Abstract
Liver regeneration proceeds under the well-orchestrated control of multiple transcription factors that lead hepatocytes to reenter the cell cycle, proliferate, and renew quiescence. Here, we found an important role of the zinc-finger transcription factor Snail in liver regeneration. Snail was typically expressed in quiescent adult hepatocytes, but was rapidly degraded when the liver needed to regenerate itself. Decreased levels of Snail induced DNA synthesis in hepatocytes through up-regulation of cell cycle-related proteins. Snail degradation was dependent on phosphorylation by glycogen synthase kinase (GSK)-3β, whose quantity and activity were immediately increased after loss of liver mass or hepatic injury. Inactivation of GSK-3β resulted in suppression of Snail degradation and DNA synthesis in hepatocytes, leading to impaired liver growth during regeneration. This GSK-3β–dependent Snail degradation occurred as a result of cytokine, growth factor, and bile acid signals that are known to drive liver regeneration. Thus, GSK-3β–dependent Snail degradation acts as a fundamental cue for the initiation of hepatocyte proliferation in liver regeneration.
Keywords: liver development, cell signaling, hydrodynamic gene delivery
Liver regeneration is a fundamental response to hepatic damage, and has been favorably studied since the two-thirds partial hepatectomy (PH) model in rodents was described (1). In this system, quiescent mature hepatocytes rapidly reenter the cell cycle after PH and proliferate to restore the original liver mass. The priming of hepatocyte proliferation depends on the cellular responses to external stimulation by specific humoral factors, such as cytokines (e.g., IL-6) and growth factors [e.g., hepatocyte growth factor (HGF)], that are increased in the liver after PH (2). In most cases, these key effectors for liver regeneration are secreted by nonparenchymal cells and lead to the proliferation and survival of hepatocytes via activation of particular intracellular signaling pathways (3). In parallel with the effects of the cytokines and growth factors on liver growth, the remnant hepatocytes after PH are also responsive to relatively increased bile acid flux and thereby initiate proliferation. This bile acid signaling pathway depends on nuclear bile acid receptors, and therefore hepatocytes lacking the bile acid receptor FXR suffer severe defects in proliferation after PH (4). These humoral factor signals alter the gene expression pattern in hepatocytes by activating transcription factors, including signal transducer and activator of transcription (STAT)-3, AP1, NF-κB, CCAAT-enhancer-binding protein (C/EBP)β, c-Myc, and forkhead box (Fox) m1b, and finally induce DNA synthesis in hepatocytes through regulation of the cell cycle machinery (5). Consequently, the cell-extrinsic and -intrinsic mechanisms underlying the induction of hepatocyte proliferation have been studied intensively. However, it is still incompletely understood how such dynamic changes in the cellular proliferation state occur during liver regeneration.
Here, we investigated the role of Snail, a zinc-finger transcription factor, in mouse liver regeneration. Snail is involved in many aspects of cellular function, including cell motility, differentiation, proliferation, and survival, during embryonic development and tumor progression (6). Until now, however, there has been no evidence regarding the contribution of Snail to normal liver regeneration. The amount of Snail in cells is controlled by glycogen synthase kinase (GSK)-3β, which binds to and phosphorylates Snail in the nucleus and cytoplasm of cells and eventually leads to β-Trcp–mediated ubiquitination and subsequent proteasomal degradation of Snail (7). Thus, in this study, we also investigated the role of GSK-3β in the regulation of Snail during liver regeneration.
Results
Snail Is Expressed in Quiescent Adult Hepatocytes, but Is Rapidly Degraded After Loss of Liver Mass or Hepatic Injury.
