Abstract
Oil-in-water emulsions have been successfully used to increase the efficacy, immunogenicity, and cross-protection of human vaccines; however, their mechanism of action is still largely unknown. Nlrp3 inflammasome has been previously associated to the activity of alum, another adjuvant broadly used in human vaccines, and MyD88 adaptor protein is required for the adjuvanticity of most Toll-like receptor agonists. We compared the contribution of Nlrp3 and MyD88 to the adjuvanticity of alum, the oil-in-water emulsion MF59, and complete Freund's adjuvant in mice using a three-component vaccine against serogroup B Neisseria meningitidis (rMenB). Although the basal antibody responses to the nonadjuvanted rMenB vaccine were largely dependent on Nlrp3, the high-level antibody responses induced by alum, MF59, or complete Freund's adjuvant did not require Nlrp3. Surprisingly, we found that MF59 requires MyD88 to enhance bactericidal antibody responses to the rMenB vaccine. Because MF59 did not activate any of the Toll-like receptors in vitro, we propose that MF59 requires MyD88 for a Toll-like receptor-independent signaling pathway.
Vaccine adjuvants target the innate immune system to enhance humoral and cellular responses to coadministered antigens. In the last decade, a better understanding of the innate immunity pathways lead to the characterization of the mechanism of action of the vaccine adjuvants deriving from microbial structures. Most of these compounds (e.g., CpG oligonucleotides, monophosphoryl lipid A) target a family of pattern-recognition receptors, called Toll-like receptors (TLRs), expressed by immune cells, including antigen presenting cells (APCs) (1). Engagement of TLRs triggers the expression of cytokines and costimulatory molecules through a signaling pathway that is largely dependent on MyD88 adaptor protein. This pathway is required for priming of naive CD4 T cells by APCs, and therefore most TLR agonists require MyD88 for cellular and humoral responses in mice. Two exceptions are represented by TLR4, which triggers MyD88 and another adaptor protein called TRIF (TLR-domain-containing adapter protein inducing IFN-β), and therefore is only partially dependent on MyD88, and by TLR3, which depends only on TRIF (1). Besides playing a central role for the TLR signaling, MyD88 is involved in other innate immune pathways. MyD88 interacts also with the IL-1 receptor (IL-1R) through a domain that is called the Toll-IL1R (TIR) domain and is required for IL-1 and IL-18 signaling (2). Recently, it has been shown that MyD88 can also interact with the receptor TACI, which triggers class-switch recombination in B cells in a new TIR-independent mechanism (3).
Adjuvants targeting TLR signaling have been licensed only recently. In contrast, particulate adjuvants, such as aluminum salts (alum) and emulsions, have been used in preclinical models and human licensed vaccines for decades, despite which, their mechanism of action is less characterized compared with the adjuvants derived from microbial compounds.
Alum has been used in several human vaccines for more than 70 y. Experiments conducted in mice double-knockout for MyD88 and TRIF have suggested that alum acts independently from TLR signaling (4). It has been shown that alum adsorption increases antigen uptake by antigen-presenting cells (5). Alum alone is a weak activator of immune cells in vitro (6–8) but synergizes with LPS for the production of mature IL-1β through the activation of the Nlrp3 inflammasome complex (9–11). The activation of Nlrp3 in vitro has also been demonstrated for QuilA and chitosan, two other particulate adjuvants (11). The requirement of Nlrp3 for the adjuvanticity of alum in vivo is more controversial. Immunization studies in mice deficient for the inflammasome cascade have initially suggested that Nlrp3 activation is required for alum adjuvanticity (9, 11). In a following study it has been proposed that deficiency in the Nlrp3 inflammasome pathway has only a partial effect, reducing IgE titers but not IgG (12). More recently, the involvement of Nlrp3 on alum adjuvanticity has been challenged altogether (13, 14).
