrane potential (∼8 mV) to GABA receptor reversal potential (∼−81 mV), and dampened excitatory potentials via shunting inhibition. In addition, both anoxia and GABA decreased excitatory postsynaptic activity, likely via GABABR-mediated inhibition of presynaptic glutamate release. In combination, these mechanisms increased the stimulation required to elicit an action potential >20-fold, and excitatory activity decreased >70% despite membrane potential depolarization. In contrast, anoxic neurons cotreated with GABAA+BR antagonists underwent seizure-like events, deleterious Ca2+ influx, and cell death, a phenotype consistent with excitotoxic cell death in anoxic mammalian brain. We conclude that increased endogenous GABA release during anoxia mediates SA by activating an inhibitory postsynaptic shunt and inhibiting presynaptic glutamate release. This represents a natural adaptive mechanism in which to explore strategies to protect mammalian brain from low-oxygen insults.
Keywords: western painted turtle, cerebral cortex, channel arrest, pyramidal neurons, natural anesthetic mechanism
When deprived of oxygen, mammalian neurons are unable to produce sufficient ATP to meet cellular demands (1, 2). As a result, the Na+/K+ ATPase (Na+ pump) fails and neuronal membrane potential (Vm) becomes unsustainable and anoxic depolarization (AD) follows, causing electrical hyperexcitability, deleterious Ca2+ influx, and spreading depression in the penumbral region (2, 3). Numerous studies have focused on the role of glutamatergic N-methyl-d-aspartate receptors (NMDARs) in this mechanism, and although NMDAR blockade prevents glutamatergic excitotoxicity (4), it does not prevent AD-mediated injury or postinsult apoptotic cell death (5). Thus, it is not surprising that clinical interventions targeting glutamate receptors alone have been largely ineffective against anoxic or ischemic damage (6), and therefore examination of alternative mechanisms to limit excitability during such insults is necessary.
A potential therapeutic alternative to directly antagonizing excitatory pathways is to up-regulate inhibitory mechanisms such as those mediated by γ-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the mature mammalian CNS (7). GABAergic mechanisms are not strongly recruited in ischemic mammalian neurons. In fact, although [GABA] is elevated by ∼30% in ischemic murine brain, GABAA receptor subunit mRNA expression is decreased by ∼85%, and GABA-evoked currents and [ATP] run down rapidly, suggesting endogenous GABAergic neuroprotection is transient and largely ineffective (8–10). Nonetheless, activating GABA receptors (GABARs) preinsult limits neuronal hyperexcitability in mammalian models of ischemic damage, and AD and cell death are not observed in the afflicted brain region (11, 12). Despite these promising results, GABA has received little attention as a clinical stroke intervention and the mode of GABAergic neuroprotection is poorly understood (13).
Unlike the modest increase observed in anoxic mammals, brain [GABA] is rapidly elevated in anoxia-tolerant species including freshwater turtles (Chrysemys picta bellii, Trachemys scripta elegans) and fish (Carassius carassius, Carassius auratus) (1, 14). These organisms achieve a large-scale reduction in cellular energy turnover and survive weeks to months of anoxic exposure without apparent detriment (1). The key to this tolerance may be an adaptive mechanism that combines depressed glutamatergic signaling with enhanced GABAergic signaling. Indeed, we have previously demonstrated that excitatory glutamatergic signaling (via NMDARs and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors; AMPARs) is down-regulated in these species during anoxia (15–17); however, the role of GABAergic signaling in anoxia tolerance has not been elucidated. In turtle brain, [GABA] is increased a remarkable 80-fold during anoxia and electrical activity is suppressed 75–95% (14, 18–20). Therefore, this organism provides a good model in which to examine the role of GABA in regulating electrical activity during anoxia. We hypothesized that increased GABAergic conductance to Cl− mediates electrical suppression (i.e., spike arrest; SA) in the anoxic turtle cortex, and that in combination with glutamatergic channel arrest prevents hyperexcitability and provides neuroprotection against anoxic insults.
Results
Spike Arrest Is Comediated by GABAA and GABAB Receptors.
We examined SA in pyramidal neurons from intact cortical sheets because synaptic connectivity and endogenous neurotransmitter release are maintained in this preparation ex vivo (18, 20, 21). In normoxia, stepwise current injections elicited action potentials (APs) at a threshold (APth) of −49.1 ± 2.0 mV and spontaneous APs occurred with a frequency (APf) of 2.1 ± 0.3 Hz (n = 15), whereas during anoxia, APth depolarized to −31.1 ± 2.9 mV and APf decreased >70% (n = 19; Fig. 1 A, B, D, and E). These changes were reversed by reoxygenation and are consistent with previous reports of SA in anoxic turtle brain (15, 18, 20). GABA perfusion reversibly mimicked SA and depolarized APth in a dose-dependent fashion (n = 3–13 for each [GABA]; Fig. 1C), and at moderate (0.5 mM) or high (2.0 mM) [GABA], APth depolarized 20–35 mV and APf decreased 60–70% (n = 10–13 each; Fig. 1 A and D). Conversely, antagonism of GABAARs (with 25 μM gabazine; GZ) or GABABRs (with 5 μM CGP55845; CGP) each partially reduced the depolarization of APth and increased APf, whereas coantagonism of GABAA+BRs abolished the anoxic depolarization of APth and increased APf 30- and 100-fold relative to normoxic and anoxic controls, respectively (n = 9–13 each; Fig. 1 A and D).