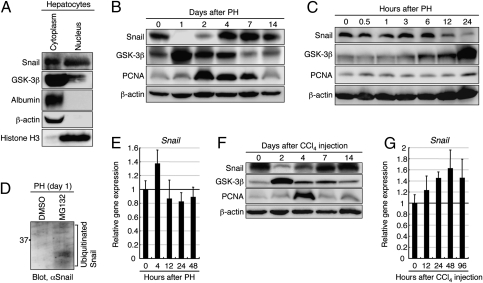
Western blot analyses revealed that both Snail and GSK-3β were expressed in quiescent adult mouse hepatocytes and present in the nucleus and cytoplasm (Fig. 1A). Intriguingly, the amount of Snail in the liver was significantly decreased within 24 h after PH, although it returned to its normal level by 4 d after PH (Fig. 1B and Fig. S1A). In contrast, the amount of GSK-3β in the liver was increased at 1 d after PH, but returned to its normal level by 7 d after PH (Fig. 1B and Fig. S1A), whereas there was no significant change in the amount of serine 9-phosphorylated GSK-3β in the liver after PH (Fig. S1 B and C). Following such drastic changes of the amounts of Snail and GSK-3β, the amount of proliferating cell nuclear antigen (PCNA), which is expressed in the nuclei of cells during DNA synthesis, was increased in the liver at 2 d after PH and then gradually decreased to its normal level (Fig. 1B and Fig. S1A). According to a decrease in Snail after PH, the mRNA expression of E-cadherin, a transcriptional target of Snail for suppression, was increased in the liver (Fig. S1D). In a detailed analysis, a decrease in Snail and an increase in GSK-3β in the liver was first observed at 12 and 6 h after PH, respectively (Fig. 1C and Fig. S1E). To determine why Snail in the liver was decreased after PH, we examined the protein degradation and changes in mRNA expression of Snail in the regenerating liver. When we directly injected the proteasome inhibitor MG132 into adult mice after PH to block proteasomal degradation of ubiquitinated proteins, ubiquitinated Snail had accumulated in the liver by 1 d after PH (Fig. 1D). However, quantitative PCR (qPCR) analyses revealed that, even after PH, Snail mRNA was stably expressed (Fig. 1E). Thus, the immediate decrease in Snail after PH was caused by degradation of ubiquitinated Snail. Moreover, as in the case of PH, the amounts of Snail and GSK-3β in the liver were also decreased and increased, respectively, at 2 d after s.c. injection of carbon tetrachloride (CCl4), a hepatotoxin that leads to fulminant hepatic failure and subsequent liver regeneration (Fig. 1F and Fig. S1F). Following this, the amount of PCNA in the liver was increased at 4 d after CCl4 injection (Fig. 1F and Fig. S1F). However, Snail mRNA expression was not decreased in the liver after CCl4 injection (Fig. 1G). Thus, Snail residing in quiescent adult hepatocytes is degraded in the early stages of liver regeneration, which is followed by activation of DNA synthesis in hepatocytes, suggesting that an immediate decrease in Snail in the damaged liver is important to induce the proliferation of hepatocytes.
Fig. 1.
Snail degradation and GSK-3β up-regulation in the liver after PH. (A) Western blot analyses of Snail and GSK-3β in the nucleus and cytoplasm of adult mouse hepatocytes. Albumin and β-actin, which reside in the cytoplasm, and histone H3, which resides in the nucleus, were evaluated to confirm the separation of the cytoplasmic and nuclear protein fractions of the hepatocytes. (B) Western blot analyses revealed that the amounts of Snail, GSK-3β, and PCNA in the liver change dramatically after PH. (C) Western blot analyses revealed that the drastic changes in the amounts of Snail and GSK-3β in the liver begin at 12 and 6 h after PH, respectively. (D) Western blot analysis of Snail in the liver of DMSO or MG132-treated mice at 1 d after PH, following purification of ubiquitin-binding proteins. (E) qPCR analyses of Snail were carried out on total RNA derived from the liver after PH. (F) Western blot analyses revealed that the amounts of Snail, GSK-3β, and PCNA in the liver change dramatically after s.c. injection of CCl4 into adult mice. (G) qPCR analyses of Snail were carried out on total RNA derived from the liver after CCl4 injection. The data shown in E and G were normalized by the value of the liver at 0 h after PH or CCl4 injection, respectively, and the fold differences are shown. The data are means ± SD (n = 3).