MF59 is an oil-in-water emulsion made of squalene, emulsified with two surfactants (polysorbate 80 and sorbitan trioleate) to obtain nanodroplets of 160 nm. MF59 has been licensed in Europe for adjuvanted seasonal Flu vaccines since 1997, and is known to increase immunogenicity and cross-protection in young children and in the elderly (15). MF59 has been licensed in Europe as well for pandemic influenza vaccines and has been widely used for the 2009 H1N1 pandemic flu campaign (16). Clinical trials conducted using avian H5 pandemic flu antigens have demonstrated that MF59 allowed for antigen dose sparing and increased seroconversion and cross-protection in vaccinees (17). A recent study has shown that in infants MF59 increases the efficacy of the Trivalent Inactivated influenza Vaccine from 43% to 89%.* As with alum, the mechanism of action of MF59 is not fully characterized. In vitro data on human cells have shown that MF59 does not activate dendritic cells (DCs), but induces the secretion of chemokines by granulocytes, macrophages, and monocytes and promotes the differentiation of monocytes toward DCs (7). MF59 is able to enhance antigen uptake by mouse dendritic cells in vivo and to promote phagocytosis in human peripheral blood mononuclear cells (7, 18). In addition to antigen delivery functions, MF59 induces the local up-regulation of cytokines, chemokines, and other innate immunity genes in the muscle (19), which promote the recruitment of immune cells like monocytes, DCs, and granulocytes (20). The innate immune pathways required for MF59 adjuvanticity are unknown.
Another particulate adjuvant—not licensed for human vaccines but largely used in preclinical models–is complete Freund's adjuvant (CFA), a mix of mineral water-in-oil emulsion and microbial products derived from Mycobacterium tuberculosis. Experiments conducted in MyD88-TRIF double-knockout mice have suggested that TLR signaling is not required for CFA-enhanced antibody responses to TNP-KLH antigen (21). In contrast, another study has shown that IgG2c responses to ovalbumin induced by CFA are affected by MyD88 deficiency (9).
In this study we assessed the contribution of Nlrp3 and MyD88 to adjuvanticity using knockout mice. We show that alum, MF59, and CFA adjuvanticity to a recombinant meningococcus B vaccine (rMenB) is independent of Nlrp3 activation. Because Nlrp3 deficiency affected the antibody response to the unadjuvanted vaccine, we propose that adjuvants can overcome the requirement for the Nlrp3 inflammasome by activating alternative innate immune pathways. In addition, we show that mutations affecting MyD88 strongly reduce the functional antibody titers to MenB induced by emulsions MF59 and CFA, although they did not affect the responses to alum. As MF59 did not engage any TLR in vitro, we suggest that MyD88 plays a TLR-independent function, which is required for MF59 adjuvanticity.
Results
MF59 Does Not Promote Inflammasome Activity in Vitro.
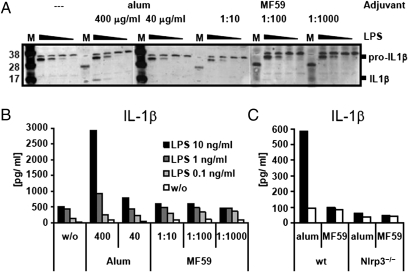
To test whether MF59, similarly to alum, can activate the inflammasome complex in vitro, we added MF59 to LPS-primed murine bone marrow-derived dendritic cells (BM-DC). Fig. 1A shows that priming with rising concentrations of LPS leads to enhanced production of pro–IL-1β and that subsequent addition of alum results in detectable levels of cleaved, mature IL-1β. In contrast, the addition of various amounts of MF59 did not induce pro–IL-1β processing. Measuring cytokine concentrations in culture supernatants of BM-DC gave similar results (Fig. 1B). By comparing BM-DC from WT and Nlrp3-deficient mice we could confirm previous data showing that alum-mediated induction of IL-1β processing is totally dependent of Nlrp3 (Fig. 1C). Our data indicate that only alum but not MF59 can lead to inflammasome activation in vitro.
Fig. 1.
IL-1β cleavage and release from BM-DC. BM-DC were primed overnight with serial dilutions of LPS (10 ng/mL, 1 ng/mL, 0.1 ng/mL, 0 ng/mL). MF59 or alum were added to the cells at the indicated concentrations and cells were incubated for further 24 h. Cleavage of pro–IL-1β was assessed by Western blotting (A) (M: protein weight marker) and cytokine release into the culture supernatant was measured by multiplex-bead ELISA (B). BM-DC from Nlrp3−/− or WT mice were primed with 10 ng/mL of LPS and incubated overnight with MF59 (1:100, vol:vol) or alum (400 μg/mL) (C).