Fig. 1.
Spike arrest in the anoxic turtle cortex is GABAA+BR-mediated. (A) Summary of neuronal AP threshold (APth) during a normoxic-to-anoxic transition with recovery (t = 10, 30, and 60 min). (B) Sample recordings of APs stimulated from electrically quiet neurons by stepwise current injection. (C) Dose–response relationship of [GABA] versus ΔAPth. (D) Summary of APf expressed as fold change relative to pretreatment baseline. (E) Sample Vm recordings of APs from D. Treatments: GABA, 25 μM gabazine (GABAAR antagonist), 5 μM CGP55845 (GABABR antagonist). Data are mean ± SEM from 9–19 separate experiments. Asterisks indicate significant difference from normoxic controls. Double daggers indicate significant difference from anoxic controls (P < 0.05).
Endogenous GABA Release Increases During Anoxia and “Resets” Vm to GABA Receptor Reversal Potential.
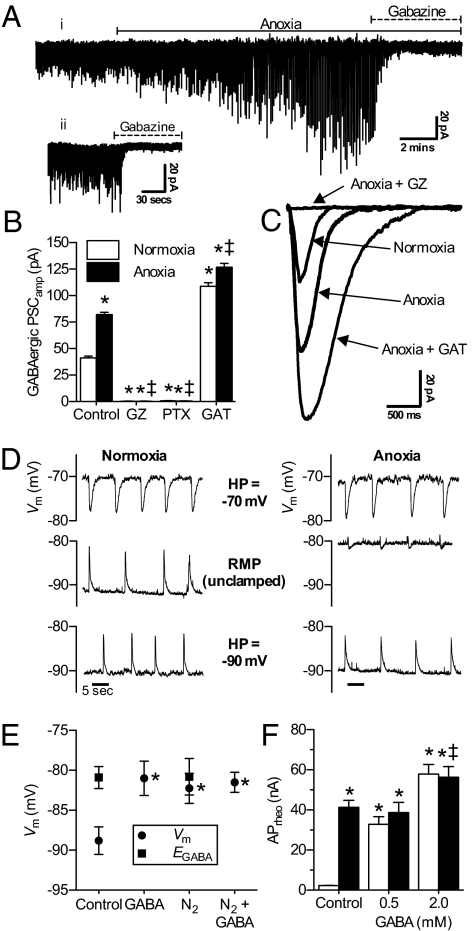
To directly assay anoxic changes in GABA release, we adapted techniques used elsewhere to examine GABAergic postsynaptic currents (PSCs) (9, 22), and used GABAARs to detect anoxic changes in extracellular [GABA] (n = 8; Fig. 2A) (for these experiments only, currents were isolated and enhanced as described in SI Materials and Methods and Fig. 2). Under these conditions, a significant tonic inhibitory current was not observed (normoxic Itonic = 1.6 ± 3.9 pA vs. anoxic Itonic = 3.1 ± 2.4 pA; n = 8; Fig. 2Aii); however, spontaneous GABAergic slow phasic PSCs were detectable during normoxia, and their amplitude doubled during anoxic perfusion (41.2 ± 1.6 to 82.1 ± 2.1 pA; n = 10; Fig. 2 Ai, B, and C), whereas PSCf was unchanged (Fig. S1A). PSCs were abolished by GABAAR antagonists (GZ or picrotoxin; PTX) and enhanced by GABA transporter antagonists (GAT; n = 4 each), confirming postsynaptic GABAARs activation by endogenous synaptic GABA release. To better understand this relationship, we measured native GABAergic postsynaptic potentials (PSPs) using the perforated-patch method to avoid perturbing intracellular [Cl−] and in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and (2R)-amino-5-phosphonovaleric acid (APV) to isolate GABAergic events. Normoxic GABAergic PSP amplitude was 10.3 ± 1.8 mV, and events were depolarizing relative to Vm (n = 7; Fig. 2D and Fig. S1B). Similar to PSCs, GABAergic PSPs were abolished by GZ but not by CGP, indicating mediation by postsynaptic GABAARs (n = 10–13 each). PSPs occurred at a rate of 0.1 ± 0.01 Hz, and this frequency was unchanged during anoxia (0.09 ± 0.02 Hz); however, anoxic PSPs were markedly smaller in amplitude (−1.47 ± 0.7 pA), and their polarity was reversed such that PSPs generally became mildly hyperpolarizing relative to Vm, indicating that the relationship between Vm and the reversal potential of Cl− (ECl) is reset during anoxia. Importantly, when we current-clamped neurons at −90 mV (near normoxic Vm) or −70 mV (depolarized relative to both normoxic and anoxic Vm), the resulting PSPs were not different between conditions, indicating that the mechanism underlying this change is an alteration of Vm homeostasis during anoxia.