Decreased Levels of Snail Induce DNA Synthesis in Hepatocytes.
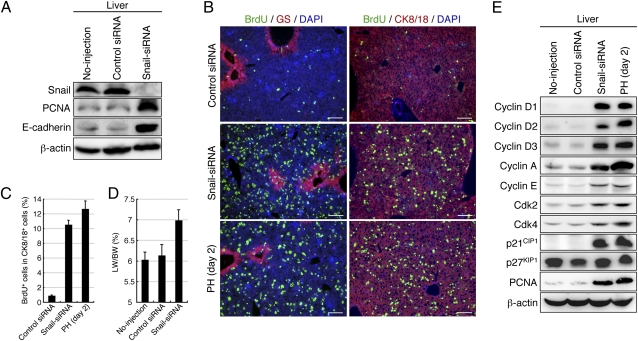
To directly address the effects of Snail degradation on hepatocyte proliferation, we introduced a Snail-specific siRNA (Snail siRNA) into normal adult mice using a hydrodynamic gene delivery system, which confirmed that most hepatocytes in the liver took up the injected siRNA in vivo (8), and induced RNA interference toward Snail transcripts. Treatment with the Snail siRNA efficiently reduced the amount of Snail in the liver and increased the amounts of PCNA and E-cadherin, whereas little effect was observed after injection of a control siRNA (Fig. 2A and Fig. S2A). Thus, the hydrodynamic gene delivery itself did not induce hepatocyte proliferation, but it temporally allowed a small increase in alanine transaminase, which indicates hepatotoxicity (Fig. S2B). Similar to the liver at 2 d after PH, the number of BrdU-incorporating mitotic cells, which were nonspecifically localized throughout the liver, was dramatically increased in cytokeratin (CK) 8/18+ hepatocytes at 2 d after Snail siRNA injection without any increase in the number of apoptotic cells (Fig. 2 B and C and Fig. S2C). This increase in the number of mitotic cells after Snail siRNA injection caused liver hypertrophy (Fig. 2D). The amounts of proteins linked to the cell cycle, such as cyclin D1, cyclin D2, cyclin D3, cyclin A, cyclin E, cyclin-dependent kinase (CDK) 2, CDK4, and the CDK inhibitor p21CIP1, were increased in the liver at 2 d after Snail siRNA injection, and similar increases in these proteins were observed at 2 d after PH (Fig. 2E and Fig. S3 A–C). Chromatin immunoprecipitation analysis revealed that Snail bound to the region in the cyclin D2 promoter, which contains an E-box consensus for Snail binding (9), in the liver of normal adult mice, whereas the level of such binding became much weaker in the liver at 1 d after PH (Fig. S3 D and E). Thus, the decreased level of Snail in hepatocytes is sufficient to trigger DNA synthesis by activating the cell cycle machinery that is normally activated by regenerative stimuli after PH.
Fig. 2.
Decreased levels of Snail induce DNA synthesis in hepatocytes through activation of the cell cycle machinery. (A) Western blot analyses of Snail, PCNA, and E-cadherin in age-matched normal mouse liver (no injection) and in the liver of adult mice at 2 d after hydrodynamic injection of a control siRNA or Snail siRNA. The Snail siRNA efficiently reduces the amount of Snail and increases the amounts of PCNA and E-cadherin in the liver. (B) Coimmunofluorescence staining of BrdU with glutamine synthetase (GS; Left) or CK8/18 (Right) in livers obtained at 2 d after injection of a control siRNA or Snail siRNA and at 2 d after PH. GS marks hepatocytes surrounding the central veins of the liver, indicating that the BrdU-incorporating cells after Snail siRNA injection and PH are nonspecifically localized throughout the liver. DNA was stained with DAPI. (Scale bars, 100 μm.) (C) The percentages of cells immunoreactive for BrdU in 2,090 ± 121 or 1,879 ± 124 CK8/18+ cells in the liver at 2 d after injection of a control siRNA or Snail siRNA, respectively, and in 1,977 ± 123 CK8/18+ cells in the liver at 2 d after PH. The data are means ± SD (n = 3). (D) Liver-to-body weight (LW/BW) ratios in age-matched normal mice (no injection) and in mice at 2 d after injection of a control siRNA or Snail siRNA. The data are means ± SD (n = 3). (E) Western blot analyses revealed up-regulation of cell cycle-related proteins, except for p27KIP1, in the liver at 2 d after Snail siRNA injection or PH.