Nlrp3-Deficient Mice Respond Well to rMenB Vaccine When Adjuvanted with Alum, MF59, and CFA.
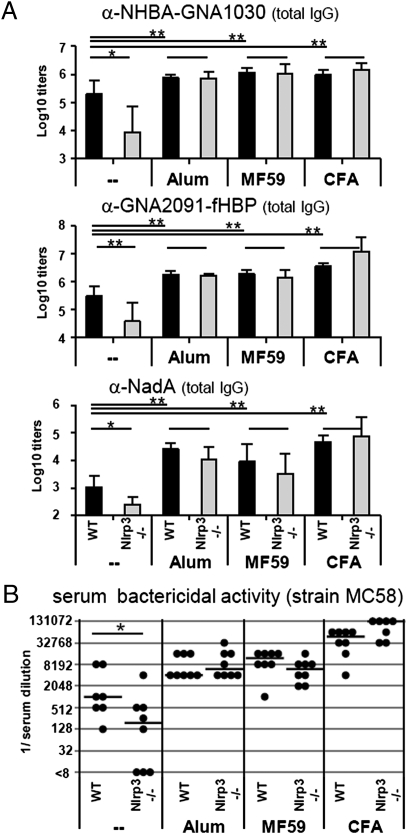
Although direct inflammasome activation in vitro by alum is well established, there is some controversy about the requirement of the Nlrp3 inflammasome pathway to alum-mediated adjuvanticity in vivo and no data were available regarding MF59. We tested the contribution of the Nlrp3 inflammasome to the generation of adaptive immune responses using three different recombinant clinical-grade proteins from the Neisseria meningitidis serotype B vaccine (rMenB): Neisseria adhesin A (NadA), a fusion protein of Neisseria heparin binding protein (NHBA) with Neisseria antigen GNA1030 (NHBA-GNA1030) and a fusion protein of factor H binding protein (fHBP) with Neisseria antigen GNA2091 (GNA2091-fHBP). All these antigens are expressed on the bacterial surface and have been selected because they elicit broad protective antibody responses (22). Groups of Nlrp3−/− (knock-out, KO) or control C57BL/6 mice (WT) received three immunizations of either plain vaccine in PBS buffer or rMenB adjuvanted with alum, MF59, or CFA. Serum antibody titers toward all antigens present in the vaccine were assessed 2 wk after a third immunization.
Nlrp3-deficient mice mount a significantly weaker antibody response toward the nonadjuvanted vaccine than WT controls (Fig. 2A). In contrast to plain vaccination, the IgG antibody titers induced by vaccines adjuvanted with alum, CFA, or MF59 are similar in WT and in KO mice. All three adjuvants tested significantly enhanced antibody production toward each antigen contained in the vaccine compared with plain vaccination.
Fig. 2.
Antibody titers in Nlrp3−/− mice and WT controls after vaccination with plain or adjuvanted MenB antigens. (A) Total IgG antibody titers to all antigens contained in the vaccine: NHBA-GNA1030, GNA2091-fHBP, and NadA. Values represent means of Log10 titers of eight mice per group plus SD. Unpaired, two-tailed Student's t test was performed comparing WT vs. Nlrp3−/− and adjuvant vs. PBS injection (in WT mice). *P < 0.05 are considered significant and **P < 0.001 highly significant. (B) SBA of mouse polyclonal antibodies in the serum of eight single mice (black symbols) and their median value (line). Statistical analysis of WT vs. KO mice was performed using unpaired, two-tailed Mann-Whitney test. *P < 0.05.
We further assessed the effect on Nlrp3 deficiency on development of the different IgG subclasses. Significant differences between KO and WT mice were only observed in the induction of IgG1 and IgG2c antibodies against NHBA-GNA1030 in the nonadjuvanted vaccine groups, but immune responses were indistinguishable between Nlrp3-deficient mice and controls for all adjuvants tested (Fig. S1).