Fig. 2.
Endogenous synaptic GABAergic activity is enhanced during anoxia and Vm resets to EGABA. (A) (i) Raw spontaneous GABAergic current activity. (ii) Raw tonic GABAergic activity. To aide current detection, pipette [Cl−] was increased to 110 mM and neurons were clamped at −100 mV and cotreated with the NMDAR antagonist APV (25 μM) and the AMPAR antagonist CNQX (25 μM). (B) Summary of GABAergic PSCamp. Neurons were examined per A. (C) Superimposed raw spontaneous GABAergic PSC recordings averaged from 30 events from three neurons per trace. (D) Sample recordings of GABAergic PSPs in normoxia and anoxia from neurons voltage-clamped at −70 mV or −90 mV (top and bottom traces, respectively) or free-running (no voltage-clamp; middle traces) in the presence of APV and CNQX. RMP, resting membrane potential [holding potential (HP)]. (E) Summary of paired Vm and EGABA measured in the perforated-patch configuration. APs were prevented by the voltage-gated Na+ channel antagonist TTX (5 μM). (F) Summary of APrheo (Iinj to elicit an AP). Treatments were as in Fig. 1, with GABA transport inhibitors SKF 89976A hydrochloride (40 μM; GAT-1) and (S)-SNAP-5114 (20 μM; GAT-2 and -3). Data are mean ± SEM from 4–19 separate experiments. Asterisks indicate significant difference from normoxic controls. Double daggers indicate significant difference from anoxic controls (P < 0.05).
GABAAR activation induces Cl− flux in the direction of ECl (GABAAR reversal potential; EGABA) (7), which has not been previously measured in adult turtle brain. Therefore, to more closely examine the relationship between Vm and GABAAR activity, we examined EGABA. In normoxic whole-cell experiments, EGABA was −80.9 ± 1.4 mV, and ∼8 mV depolarized relative to Vm in the same neurons (−88.8 ± 1.7 mV; n = 10; Fig. 2E). EGABA was unchanged during anoxic perfusion (−80.8 ± 2.3 mV; n = 8; Fig. 2E and Fig. S1C); however, Vm depolarized to −80.2 ± 1.9 mV. Consistent with an underlying GABAergic mechanism, normoxic GABA perfusion depolarized Vm in a dose-dependent fashion (n = 3–13 each [GABA]; Fig. S1D), and 2 mM GABA depolarized Vm to EGABA in normoxia and anoxia (−81.0 ± 2.1, n = 12, and −81.5 ± 1.2 mV, n = 7, respectively). In separate experiments, we examined the effect of normoxic GABAR modulation on Vm and EGABA in the perforated-patch configuration. Consistent with our whole-cell results, EGABA was depolarized relative to Vm (−85.6 ± 0.8 and −91.8 ± 0.7 mV, respectively; n = 7; Fig. S1E), whereas during GABA perfusion, Vm depolarized to −84.3 ± 1.3 mV (n = 10) in a GABAAR-dependent fashion (n = 7). [Note: EGABA could not be determined during GABA or GZ perfusion due to GABAAR saturation or antagonism, respectively (Fig. 2E and Fig. S1 E and F).]
When EGABA is depolarized relative to Vm, such as in the early stages of mammalian development and to a lesser degree in the anoxic turtle cortex, the Cl− gradient is such that postsynaptic GABAAR activation induces Cl− efflux accompanied by reductions in cell volume (7). Therefore, to confirm the relationships between Vm and EGABA observed in our electrophysiology experiments, we examined Cl− movement and neuronal volume regulation using noninvasive assays [6-methoxy-N-ethylquinolinium iodide (MEQ) and calcein-AM fluorescence, respectively] (23, 24). Anoxia or GABA perfusion reversibly increased MEQ fluorescence 30–40% in a GABAAR-dependent fashion, reflecting Cl− efflux, because intracellular Cl− quenches MEQ fluorescence (n = 5 each; Fig. S1 G and H). Similarly, calcein fluorescence increased 22.1 ± 4.0 and 9.0 ± 3.0% during anoxia or GABA perfusion, respectively, reflecting cell shrinkage, whereas GABAA+BR antagonism reduced the anoxic increase by ∼80%, to 5.0 ± 7.7% (n = 4 each; Fig. S1 I–K). Therefore, unlike in most adult mammalian neurons, EGABA is depolarized relative to normoxic Vm in adult turtle cortex. Furthermore, the GABAAR-mediated increase in Cl− conductance (GCl) is sufficiently large that ECl (EGABA) becomes the primary determinant of Vm during anoxia, and Vm resets to EGABA. Despite this initial mild depolarization of Vm, this mechanism strongly resisted further Vm depolarization beyond EGABA because rheobase (APrheo; i.e., stimulation required to generate an AP) increased >15-fold during anoxia or GABA perfusion (n = 10–17 each; Fig. 2F).