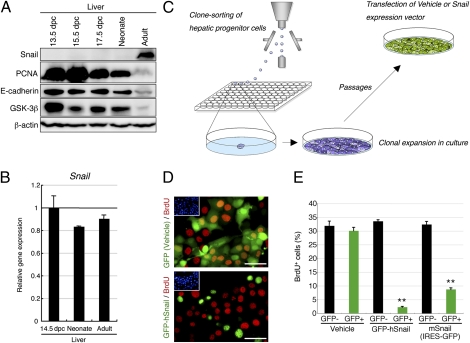
To further investigate the function of Snail in the liver, we investigated hepatic progenitor cells in the developing mouse liver. During liver growth in mouse embryos and newborns, the amounts of Snail in the liver were extremely low (Fig. 3A and Fig. S4A), whereas these mice strongly expressed Snail mRNA in the liver as adult mice (Fig. 3B). Thus, Snail may be degraded in the developing liver, as well as in the liver after PH or hepatic injury. Hepatic progenitor cells were isolated from the liver of 13.5-d postcoitum (dpc) mouse embryos and clonally cultured as described previously (10). Next, we introduced a construct that highly expressed mouse or human Snail with GFP into the cultured cells and analyzed the effects on proliferation (Fig. 3C). The forced expression of mouse or human Snail in hepatic progenitor cells allowed the accumulation of Snail in the nuclei of these cells and resulted in a significant reduction in the number of BrdU-incorporating mitotic cells, whereas introduction of a control construct that expressed GFP had little effect (Fig. 3 D and E). Regardless of Snail expression, these cells expressed E-cadherin that was typically localized at sites of cell–cell junctions (Fig. S4B). In the early stages of subculture, the Snail-expressing cells stopped their proliferation and gradually disappeared from the cultures. Thus, Snail inhibits DNA synthesis in hepatic progenitor cells and their subsequent proliferation without alteration in the morphology of cells.
Fig. 3.
Snail inhibits the proliferation of hepatic progenitor cells in the developing mouse liver. (A) Western blot analyses of Snail, PCNA, E-cadherin, and GSK-3β in the liver of embryonic (13.5, 15.5, and 17.5 dpc), neonatal (postnatal day 1), and 10-wk-old adult mice. The amounts of PCNA, E-cadherin, and GSK-3β in the embryonic and neonatal mouse livers are consistently much higher than those in the adult mouse liver, whereas the amounts of Snail are the opposite. (B) qPCR analyses of Snail using total RNA derived from 14.5-dpc, neonatal (postnatal day 1), and 10-wk-old adult mouse livers. All data were normalized by the value of the 14.5-dpc mouse liver, and the fold differences are shown. The data are means ± SD (n = 3). (C) Experimental procedures to elucidate the functions of Snail in hepatic progenitor cells. Hepatic progenitor cells were isolated from the liver of 13.5-dpc mouse embryos and clonally cultured. Cells propagated in culture were then replated onto new culture dishes and transiently transfected with a construct that expressed mouse or human Snail with GFP. Vehicle-transfected cells were evaluated as a control. (D) BrdU immunofluorescence images of hepatic progenitor cells after transfection. BrdU-incorporating mitotic cells are observed among the control cells expressing GFP only, but not among the cells expressing GFP-tagged human Snail (GFP-hSnail). (Insets) Individual cells labeled with DAPI. (Scale bars, 50 μm.) (E) Hepatic progenitor cells were transfected with a construct that expressed mouse or human Snail with GFP. The percentages of cells immunoreactive for BrdU in transfected (GFP+) and nontransfected (GFP−) cells were then analyzed by flow cytometry. Vehicle-transfected cells and nontransfected (GFP−) cells were evaluated as controls. The data are means ± SD (n = 3). The statistical significance was determined with Student's t test. **P < 0.01.