Immunity toward infection with N. meningitidis correlates with the presence of bactericidal antibodies, which kill the bacterium in the presence of complement (23). Therefore, we further addressed functionality and breadth of antibodies by performing bactericidal killing assays using three different N. meningitidis strains (MC58, M4407, and NZ98/254). The serum bactericidal titers (SBAs) for each MenB strain depend not only on the immunogenicity of the vaccine but also on the level of expression and on the sequence similarity of the three vaccine antigens used (23). Vaccination with the plain vaccine induced high SBA titers for strain MC58, expressing high levels of a conserved fHBP antigen in WT mice; however, this response was reduced by four times in Nlrp3-deficient mice (Fig. 2B). In addition, plain vaccines induced low but detectable SBA titers for all other strains in WT mice, but in Nlrp3-deficient mice SBA titers were below detection limit. In agreement with the data on IgG titers, SBA titers toward all Neisseria strains induced by alum-, MF59-, and CFA-adjuvanted vaccines were increased compared with plain vaccine and not affected by Nlrp3 deficiency (Fig. 2B and Table 1).
Table 1.
SBA of mouse polyclonal antibodies from Nlrp3−/− mice and WT controls
|
Neisseria strains |
||||||
| MC58 |
M4407 |
NZ98/254 |
||||
| WT | Nlrp3−/− | WT | Nlrp3−/− | WT | Nlrp3−/− | |
| Plain | 4,096 | 512 | 64 | <16 | 128 | <16 |
| Alum | 8,192 | 8,192 | 64 | 128 | 64 | 64 |
| MF59 | 8,192 | 8,192 | 512 | 512 | 256 | 256 |
| CFA | >32,768 | >32,768 | 2,048 | 4,096 | 1,024 | 4,096 |
Serum (Post 3) from eight mice per group were pooled and serial dilutions (1:2) were assessed for their bactericidal activity. Differences in dilution steps ≥ 2 were considered significant and are highlighted in bold.
Next, we assessed how Nlrp3 deficiency impacts on local innate immune responses induced by adjuvant administration. We found that alum, MF59, or CFA injection led to cytokine release, activation of resident macrophages, and recruitment of monocytes and neutrophils in the peritoneum. In agreement with antibody production data, all these innate immune events were not affected by Nlrp3 deficiency (Fig. S2).
Taken together, these data show that adaptive and innate immune responses induced by all vaccine adjuvant tested are independent of Nlrp3.
MF59 Adjuvanticity Depends on MyD88.
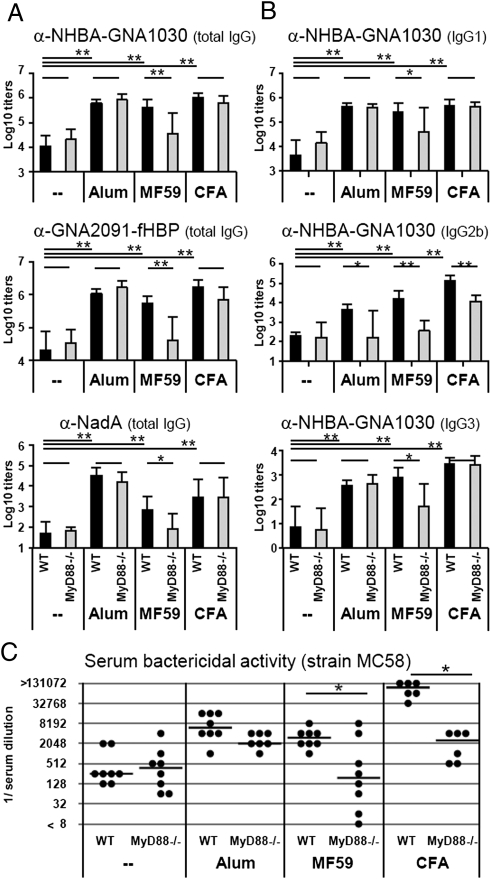
Next we investigated the effect of MyD88 deficiency on adjuvanticity. Groups of MyD88−/− or control C57BL/6 mice received three immunizations of rMenB either as plain vaccine or adjuvanted with MF59, alum, or CFA. As expected, all adjuvants tested lead to significantly enhanced total IgG antibody titers in WT mice for all antigens (Fig. 3A). Alum- or CFA-enhanced total IgG responses were not affected by MyD88 deficiency. In contrast, MyD88 KO mice that received MF59-adjuvanted vaccine displayed significantly reduced IgG antibody titers similar to nonadjuvanted vaccine. A similar picture was observed for IgG1 and IgG3 subclasses (Fig. 3B). Instead, the production of IgG2b antibodies was reduced in all groups of MyD88 mutant mice, independently of the adjuvant used (Fig. 3B).