GABAA and GABABRs Mediate Distinct Post- and Presynaptic Mechanisms of SA.
Because anoxia or GABA perfusion induced Cl− efflux and Vm depolarization, we hypothesized that GABAR activation increased neuronal conductance to Cl−. Not surprisingly, we found that anoxic perfusion reversibly increased neuronal whole-cell conductance (Gw) from 4.8 ± 0.3 to 6.7 ± 0.4 nS (n = 17 for each; Fig. 3 A and B) (see comment on previous Gw measurements in anoxic turtle neurons in SI Materials and Methods). This change was abolished by GZ but not by CGP (n = 7 each), whereas GABA perfusion increased Gw in a dose-dependent fashion (n = 3–13 for each [GABA]; Fig. 3C). Importantly, pretreatment with 0.5 mM GABA raised baseline Gw to 8.1 ± 0.6 nS (n = 9; Fig. 3A); however, following the transition to anoxia, Gw decreased to 6.6 ± 0.4 nS and this change was prevented by GZ (n = 9 each). This suggests the magnitude of the anoxic change in GCl was partially masked in our experiments, because Gw is a global cellular measurement that includes the conductance of all open channels and receptors. This is particularly relevant in this model because previous studies have demonstrated channel arrest of AMPARs (GNa) and NMDARs (GNa/Ca), decreased K+ leakage, and reduced K+ and Na+ channel and NMDAR density in anoxic turtle brain (1, 15), all of which would mask the GABAAR-mediated GCl. To isolate ΔGCl from these confounding anoxic changes, we examined the effects of GABA on normoxic input resistance (Rin; the inverse of Gw) using the gramicidin perforated-patch technique. In support of our hypothesis that ΔGCl was partially obscured during anoxia, GABA reversibly decreased Rin ∼85%, from 314.5 ± 50.2 to 72.1 ± 13 MΩ (n = 10 and 7, respectively; Fig. S2 A and B), and this change was prevented by GZ but not by CGP (n = 8 and 6, respectively). Therefore, increased GABA release enhanced GABAAR-mediated PSP activity and activated a large GCl, which was the primary determinant of Gw homeostasis during anoxia, causing Vm to depolarize to EGABA.
Fig. 3.
GABAAR activation induces a shunt-like increase in plasma membrane permeability to Cl−; GABABR activation decreases EPSP activity via a presynaptic mechanism. (A) Summary of Gw measured in the whole-cell configuration. (B) I–V relationships from A. (C) Dose–response relationship of [GABA] versus Gw. (D) Summary of EPSPf expressed as fold change relative to pretreatment baseline. (E) Sample Vm recordings from neurons treated as indicated. Black bars represent duration of treatment. Treatments were as in Figs. 1 and 2. Data are mean ± SEM from 6–19 separate experiments. Asterisks indicate significant difference from normoxic controls. Double daggers indicate significant difference from anoxic controls (P < 0.05).
CGP perfusion increased action potential frequency approximately fivefold in both normoxia and anoxia (Fig. 1D) but, unlike GZ, had no effect on Gw (Fig. 3A). This suggests that GABABR-mediated inhibition occurs at the presynapse. Excitatory postsynaptic potentials (EPSPs) are mediated by presynaptic glutamate release, and unlike the dramatic increase characteristic of ischemic mammalian brain, [glutamate] decreases 47% in anoxic turtle brain due to a combination of sustained reuptake and reduced release, and glutamatergic EPSPf and amplitude decrease (1, 2, 15). Glutamate release can be inhibited by agonism of presynaptic GABABRs, which activate K+ channels that hyperpolarize presynaptic cells and also inhibit Ca2+ channels that mediate vesicular glutamate release (25). Because [GABA] is elevated in anoxic turtle brain (14), such presynaptic mechanisms likely contribute to SA by decreasing postsynaptic glutamatergic drive. In support of this, we found that CGP enhanced AMPAergic EPSPf in both normoxia and anoxia (n = 9–13 each; Fig. 3D). Furthermore, antagonizing NMDARs with APV prevented hyperexcitability in anoxic CGP-challenged neurons, and APs were prevented despite the CGP-mediated enhancement of EPSP activity during anoxia (n = 5; Fig. 3E).
GABAergic SA Is Critical to Anoxia Tolerance in Turtle Neurons.
To assess the importance of GABAergic mechanisms in anoxia tolerance, we measured hallmarks of excitotoxic cell death (ECD) characteristic of ischemic mammalian neurons, including hyperexcitability, AD, deleterious Ca2+ influx, and cell death (2, 3). Similar to previous examinations in turtle cortex, these parameters were unchanged in normoxia, whereas during anoxia, Vm depolarized to EGABA (n = 12) and cytosolic calcium concentration ([Ca2+]c) increased ∼30% (n = 5; Fig. 4 A and B and Fig. S3 A and B) (1, 17, 26). Anoxic changes were reversed by reperfusion, indicating turtle neurons were not significantly stressed. Similarly, propidium iodide (PI) uptake [assessed relative to maximum uptake in metabolically arrested neurons treated with iodoacetate (IAA) and sodium cyanide (NaCN); Fig. S3 D and E] was unchanged during 4 h of normoxia or anoxia (n = 3 each; Fig. 4 A and B and Fig. S3C).