These findings demonstrate that quiescent adult hepatocytes, but not hepatic progenitor cells in the developing liver, contain Snail to block proliferation, and that a transient decrease in Snail in the damaged liver immediately induces DNA synthesis in hepatocytes for proliferation.
Increases in the Quantity and Activity of GSK-3β in the Damaged Liver Induce Snail Degradation and DNA Synthesis in Hepatocytes.
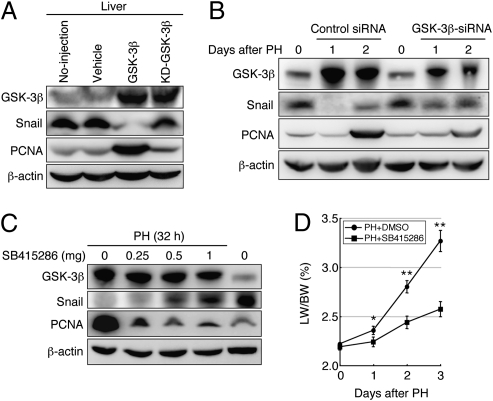
Next, we sought to determine why Snail degradation occurs after PH. As described above, the amount of GSK-3β in the liver began to increase at 6 h after PH and peaked at 24 h, followed by degradation of ubiquitinated Snail in the liver. It is known that GSK-3β–mediated phosphorylation of Snail leads to ubiquitination and subsequent proteasomal degradation of Snail (7). Thus, an immediate increase in GSK-3β in the damaged liver may be important for inducing Snail degradation. To address this issue, we introduced a construct that highly expressed wild-type or kinase-dead (KD) GSK-3β (11) into normal adult mice by hydrodynamic injection. The forced expression of these genes increased the amount of GSK-3β in the liver at 1 d after injection (Fig. 4A and Fig. S5A). However, a decrease in Snail and an increase in PCNA only occurred when wild-type GSK-3β was overexpressed (Fig. 4A and Fig. S5A). In addition, with an increase in GSK-3β, the amount of phosphorylated serine residues within Snail, which are known to undergo phosphorylation by GSK-3β (7), was increased at 8 h after injection of the wild-type GSK-3β expression construct (Fig. S5B). Thus, an increase in GSK-3β in hepatocytes is sufficient to cause Snail degradation and induce cell proliferation through GSK-3β–mediated phosphorylation of Snail. To examine whether increased GSK-3β is important for liver regeneration to proceed, we introduced a GSK-3β–specific siRNA (GSK-3β siRNA) into adult mice by hydrodynamic injection at 1 d before PH. This GSK-3β siRNA efficiently reduced the amount of GSK-3β and increased the amount of Snail upon injection into the liver (Fig. S5 C and D). Treatment with the GSK-3β siRNA was effective for suppressing not only the increase in GSK-3β but also the decrease in Snail and increase in PCNA in the liver at 1 and 2 d after PH (Fig. 4B and Fig. S5E). Moreover, the GSK-3β inhibitor SB415286, which was i.p. injected into adult mice after PH, also dose-dependently suppressed the decrease in Snail and increase in PCNA in the liver and strongly inhibited liver growth in the early stages of regeneration (Fig. 4 C and D and Fig. S5F). Thus, increases in the quantity and activity of GSK-3β in the damaged liver are essential for liver regeneration through the induction of Snail degradation.
Fig. 4.
