Abstract
The molecular basis of the thermal sensitivity of temperature-sensitive channels appears to arise from a specific protein domain rather than integration of global thermal effects. Using systematic chimeric analysis, we show that the N-terminal region that connects ankyrin repeats to the first transmembrane segment is crucial for temperature sensing in heat-activated vanilloid receptor channels. Changing this region both transformed temperature-insensitive isoforms into temperature-sensitive channels and significantly perturbed temperature sensing in temperature-sensitive wild-type channels. Swapping other domains such as the transmembrane core, the C terminus, and the rest of the N terminus had little effect on the steepness of temperature dependence. Our results support that thermal transient receptor potential channels contain modular thermal sensors that confer the unprecedentedly strong temperature dependence to these channels.
Keywords: chimera, temperature gating, temperature jump, thermosensation, pain
The ability to sense temperature is vital to living organisms. In mammals, the neural input on ambient temperature results from specialized groups of neurons that project to the skin. The transducers involve ion channels known as transient receptor potential (TRP) channels (1, 2), which constitute an array of biological thermometers responsive over a broad temperature gradient from noxious cold to noxious hot (3).
The molecular mechanism by which temperature changes induce channel opening is not yet known, but the phenomenological tools to analyze the system are known from classical thermodynamic theory. The probability of channel opening follows a Boltzmann relationship to temperature. The enthalpy change (ΔH) between closed and open determines the slope sensitivity of the curve, whereas the entropy change (ΔS) affects its midpoint (T1/2). The term “threshold” is also commonly used in studies of temperature-sensitive channels to represent the change in temperature required for the response to be larger than the noise level of the recording. Changes in threshold could occur by changes in ∆H or T1/2 or the recording noise level.
Thermodynamic analyses reveal that thermal TRP channels undergo large enthalpy changes, which accounts for their high temperature sensitivity (4–8). The opening of TRPV1, for example, involves an activation enthalpy of approximately 100 kcal/mol (7), five times the enthalpy change for ligand- or voltage-dependent gating [Q10 ∼ 2–3 (ref. 9), equivalent to an enthalpy of approximately 20 kcal/mol]. If the free energy change were determined by enthalpy alone, the rate of gating would be very slow because the barrier would be too high. However, thermal TRP channels have evolved to have tightly coupled enthalpy and entropy changes so that the free energy change is relatively small (7). The threshold of activation summarizes the influence of all the temperature-insensitive processes that can regulate gating including the membrane potential (5, 6, 10) and any other allosteric sources such as ligand binding.
The strong temperature dependence of thermal TRP channels suggests that the channels evolved specific mechanisms for temperature sensing. In support of the hypothesis, single-channel and macroscopic kinetic analyses reveal that temperature changes only influence specific gating components (4, 7, 11). Although membrane lipids are prone to temperature-dependent phase transitions, both TRPV1 and TRPM8 remain activated by temperature following perturbations of membrane fluidity by either cholesterol changes (4) or lipid bilayer reconstitution (8). TRP channels have a membrane topology similar to voltage-gated K+ channels (12), albeit with relatively large intracellular termini (13). Both the pore domain (11, 14, 15) and the C terminus (16, 17) have been implicated in temperature gating. For example, single residue mutations at the outer pore of TRPV3 abrogated its heat activation (14), whereas alterations of the pore turret compromised the gating of TRPV1 (15). Exchanges of the C termini between TRPV1 and TRPM8 reversed the polarity of thermal sensitivity of the channels (16). Thus, the gating by temperature involves extensive structural changes, but the experiments did not distinguish whether these sites directly mediate temperature sensing or downstream gating.
In this study, we present a chimeric analysis on a group of heat-activated channels, taking advantage of a rapid temperature jump technique (7) that permits kinetic analysis of the open-closed transitions. We measured ∆H for swaps of different regions of the channels including the transmembrane (TM) core and the N and C termini. This fundamental measure of the kinetics separates mutations better than measures that combine the parameters into a single threshold estimate or loss of functions. We have found that a portion of the N terminus is capable of significantly altering the enthalpy of activation. Furthermore, swapping that region between temperature-sensitive and insensitive isoforms transfers the sensitivity of the donor. Our results demonstrate that a localized structural domain exists for temperature sensing in thermal TRP channels.
Results
TRPV1 and TRPV2 Have Distinct Temperature Dependence.
In search of wild-type templates for chimeric analysis, we compared thermal activation of rat rTRPV1 (18) and rTRPV2 (19). These two channels share an amino acid identity of approximately 70% (19), but they exhibited markedly different activation properties to submillisecond temperature jumps (Fig. 1). The response of TRPV1 followed single exponentials with an activation threshold between 40–45 °C as previously observed (7), whereas TRPV2 exhibited two well separated kinetic components and had a threshold between 50–53 °C. More importantly, their responses showed distinct slope sensitivity to temperature (Fig. 1D). This difference was persistent irrespective of variations in the threshold of activation.
Fig. 1.