Fig. 3.
Antibody titers in MyD88−/− mice and WT controls after vaccination with plain or adjuvanted MenB antigens. (A) Total IgG antibody titers to all antigens contained in the vaccine: NHBA-GNA1030, GNA2091-fHBP, and NadA. (B) IgG subclasses IgG1, IgG2b, and IgG3 for one representative antigen (NHBA-GNA1030). Values represent means of Log10 titers of eight mice per group plus SD. Unpaired, two-tailed Student's t test. *P < 0.05; **P < 0.01. (C) SBA of mouse polyclonal antibodies in the serum of eight single mice (black symbols) and their median value (line). Unpaired, two-tailed Mann-Whitney test. *P < 0.05.
We further assessed functionality of antibody titers and found that all adjuvanted vaccines lead to significantly higher SBA titers in WT mice (Fig. 3C). In MyD88 mutants, the SBA titers induced by nonadjuvanted or by alum-adjuvanted vaccines to all of the MenB strains tested were not affected. In contrast, the MyD88 deficiency resulted in a significant reduction of bactericidal activity elicited by both MF59- and CFA-adjuvanted vaccines (Fig. 3C and Table 2).
Table 2.
SBA of mouse polyclonal antibodies from MyD88−/− mice and WT controls
|
Neisseria strains |
||||||
| MC58 |
M4407 |
NZ98/254 |
||||
| WT | MyD88−/− | WT | MyD88−/− | WT | MyD88−/− | |
| Plain | 256 | 256 | <16 | <16 | <16 | <16 |
| Alum | 4,096 | 2,048 | 128 | 256 | 64 | 128 |
| MF59 | 2,048 | 512 | 128 | 16 | 64 | 16 |
| CFA | 65,536 | 2,048 | 4,096 | 512 | 1,024 | 256 |
Serum (Post 3) from eight mice per group were pooled and serial dilutions (1:2) were assessed for their bactericidal activity. Differences in dilution steps ≥ 2 were considered significant and are highlighted in bold. The difference in SBA titers measured in WT mice in Tables 1 and 2 may be explained by the fact that female and male mice were used, respectively.
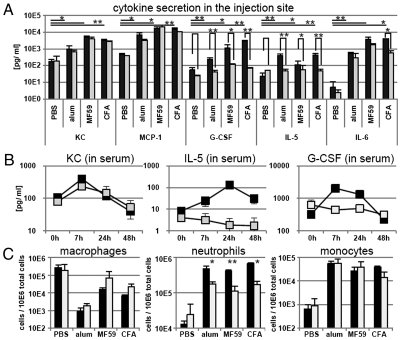
To better understand the role of MyD88 in adjuvanticity, we compared the innate immune responses induced by adjuvant injection in WT and MyD88 KO mice. Injection of alum, MF59, and CFA lead to release of cytokines, like the neutrophil-attracting chemokine KC, the monocyte chemoattractant protein-1 (MCP-1), G-CSF, IL-5, and IL-6 in the peritoneum. We found that G-CSF and IL-5 cytokine levels in the peritoneum were significantly diminished in MyD88 KO mice compared with WT mice after injection of all three adjuvants (Fig. 4A). Cytokine release in the vaccination site subsequently leads to higher levels of the respective cytokines in the serum and recruitment of responsive cell types, like neutrophils and monocytes, from the blood stream into the injection site. Consistently, we found that chemokine levels in the serum in response to MF59 were reduced in MyD88 KO mice (Fig. 4B). All adjuvants lead to macrophage activation and neutrophil and monocyte influx into the peritoneum (Fig. 4C). However, only neutrophil recruitment in response to adjuvant injection was affected by MyD88 deficiency. In summary, we could detect some effects of MyD88 deficiency on the innate immune responses induced by adjuvant injection, but none of these effect was specific for MF59.
Fig. 4.