Increases in the quantity and activity of GSK-3β are essential for liver regeneration through the induction of Snail degradation. (A) Western blot analyses of GSK-3β, Snail, and PCNA in age-matched normal mouse liver (no injection) and in the liver of adult mice at 1 d after hydrodynamic injection of vehicle, wild-type GSK-3β expression construct, or KD-GSK-3β expression construct. (B) Western blot analyses of GSK-3β, Snail, and PCNA in the liver after PH, following the introduction of a control siRNA or GSK-3β siRNA into adult mice by hydrodynamic injection at 1 d before PH. (C) Western blot analyses of GSK-3β, Snail, and PCNA in the liver of SB415286-treated mice at 32 h after PH and in age-matched normal mouse liver. (D) Liver-to-body weight (LW/BW) ratios in mice administered DMSO (●) or SB415286 (■) after PH. The data are means ± SD (n = 3). The statistical significance was determined with Student's t test. *P < 0.05, **P < 0.01.
GSK-3β–Dependent Snail Degradation Occurs as a Result of the Initial Regenerative Responses to Hepatic Damage.
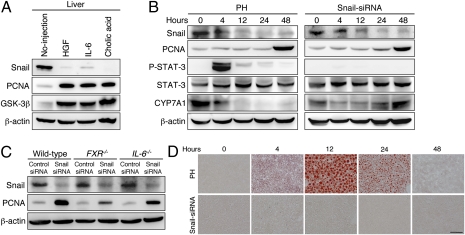
Our findings demonstrate that GSK-3β–dependent Snail degradation directs hepatocyte proliferation in normal liver regeneration, but do not address the relationship between this event and the well-defined regenerative responses to PH. To clarify this issue, we directly injected IL-6, HGF, and cholic acid into adult mice and examined their impact on Snail and GSK-3β in the liver. Concomitant with an increase in PCNA, i.p. injection of these regenerative factors induced a decrease in Snail and an increase in GSK-3β in the liver (Fig. 5A and Fig. S6A). However, treatment with the Snail siRNA was capable of inducing hepatocyte proliferation without phosphorylation of STAT-3, a downstream transcription factor in the IL-6 signaling pathway, and a decrease in cholesterol 7a hydroxylase (CYP7A1), a downstream negative target of FXR, both of which are normally observed after PH (Fig. 5B). Moreover, even in FXR−/− and IL-6−/− mice, a decrease in Snail after Snail siRNA injection was sufficient to induce hepatocyte proliferation, as well as in wild-type mice (Fig. 5C and Fig. S6B). In the early stages of liver regeneration, lipid droplet formation and accumulation in hepatocytes are known to be essential parts of the proliferative response (12). However, these alterations were not required for the hepatocyte proliferation induced by Snail siRNA injection (Fig. 5D). These findings demonstrate that GSK-3β–dependent Snail degradation occurs as a result of the initial regenerative responses to hepatic damage, rather than as a cause of these responses.
Fig. 5.
GSK-3β–dependent Snail degradation occurs as a result of humoral factor signals that drive liver regeneration. (A) Western blot analyses of Snail, PCNA, and GSK-3β in age-matched normal mouse liver (no injection) and in the liver of adult mice at 1 d after injection of HGF, IL-6, and cholic acid. (B) Western blot analyses of total cell lysates derived from the liver after PH or hydrodynamic injection of Snail siRNA into adult mice. P, phosphorylated. (C) Western blot analyses of Snail and PCNA in the liver of wild-type, FXR−/−, and IL-6−/− mice at 2 d after hydrodynamic injection of a control siRNA or Snail siRNA into adult mice. (D) Oil red O staining of the liver after PH or hydrodynamic injection of Snail siRNA into adult mice to detect lipid droplet formation and accumulation in hepatocytes. (Scale bar, 50 μm.)