Fig. 4.
Pharmacological inhibition of endogenous inhibitory GABAergic mechanisms during anoxia is excitotoxic to turtle neurons. Sample Vm recordings (upper trace in each grouping), fura-2 Ca2+ fluorescence recordings (lower trace), and paired raw PI fluorescence images before treatment onset (t = 0 h) and following 4 h of treatment from neurons treated with normoxia (A), anoxia (B), anoxia + GABAA+BR antagonists (C), and anoxia + GABAA+BR antagonists in the presence of TTX (D). Black bars represent duration of treatment. Treatments were as in Figs. 1 and 2.
In striking contrast, neurons cotreated with GZ and CGP underwent recurrent seizure-like events (SLEs) and terminal depolarization of Vm (n = 17), a fourfold increase in [Ca2+]c during anoxia that became further elevated to >1 μM during reperfusion (n = 4), and an increase in PI uptake within 2 h of treatment onset that at 4 h was elevated four- and sevenfold above normoxic and anoxic controls (n = 3; Fig. 4C and Fig. S3 A–C). This suggests SA is critical to anoxia tolerance because blocking the inhibitory GABAergic activity sensitized Vm to depolarizing inputs, resulting in unsustainable electrical hyperexcitation and terminal depolarization of Vm. We hypothesized that SA is predominately mediated by a GABAAR-dependent electrical shunt mechanism that is passively recruited to oppose spontaneous excitatory events. If correct, pharmacological inhibition of APs with voltage-gated Na+-channel antagonist tetrodotoxin (TTX) should limit electrical excitability in GABAA+B R-antagonized neurons, eliminate the requirement of SA as critical for anoxia tolerance, and ameliorate cytotoxicity despite persistent GABAAR blockade and enhanced presynaptic glutamate release. In support of this, TTX inhibited APs and prevented SLEs (n = 6), deleterious [Ca2+]c accumulation (n = 4), and PI uptake by GABAA+BR-antagonized anoxic neurons (n = 4; Fig. 4D and Fig. S3 A–C).
Discussion
Using a naturally anoxia-tolerant model, we show that GABAA/BRs are critical to SA and neuronal survival against low-oxygen insult in an anoxia-tolerant species. Importantly, we also show through the use of GABA uptake blockers and GABAA/BR antagonists that the anoxic response is sensitive to endogenous synaptic GABA release and activates distinct inhibitory mechanisms on pre- and postsynaptic cells. The primary mechanism of SA is an increase in slow phasic GABAAR-mediated PSP activity, which opens a postsynaptic GCl that dominates Gw during anoxia. We propose that this conductance acts as an inhibitory electrical shunt that effectively clamps Vm at EGABA during anoxia, opposing further Vm depolarization due to glutamatergic EPSPs. This is likely the primary mechanism underlying anoxic SA, because GABAAR antagonism is significantly more excitatory than GABABR antagonism. Nonetheless, GABABR activation contributes to SA by decreasing postsynaptic EPSPf during anoxia, presumably via inhibition of presynaptic glutamate release. Importantly, Vm depolarization, such as the shift to EGABA we report here, is usually excitatory in neurons (7); however, the voltage separation between Vm and APth paradoxically increases during anoxia because APth depolarizes twofold further than Vm. Therefore, although anoxic neurons depolarize to EGABA, Vm is hyperpolarized relative to APth, and the net effect of these changes is inhibitory. Indeed, despite Vm depolarization, rheobase increases ∼20-fold during anoxia and fewer APs are elicited by each supra-APth stimulus train.
EGABA is primarily determined by the Cl− gradient, which is in turn dependent upon the balance in expression and activity of Na+-K+-Cl− (NKCC1) (Cl− influx) and K+-Cl− (KCC2) (Cl− efflux) cotransporters (7). In developing mammalian brain, KCC2 expression is low relative to NKCC1, and GABA is the primary excitatory neurotransmitter. As a result, mammalian neonate brain is particularly prone to hyperexcitability and seizures, but can be protected by simultaneously inhibiting NKCC1 and antagonizing postsynaptic GABAARs to temporarily switch the Cl− gradient and render GABA inhibitory (27). Conversely, in adult mammalian brain, KCC2 expression is high and EGABA is generally hyperpolarized relative to Vm (7). Therefore, GABA perfusion opposes AP generation by hyperpolarizing Vm away from APth via GABAAR-mediated Cl− influx; however, this form of inhibition requires increased Na+-pump activity to maintain ion gradients and is therefore not an effective strategy (1).