Innate immune responses after adjuvant stimulation in MyD88−/− mice or WT controls. (A) Cytokine content in intraperitoneal washfluid 4 h after intraperitoneal adjuvant injection. Black bars: WT mice; gray bars: MyD88−/− mice. Values represent mean of three mice plus SD. Unpaired, two-tailed Student's t test was performed comparing WT vs. MyD88−/− and adjuvant vs. PBS injection (in WT mice). *P < 0.05 **P < 0.001. (B) Cytokine content in serum at different timepoints postinjection of MF59. Black symbols: WT mice; gray symbols: MyD88−/− mice. Values represent mean of two to three mice ± SD. (C) Cell recruitment events in the peritoneal cavity 4 h after adjuvant injection. Values are given per 10E6 total peritoneal cells and represent means of three mice per group plus SD.
Because the adaptor molecule MyD88 is involved in signaling of most TLRs, we tested MF59 and the M. tuberculosis extract that is used to make CFA for their ability to activate TLRs in vitro. Different TLR-reporter cell lines (stable TLR-2, -3, -4, -5, -7, -8, or -9–expressing 293 cells) were stimulated with increasing concentrations of MF59 (Fig. S3A), M.tuberculosis extract (Fig. S3B), or a combination of both (Fig. S3C). MF59 did not activate any TLR but M. tuberculosis extract activated TLR2. The addition of MF59 did not affect TLR stimulation by M. tuberculosis (Fig. S3C). Similarly, no interference was found by costimulation of all TLR reporter cells with MF59 and their respective TLR agonists (Pam3CSK4; PolyI:C; Flagellin; R848, CpG) (Fig. S4). These data demonstrate that, in contrast to CFA, which contains TLR2 elicitors, MF59 is not a TLR agonist and does not interfere with TLR engagement.
Discussion
It has been proposed that particulate adjuvants, such as alum, can induce inflammasome activation through a mechanism involving phagosomal destabilization (24). However, the requirement of inflammasome activation for alum adjuvanticity is more controversial and probably depends on the experimental model used (9, 11).
In the first part of this study we addressed the requirement of Nlrp3 inflammasome for three particulate adjuvants: a mineral salt (alum), a squalene-based oil-in-water emulsion (MF59), and a mineral water-in-oil emulsion mixed with microbial structures (CFA). As antigen, we used a mix of three clinical-grade proteins that represent five surface-exposed antigens of meningococcus B known to require an adjuvant for an optimal antibody response, which allows killing of the bacteria in the presence of complement. We found that the antibody responses (total IgG, IgG isotypes, and functional SBA titers) were independent of Nlrp3 inflammasome for all adjuvants tested. Consistent with the adaptive immunity data, we found that Nlrp3 mutation had no effect on all innate immune reactions induced by the injection of alum, MF59, and CFA. These data are in agreement with some recent reports on alum adjuvanticity and suggest that adjuvants based on oil emulsions are also independent of inflammasome activation (13, 14). In addition, our data are consistent with a recent study showing that MF59 adjuvanticity to a H5 influenza vaccine is independent of Nlrp3 and caspase 1, but depends on the adaptor protein ASC (25). We cannot exclude that other inflammasome complexes may play a redundant role with Nlrp3 in vivo after the injection of emulsions (26). However, the inability of MF59 to induce any processing of IL-1β in vitro in mouse and human LPS-primed cells strongly suggests that it is inflammasome-independent.
Interestingly, we found that Nlrp3 is required for an optimal response to unadjuvanted vaccine to all three proteins used. These data suggest that, in the absence of adjuvants, the activation of Nlrp3 inflammasome induced by the stress and the damage caused by injection plays a role to trigger an adaptive immune response to the vaccine. The addition of an adjuvant can overcome Nlrp3 requirement, probably by activating other inflammasome complexes or alternative signaling pathways that are redundant with Nlrp3 activation.