Discussion
In this study, we have shown that Snail degradation is essential for priming hepatocytes to proliferate in normal liver regeneration. Snail is normally expressed in epithelial cells to progress the acquisition of a mesenchymal phenotype, and confers migratory and invasive properties on these cells during embryonic development and tumor progression (6). In the adult mouse liver, however, Snail is consistently expressed in quiescent mature hepatocytes, a subpopulation of epithelial cells. Nonparenchymal cells within the liver, which include mesenchymal lineage cells, also expressed Snail, but the expression levels were lower than that in hepatocytes (Fig. S7). Despite Snail expression, hepatocytes do not lose their epithelial phenotype or acquire the potential for motility. Thus, Snail in hepatocytes may be quantitatively insufficient to induce a mesenchymal phenotype and/or function for other purposes. The present data suggest that Snail resides in hepatocytes to retain their quiescent state, rather than introduce mesenchymal features. In support of this idea, Snail was not observed during liver growth in mouse embryos and newborns. Moreover, siRNA-mediated Snail knockdown was sufficient to induce DNA synthesis in hepatocytes through up-regulation of cell cycle-related proteins. Consistent with these results, Snail can block the cell cycle through repression of cyclin D1, cyclin D2, and CDK4 in cell lines (13). Therefore, Snail may impair cell cycle progression in hepatocytes by directly contributing to the repression of cell cycle-related gene expressions.
The mechanism controlling the amount of Snail in hepatocytes may also be relevant to the termination of liver regeneration. This process depends on the effects of hepatocyte antiproliferative factors, including TGFβ and related TGFβ-family members such as activin (2, 3). Because TGFβ induces Snail expression in hepatocytes (14), it can be speculated that TGFβ increases in the liver leads to up-regulation of Snail following its transient degradation. However, the TGFβ type 1 receptor kinase inhibitor SB431542 could not suppress an increase of Snail at 4 d after PH (Fig. S8). Our present data suggest that the arrest of hepatocyte proliferation is at least partly attributable to the return of the amounts of Snail and GSK-3β to their basal levels in response to a gradual decrease of the external regenerative stimuli, whereas the effects of TGFβ and other inhibitory factors in this process remain to be further studied. However, our findings also suggest the possibility that Snail degradation in hepatocytes becomes a trigger for repressing their proliferation. We observed an increase of p21CIP1 in the liver after PH, in accord with a previous report (15). Likewise, siRNA-mediated Snail degradation led to an increase in p21CIP1 in the liver. In Snail-expressing cell lines, however, high levels of p21CIP1 are stably expressed to retain a growth-arrested state (13). Thus, the increases in p21CIP1 after PH and Snail siRNA injection may occur as a result of other intracellular reactions caused by Snail degradation. Taken together, GSK-3β–dependent Snail degradation may be a critical event to control the cellular proliferation state in both the initiation and termination of liver regeneration.
GSK-3β phosphorylates a number of substrates involved in diverse cellular functions, including cell metabolism, proliferation, differentiation, and survival (16). During liver regeneration, GSK-3β may regulate β-catenin activity, which is important for hepatocyte proliferation (17). Here, we have shown that increases in the quantity and activity of GSK-3β in the early stages of liver regeneration facilitate the phosphorylation of Snail and its subsequent degradation. Inactivation of GSK-3β using a siRNA or SB415286 resulted in the suppression of Snail degradation and DNA synthesis in hepatocytes, leading to impaired liver growth during regeneration. Thus, an immediate, but transient, increase in GSK-3β is required for priming hepatocyte proliferation in liver regeneration. In support of this finding, the amounts of GSK-3β during liver growth in mouse embryos and newborns were consistently much higher than that in the adult liver, whereas the amounts of Snail were the opposite (Fig. 3A). In addition, a recent study indicated that GSK-3β contributes to hepatocyte proliferation and survival during rat liver regeneration (18). Moreover, the age-associated decline in GSK-3β is correlated with a reduced regenerative capacity of aged mouse liver (19). Taken together, our present results and these previous data imply that the mechanisms controlling the amount of GSK-3β in hepatocytes are important for regulating their proliferation state during the processes of development, regeneration, and aging. Therefore, how is the amount of GSK-3β controlled in the liver? Because GSK-3β was rapidly increased after PH, it was supposed to be posttranscriptionally regulated. The unchanged expression levels of GSK-3β mRNA during liver development and regeneration supported this idea (Fig. S9). Our present findings have shown that cytokine, growth factor, and bile acid signals are potential regulators of GSK-3β in hepatocytes. Interestingly, previous results in cell lines indicated that GSK-3β appears to be regulated by regulator of calcineurin 1 at the posttranscriptional level, although the mechanism remains to be addressed (20).