In adult mammalian brain, endogenous synaptic GABA release in the first few minutes of ischemia slows the onset of AD; however, this limited inhibition is insufficient to preserve [ATP] and maintain ion pumping >2–3 min (9). As a result, ion gradients are lost and extracellular K+ concentration ([K+]o) increases sharply, causing AD within ∼6 min of insult (2, 3, 9). Extracellular neurotransmitter levels also increase rapidly in the first few minutes of ischemia (2, 9, 22), and glutamatergic Na+ and Ca2+ influx quickly depolarizes Vm away from EGABA, thereby enhancing AD and also the inward-driving force on Cl−. When extracellular GABA activates GABAARs, large-scale Cl− and associated water influx occurs (9, 23). This abrupt osmotic shift causes ischemic cells to swell and, depending on the severity of AD, undergo necrotic membrane rupture (28). This problem is compounded by impaired cerebral blood flow (CBF), which limits the removal of deleterious ions and neurotransmitters, the extracellular accumulation of which are required for ischemia-related cell swelling (2, 29). In contrast, CBF is maintained during prolonged anoxia in adult turtle brain (1), and synaptic transmission persists in vivo and in vitro (18, 19, 30; present report). Furthermore, [K+]o, and cellular [ATP] are maintained for at least 6 h in anoxic turtle brain (30), and cells do not exhibit a necrotic phenotype. Finally, whereas ischemic adult mammalian neurons swell, anoxic turtle neurons shrink because EGABA is depolarized relative to normoxic Vm, and therefore anoxic activation of GABAARs causes Cl− and water efflux and thus cell-volume reduction. To our knowledge, the inhibitory depolarized relationship between EGABA and Vm we report in adult turtle neurons is a unique phenotype and may represent an evolutionary adaptation that allows this species to retain neuronal function while reducing energy expenditure during prolonged anoxia.
This unique phenotype of GABAergic inhibition contributes crucial energy savings by reducing the demand on ATPases and related cotransporters during anoxia. The bulk of these savings is attributable to the GABA-mediated decrease in AP firing (i.e., SA), which reduces activity-induced perturbations of ionic gradients and the requirement for compensatory Na+-pump activity (1). In addition, Vm resets to EGABA during anoxia, and is depolarized relative to normoxia, which further reduces pump demand. Also, because GABAergic GCl dominates Gw during anoxia, the importance of GK in determining Vm is reduced. As a result, K+-channel expression and GK are decreased without detriment in anoxic turtle brain, and the pump activity required to maintain K+ gradients is inherently reduced (1, 30). Finally, glutamate reuptake is sustained during anoxia in turtle brain and is also primarily dependent on ATPase activity (31). GABABR-mediated inhibition of presynaptic glutamate release limits the workload on these pumps, further reducing ATP consumption. Indeed, turtle brain Na+-pump activity is known to decrease ∼35% during anoxia (1); therefore, when combined, these effects provide significant energy savings and suppress neuronal excitability sufficiently to maintain [ATP] and Vm despite a >90% reduction in ATP production during anoxia (1). This conclusion is supported by the observations that: (i) GABAA+BR inhibition prevents anoxia-mediated SA and neurons exhibit hallmarks of ECD, indicating that in the absence of GABAergic inhibition, anaerobic ATP production is insufficient to sustain Vm homeostasis; and (ii) simultaneous inhibition of GABAA+BRs and voltage-gated Na+ channels prevented the GABAA+BR antagonist-mediated increase in excitatory activity and ECD during anoxia.
The lack of a measurable tonic inhibitory current in turtle cortical neurons is an important finding. Tonic currents are mediated by extrasynaptic GABAARs and set the excitability threshold for neurons in adult mammalian brain (32). Baseline tonic inhibition is typically >50 pA, and this activity increases greater than twofold postischemic insult in mammals and impairs functional recovery following stroke (9, 33). Conversely, anoxia-tolerant turtle cortical neurons likely do not express extrasynaptic GABAARs because they lack an observable tonic current. This is not uncommon; many cell types do not express these receptors (32). More importantly, because EGABA is depolarizing relative to Vm in normoxic turtle brain, any tonic GABAergic activity would be excitatory, and therefore the absence of such a tone in this model is not surprising. Indeed, the lack of any tonic inhibition minimizes the cellular signal-to-noise ratio of synaptic GABAergic inputs, and would therefore acutely sensitize turtle neurons to slow phasic (i.e., synaptic GABAAR-mediated) inhibition.
The phenotype of phasic GABAergic inhibition in this model is unique: Slow phasic GABAergic activity occurs during normoxia at a frequency that is ∼1/15th that of resting mammalian neurons (9). This activity doubles in amplitude but is unchanged in frequency throughout >30 min of anoxic exposure, whereas phasic frequency in ischemic mammalian neurons increases 20-fold (and 300-fold relative to anoxic turtle neurons) within seconds of insult onset, and GABAARs quickly become desensitized to extracellular GABA binding after ∼10 min due to NMDAR-mediated Ca2+ accumulation (9). In comparison, the phasic GABAergic response in anoxic turtle brain is sustained, likely because Ca2+-mediated GABAAR desensitization is prevented in turtle by concurrent glutamatergic channel arrest and maintenance of [Ca2+]c homeostasis during prolonged anoxia (15, 17), and because the lack of an underlying tonic baseline allows for considerably greater inhibition from a relatively modest increase in phasic activity.