In the second part of the study we showed that a knockout of the adaptor protein MyD88 did not affect alum, but affected MF59 and CFA adjuvanticity to rMenB. Interestingly, we have shown that MyD88 deficiency did not affect the total IgG Ab response to CFA. These data are in agreement with previous results obtained using a TNP antigen (21) but not with a following study that used ovalbumin (9). In our experimental system, MyD88 deficiency strongly reduced the ability of CFA to induce antibodies that kill bacteria in a complement dependent assay. This difference could be explained by the effect of MyD88 on Th1 responses induced by CFA and measured by IgG2b/c isotype. Alternatively, it is possible that MyD88 is required for generation of high avidity antibodies by CFA. The effect of MyD88 deletion on CFA adjuvanticity is not surprising, considering our in vitro data showing that M. tuberculosis extract can activate the TLR2/MyD88 signaling pathway.
MyD88 deficiency abolished secretion of G-CSF and IL-5 in response to all adjuvants and, accordingly, adjuvant-induced neutrophil recruitment in peritoneum was diminished. Several recent publications find that neutrophils can act as professional antigen-presenting cells, and Abi Abdallah et al. state that such neutrophils would lead to a T-cell differentiation with a Th1/Th17 phenotype (27). The diminished neutrophil recruitment observed in MyD88 KO mice might explain why IgG2b/c isotype responses were affected in these mice in response to all adjuvants tested.
Surprisingly, total IgG and subclasses (IgG1, IgG2b/c, and IgG3) responses to MF59 were all strongly affected by MyD88 deficiency, suggesting that MyD88 is required for both Th1 and Th2 adaptive responses generated by MF59. In addition, the functional SBA titers induced by MF59 were reduced in all MenB strains tested. MyD88 plays a central role in MF59 adjuvanticity; however, this adaptor protein is required for multiple innate immune signaling pathways. The next question will be to identify which of these pathways is required for MF59 adjuvanticity. Because we show that MF59 is not able to activate any TLR in vitro, it is very unlikely that a TLR-dependent pathway would be involved. However, we cannot exclude that the injection of MF59 in vivo induces the secretion of endogenous TLR agonists such as DNA, RNA, or lipids. Alternatively, MF59 adjuvanticity may depend on the activity of cytokines that target the IL-1R family, such as IL-1β or IL-18. If this hypothesis is true, the processing of these cytokines should be Nlrp3-independent. More recently is has been shown that MyD88 can play TIR-independent function in B-cell signaling, by interacting with TACI, a receptor involved in antibody class switch (3). Therefore, it can be hypothesized that MF59 boosts the antibody responses to rMenB by activating an intrinsic B-cell pathway. More work in different mutant mouse strains needs to be performed to address these hypotheses.
Materials and Methods
Mouse Strains.
Male MyD88−/− mice (C57B/6 background) aged 4 to 6 wk were acquired from Oriental BioService, Inc.. Female Nlrp3−/− mice (in C57B/6 background) aged 4 to 6 wk were acquired from J. Tschopp, University of Lausanne, Switzerland. Male or female C57B/6 control mice were purchased from Harlan, Holland. Mice were used in agreement with institutional and European guidelines.
Vaccine Antigen Composition.
The recombinant protein vaccine against MenB (rMenB) contains three recombinant antigens representing five different surface-expressed Neisseria proteins: NadA, a single polypeptide, and NHBA-GNA1030 and GNA2091-fHBP expressed as fusion proteins, as previously described (22, 28, 29). We confirmed that antigens are endotoxin-free by Limulus test and TLR-reporter cell lines.
Adjuvant Preparation.
MF59 was prepared as described (20). Desiccated Mycobacterium tuberculosis H37 Ra (Difco) was dissolved in PBS and used with Freund's Incomplete Adjuvant to give CFA.
Vaccine Formulation.
Alum-adjuvanted vaccine formulations were prepared by adsorbing each recombinant protein on aluminum hydroxide at 100 μg/mL protein, 3 mg/mL aluminum, and 9 mg/mL NaCl in 10 mM histidine pH 6.5. All components were mixed and left for 15 min under stirring at room temperature and than stored overnight at 4 °C before the immunization. MF59- or CFA-containing vaccine formulations were prepared by adding an equal volume of MF59 or CFA to the antigen formulations just before immunization. All formulations contained 10 mM histidine buffer pH 6.5 and NaCl to reach a final osmolality of 0.300 mOsmol/kg and 20 μg of each antigen.
When unadjuvanted formulations were prepared, antigens were diluted in PBS at 100 μg/mL just before injection; in these cases, final dose for each antigen was 20 μg.
Immunization.