Overall, we postulate that hepatocytes in the normal adult liver are conventionally ready to proliferate, but this is usually blocked by Snail activity. Once the liver is damaged, the initial regenerative responses bring about an increase in GSK-3β as a downstream target in activated intracellular signaling pathways, which leads to Snail degradation and the subsequent induction of hepatocyte proliferation. Although many other molecules are also involved in this process, GSK-3β–dependent Snail degradation can be considered to be a fundamental cue for the initiation of hepatocyte proliferation in liver regeneration.
Materials and Methods
Mice.
C57BL/6J wild-type mice (Clea), FXR−/− mice (a generous gift from Frank Gonzalez, National Cancer Institute), and IL-6−/− mice (Jackson Laboratory) were used in this study.
Expression Constructs.
The GFP-tagged human Snail gene and HA-tagged mouse Snail gene were kindly provided by Mien-Chie Hung (University of Texas M.D. Anderson Cancer Center, Houston) and Antonio García de Herreros (Institut Municipal d'Investigació Mèdica, Barcelona), respectively. The HA-tagged mouse Snail gene was inserted into pIRES2-GFP (Clontech).
Supplementary Material
Acknowledgments
We thank Mien-Chie Hung and Antonio García de Herreros for sharing reagents; Frank Gonzalez, Yasushi Yamazoe, and Masaaki Miyata for providing the FXR−/− mice; and Eriko Gunshima, Hiromi Kuboyama, and Ayako Kaneyuki for excellent technical assistance. This work was supported in part by the Program for Improvement of the Research Environment for Young Researchers from the Special Coordination Funds for Promoting Science and Technology commissioned by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a Grant-in-Aid for Scientific Research from the MEXT of Japan; the Precursory Research for Embryonic Science and Technology Program of the Japan Science and Technology Agency; and the Uehara Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016122108/-/DCSupplemental.
References
- 1.Higgins GM, Anderson RM. Experimental pathology of the liver: I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol (Chic) 1931;12:186–202. [Google Scholar]
- 2.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 3.Taub R. Liver regeneration: From myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 5.Costa RH, Kalinichenko VV, Holterman AX, Wang X. Transcription factors in liver development, differentiation, and regeneration. Hepatology. 2003;38:1331–1347. doi: 10.1016/j.hep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Nieto MA. The Snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 7.Zhou BP, et al. Dual regulation of Snail by GSK-3β–mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 8.Song E, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 9.Mauhin V, Lutz Y, Dennefeld C, Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21:3951–3957. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki A, et al. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinfeld B, et al. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 12.Fernández MA, et al. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- 13.Vega S, et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spagnoli FM, Cicchini C, Tripodi M, Weiss MC. Inhibition of MMH (Met murine hepatocyte) cell differentiation by TGF(β) is abrogated by pre-treatment with the heritable differentiation effector FGF1. J Cell Sci. 2000;113:3639–3647. doi: 10.1242/jcs.113.20.3639. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht JH, et al. Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene. 1998;16:2141–2150. doi: 10.1038/sj.onc.1201728. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 17.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of β-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, et al. Inhibition of GSK-3β decreases NF-kappaB-dependent gene expression and impairs the rat liver regeneration. J Cell Biochem. 2007;102:1281–1289. doi: 10.1002/jcb.21358. [DOI] [PubMed] [Google Scholar]
- 19.Jin J, Wang GL, Shi X, Darlington GJ, Timchenko NA. The age-associated decline of glycogen synthase kinase 3β plays a critical role in the inhibition of liver regeneration. Mol Cell Biol. 2009;29:3867–3880. doi: 10.1128/MCB.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ermak G, Harris CD, Battocchio D, Davies KJ. RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3β. FEBS J. 2006;273:2100–2109. doi: 10.1111/j.1742-4658.2006.05217.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.