It is important to note that although GABAergic pathways are pivotal to the anoxia tolerance of turtle neurons, several other mechanisms act in concert to provide this neuroprotection. Indeed, in turtle cortical neurons in which channel arrest of NMDARs is prevented, excitotoxicity is also observed (34), suggesting multiple mechanisms may provide critical contributions to anoxia tolerance in parallel. Nonetheless, these mechanisms combine to reduce whole-animal energy consumption >90% during anoxia, which matches the decrease in energy produced anaerobically relative to aerobic (normoxic) respiration (for a review, see ref. 1) and allows these animals to tolerate long-term anoxic insults. Many of these mechanisms cannot easily be adapted to mammals; however, cell-level mechanisms such as glutamatergic channel arrest and GABAergic up-regulation are more readily mimicked in mammalian brain and would provide significant energy savings during low-oxygen stresses. Indeed, in normoxic rat brain, AP generation and propagation and EPSPs account for ∼81% of neuronal energy expenditure (35).
In conclusion, we report that SA of turtle cortical neurons is key to prolonged anoxia tolerance and is mediated by unique inhibitory GABAergic mechanisms that combine increased GABAA+BR activity with a mildly depolarized EGABA to reduce AP firing and ATP demand. Although this phenotype appears to be unique to the adult turtle CNS, the elucidation of these endogenous inhibitory systems provides insight into effective neuroprotective strategies against low-oxygen insult that may be adapted to protect hypoxia-sensitive mammalian brain against stroke. Mimicking the combined GABAergic and glutamatergic phenotype of anoxic turtle brain by activating GABAA+BRs while inhibiting KCC2 activity and AMPAR and NMDAR conductance may prove to be neuroprotective in mammalian stroke models, and this hypothesis deserves further evaluation. Finally, the turtle enters into a comatose state during prolonged anoxia and EEG activity is decreased to basal levels (19). Because general anesthetics are hypothesized to induce a comatose state through the inhibition of excitatory glutamatergic and enhancement of GABAergic neurotransmission (36), as we have shown in the anoxic turtle, it is tempting to speculate that this model represents a natural anesthetic mechanism.
Materials and Methods
This study was approved by the University of Toronto Animal Care Committee. Cortical slice dissection and anoxic whole-cell patch-clamp recording methods are described elsewhere (21, 26). Briefly, cortical sheets were dissected and placed in a bath with a flow-through perfusion system. Anoxia was achieved by gassing the perfusate with 95% N2/5% CO2. Perfusion lines were double-jacketed, the bath chamber was covered, and all air spaces were similarly gassed. Neurons were perfused with pharmacological modifiers and/or anoxia as specified in Results. Electrical activity was recorded for up to 2 h from pyramidal neurons. For perforated-patch experiments, electrodes were backfilled with a KCl solution containing 25 μg/mL gramicidin.
Methods for dye-loading and fluorescence experiments are described previously (17). Baseline fluorescence was recorded for 10–20 min and then the tissue was exposed to treatment for up to 80 min and then reperfused. Ca2+ changes were assessed using 5 μM fura-2. Cl− changes were assessed using MEQ. Changes in MEQ fluorescence were calibrated using the Stern–Volmer equation as described elsewhere (23). Cell-volume changes were measured using calcein-AM (24). Cell death was assessed using a PI exclusion assay. PI fluorescence due to IAA plus NaCN treatment was assessed as maximal cell death, and each treatment group was normalized to this value.
Statistical Analysis.
Normalized data were analyzed using two-way ANOVA with a Student–Newman–Keuls post hoc test to compare within and against treatment and normoxic values. Significance was determined at P < 0.05.
Supplementary Material
Acknowledgments
A Natural Sciences and Engineering Research Council of Canada Discovery grant, a Discovery Accelerator award to L.T.B., and Ontario Graduate Scholarships in Science and Technology to M.E.P. and D.H. supported this work.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102429108/-/DCSupplemental.