Groups of eight mice were immunized intraperitoneally. For each injection, mice received a total dose of 60 μg of rMenB vaccine (trivalent, 20 μg each Ag) in 0.2 mL volume. Three injections were given at 0, 21, and 35 d.
Serology.
Serum samples from individual mice were drawn at 21 d (post1), 35 d (post2), and 49 d (post3). Total IgG (IgG) antibody titers to the proteins contained in the vaccine and IgG1, IgG2b, IgG2c, and IgG3 subclasses were assessed by ELISA.
Complement-Mediated Bactericidal Activity.
SBA against N. meningitidis strains was evaluated as previously described by Borrow et al., with minor modifications (30), using pooled baby rabbit serum (Cedarlane Laboratories) as complement source.
In Vitro Inflammasome Activation Assays.
Assays were performed as described in Li et al. (11). BM-DC cultured for 8 d in medium supplemented with 200 U/mL murine GM-CSF were plated and incubated overnight with serial dilutions of LPS. Adjuvants were added to the cells, which were further incubated for 24 h. Cleavage of pro–IL-1β was assessed by Western blotting and cytokine release into the culture supernatant was measured by multiplex-bead ELISA.
Peritoneal Wash.
Mice were injected intraperitoneally with 200 μL of PBS, 3 mg/mL alum, MF59 (1:1, vol:vol), or CFA (1:1, vol:vol). At different timepoints postinjection, peritoneal wash was performed with 2 mL of PBS. Cells in the lavage fluid were harvested by centrifugation and analyzed by multicolor-FACS analysis. The lavage fluid was stored at −20 °C until analysis of cytokine content.
FACS Analysis.
The following antibodies were used: α-Ly6C-FITC, α-CD11b-PE-Cy7, α-CD19-APC, α-CD86-PE (all from BD Pharmingen) and α-I-A/I-E-AlexaFluor700, α-F4/80-PacificBlue, α-CD11c-APC-AlexaFluor750 (all from eBioscience).
Cytokine Analysis.
Cytokine concentrations in peritoneal lavage fluid or mouse serum was measured by multiplex-bead ELISA (Milliplex, Millipore) according to the manufacturer's instructions, using a mouse 13-plex panel. The cytokines analyzed were IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12(p40), IL-12(p70), IL-17, G-CSF, IFN-γ, KC, MCP-1, and TNF-α.
In Vitro Activation of TLR-Reporter Cell Lines.
Individual HEK293-NF-κB-Luc cell lines stably expressing single human TLRs were incubated with different concentration of their respective TLR agonist (e.g., Pam3CSK4/ TLR2; PolyI:C/ TLR3; LPS/ TLR4; Flagellin/ TLR5; R848/ TLR7; R848/ TLR8; CpG/ TLR9), or the Mycobacterium extract, with different percentages (vol/vol) of MF59 or different concentration of the TLR agonist or the Mycobacterial extract in the presence of MF59. After 6 h, luciferase expression was measured and expressed as fold induction compared with cells incubated with vehicle alone.
Supplementary Material
Acknowledgments
We thank Marianna Taccone and the Novartis FACS and Animal facilities for their technical assistance, and Sylvie Bertholet for intellectual contributions and careful reading of this manuscript.
Footnotes
The authors declare no conflict of interest.
*T. Vesikari, et al., Poster presentation at the 48th Annual Meeting of the Infectious Diseases Society of America, Vancouver, BC, Canada.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107941108/-/DCSupplemental.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 3.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: Toll-like receptors and antibody responses. Nature. 2006;441(7091) doi: 10.1038/nature04875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morefield GL, et al. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588–1595. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Rimaniol AC, et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;22:3127–3135. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 7.Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 8.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The Common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69:1151–1159. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kool M, et al. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 13.McKee AS, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podda A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19:2673–2680. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 16.Clark TW, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 17.Banzhoff A, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE. 2009;4:e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupuis M, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186(1):18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 19.Mosca F, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calabro S, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 21.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuliani MM, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci USA. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly J, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA. 2010;107:19490–19495. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellebedy AH, et al. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci USA. 2011;108:2927–2932. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 27.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comanducci M, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–1454. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizza M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 30.Borrow R, et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12:970–976. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.