References
- 1.Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu Rev Physiol. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- 2.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 3.Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 4.Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- 5.Buchan A, Pulsinelli WA. Hypothermia but not the N-methyl-d-aspartate antagonist, MK-801, attenuates neuronal damage in gerbils subjected to transient global ischemia. J Neurosci. 1990;10:311–316. doi: 10.1523/JNEUROSCI.10-01-00311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 7.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Erecińska M, Nelson D, Wilson DF, Silver IA. Neurotransmitter amino acids in the CNS. I. Regional changes in amino acid levels in rat brain during ischemia and reperfusion. Brain Res. 1984;304:9–22. doi: 10.1016/0006-8993(84)90857-6. [DOI] [PubMed] [Google Scholar]
- 9.Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J Neurosci. 2004;24:3837–3849. doi: 10.1523/JNEUROSCI.5539-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Siegel RE, Schwartz RD. Rapid decline of GABAA receptor subunit mRNA expression in hippocampus following transient cerebral ischemia in the gerbil. Hippocampus. 1993;3:527–537. doi: 10.1002/hipo.450030412. [DOI] [PubMed] [Google Scholar]
- 11.Galeffi F, Sinnar S, Schwartz-Bloom RD. Diazepam promotes ATP recovery and prevents cytochrome c release in hippocampal slices after in vitro ischemia. J Neurochem. 2000;75:1242–1249. doi: 10.1046/j.1471-4159.2000.0751242.x. [DOI] [PubMed] [Google Scholar]
- 12.Costa C, et al. Coactivation of GABA(A) and GABA(B) receptor results in neuroprotection during in vitro ischemia. Stroke. 2004;35:596–600. doi: 10.1161/01.STR.0000113691.32026.06. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson GE, Lutz PL. Release of inhibitory neurotransmitters in response to anoxia in turtle brain. Am J Physiol. 1991;261:R32–R37. doi: 10.1152/ajpregu.1991.261.1.R32. [DOI] [PubMed] [Google Scholar]
- 15.Pamenter ME, Shin DS, Buck LT. AMPA receptors undergo channel arrest in the anoxic turtle cortex. Am J Physiol Regul Integr Comp Physiol. 2008;294:R606–R613. doi: 10.1152/ajpregu.00433.2007. [DOI] [PubMed] [Google Scholar]
- 16.Wilkie MP, et al. Evidence of anoxia-induced channel arrest in the brain of the goldfish (Carassius auratus) Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:355–362. doi: 10.1016/j.cbpc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Bickler PE, Donohoe PH, Buck LT. Hypoxia-induced silencing of NMDA receptors in turtle neurons. J Neurosci. 2000;20:3522–3528. doi: 10.1523/JNEUROSCI.20-10-03522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Pinzón MA, Chan CY, Rosenthal M, Sick TJ. Membrane and synaptic activity during anoxia in the isolated turtle cerebellum. Am J Physiol. 1992;263:R1057–R1063. doi: 10.1152/ajpregu.1992.263.5.R1057. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes JA, et al. Electroencephalogram activity in the anoxic turtle brain. Am J Physiol. 1997;273:R911–R919. doi: 10.1152/ajpregu.1997.273.3.R911. [DOI] [PubMed] [Google Scholar]
- 20.Feng ZC, Rosenthal M, Sick TJ. Suppression of evoked potentials with continued ion transport during anoxia in turtle brain. Am J Physiol. 1988;255:R478–R484. doi: 10.1152/ajpregu.1988.255.3.R478. [DOI] [PubMed] [Google Scholar]
- 21.Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 22.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 23.Inglefield JR, Schwartz-Bloom RD. Fluorescence imaging of changes in intracellular chloride in living brain slices. Methods. 1999;18:197–203. doi: 10.1006/meth.1999.0772. [DOI] [PubMed] [Google Scholar]
- 24.Hamann S, et al. Measurement of cell volume changes by fluorescence self-quenching. J Fluoresc. 2002;12:139–145. [Google Scholar]
- 25.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 26.Pamenter ME, Buck LT. Neuronal membrane potential is mildly depolarized in the anoxic turtle cortex. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:410–414. doi: 10.1016/j.cbpa.2008.04.605. [DOI] [PubMed] [Google Scholar]
- 27.Dzhala VI, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 28.Otsubo K, Katayama Y, Terashi A. Protective effects of glycerol and mannitol on delayed neuronal death in the gerbil hippocampus Translated from Japanese. Nippon Ika Daigaku Zasshi. 1993;60:231–240. doi: 10.1272/jnms1923.60.231. [DOI] [PubMed] [Google Scholar]
- 29.Yi CS, Fogelson AL, Keener JP, Peskin CS. A mathematical study of volume shifts and ionic concentration changes during ischemia and hypoxia. J Theor Biol. 2003;220:83–106. doi: 10.1006/jtbi.2003.3154. [DOI] [PubMed] [Google Scholar]
- 30.Chih CP, Feng ZC, Rosenthal M, Lutz PL, Sick TJ. Energy metabolism, ion homeostasis, and evoked potentials in anoxic turtle brain. Am J Physiol. 1989;257:R854–R860. doi: 10.1152/ajpregu.1989.257.4.R854. [DOI] [PubMed] [Google Scholar]
- 31.Milton SL, Thompson JW, Lutz PL. Mechanisms for maintaining extracellular glutamate levels in the anoxic turtle striatum. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1317–R1323. doi: 10.1152/ajpregu.00484.2001. [DOI] [PubMed] [Google Scholar]
- 32.Mody I, Glykys J, Wei W. A new meaning for “gin & tonic”: Tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41:145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pamenter ME, Buck LT. δ-Opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J Exp Biol. 2008;211:3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- 35.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.