TRPV1 and TRPV2 have distinct temperature dependence. (A) Putative TM topology of vanilloid receptors (Upper) and similarity of TRPV1 and TRPV2 (Lower), plotted as the degree of conservation of residues resulting from sequence alignment by ClustalX2. NT, N terminus; CT, C terminus. (B) Examples of temperature pulses generated by laser irradiation and whole-cell currents evoked by temperature from an HEK 293 cell heterologously expressing rat TRPV1. The temperature pulses, stepped from room temperature (22 °C), were 100 ms long and had a rise time of 0.75 ms. Membrane potential was -60 mV. (C) Fitting of activation time course. To extract temperature dependence, the current traces were fitted to appropriate models (two states for TRPV1 and three states for TRPV2; see text for more details). The dotted lines (red) represent the best fits. (D) Temperature response curves, measured from the maximal currents at the end of temperature steps. Each curve represents measurements from an individual cell. (E) Comparison of temperature dependence of TRPV1 and TRPV2. The activation enthalpy of the endothermic step in the fitted models is shown.
To quantify the temperature dependence of the channels, we exploited appropriate models to globally fit the activation time courses over all test temperatures (Fig. 1C). We found that a simple two-state model (C-O) in which temperature drives the opening rate was adequate to fit the response of TRPV1. The simplest model that described TRPV2 required three states (e.g., C1-C2-O) where the transition from C1 to C2 was driven by temperature, whereas other rates had nominal temperature dependence (see SI Discussion for discussions on alternative models). Fig. 1C shows the predicted currents superimposed on the experimental traces. The models provided adequate fits over different temperatures. From the fits, we estimated the temperature dependence of the channels in terms of the activation enthalpy of the endothermic transition, which led to ΔH ≈ 90 kcal/mol for TRPV1 and approximately 200 kcal/mol for TRPV2 (Fig. 1E; see Tables S1 and S2 for full model parameters). Thus, TRPV2 was more than twice as sensitive to temperature as TRPV1. Notably, the enthalpic difference between them was > 100 kcal/mol, which far exceeds the enthalpy of typical ion channel gating [ΔH ∼ 20 kcal/mol (9)], implying that it is pertinent to temperature sensing rather than downstream gating because the latter has only nominal temperature dependence.
N Terminus Mediates Temperature Sensing.
To unravel the molecular determinants underlying the large difference in their enthalpy, we constructed chimeras between rTRPV1 and rTRPV2 (Fig. 2A). First, we exchanged the whole TM core (S1–S6) between them. This relatively large exchange caused little changes in the enthalpy of either wild-type channel. The chimera of TRPV1 remained fit by a simple two-state model, whereas the chimera of TRPV2 was also fit by a three-state model (Fig. 2B). The fitting resulted in ΔH ∼ 97 kcal/mol for the TRPV1 chimera and approximately 203 kcal/mol for the TRPV2 chimera. Both chimeras essentially preserved the enthalpy of their wild-type parentals. Consistently, the temperature response curves of the chimeras showed slopes similar to that of the respective wild-types (Fig. 2B).
Fig. 2.
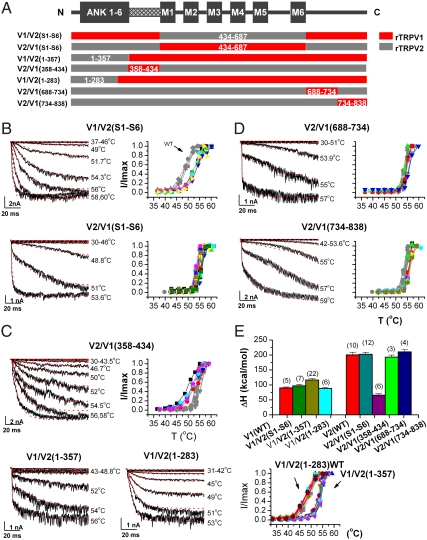
Thermal sensitivity involves the membrane-proximal N terminus. (A) Schematic representation of composition of chimeric channels between rat TRPV1 (red) and TRPV2 (gray). The first two chimeras on the top contain the exchange of the entire TM segments. The three chimeras in the middle involve changes in the N terminus. The last two chimeras correspond to TRPV2 with its C-terminal domains replaced by the cognate parts of TRPV1. Only the functional chimeras are shown. Residue numbers correspond to rat TRPV1. (B–D) Activation time courses and temperature response curves of chimeric channels (B, TM; C, N terminus; D, C terminus). V1/V2(S1–S6), V2/V1(358–434), and V1/V2(1–283) were fit by a two-state model and others by a three-state model. For comparison, the temperature response curves of wild-type channels are displayed in gray on background. (E) Activation enthalpy of chimeric channels compared with their parental wild-type channels.
On the other hand, the substitutions altered the threshold and rate of activation (Fig. 2B). For example, the TRPV1 chimera was significantly activated only above approximately 49 °C, as compared to approximately 42 °C for TRPV1 (Fig. 1). The fast activation component that occurred in TRPV2 became slower in the TRPV2 chimera. Following exposures to extreme temperatures (> 57 °C), the wild-type rTRPV2 often exhibited a small “leak” current at the baseline (Fig. 1C), suggesting that the channel did not close completely. This leak became absent in the TRPV2 chimera, indicating that the exchange of the TM domains altered the intrinsic opening and closing equilibria of the channel. The threshold and kinetics of activation had changed in the chimeric channels presumably because they are determined by free energies, which are small as compared to enthalpies and thus more sensitive to structural perturbations.
We next examined the effects of altering the cytoplasmic domains of the channels. Unfortunately, a direct exchange of the whole N terminus or C terminus did not lead to functional channels. The N terminus of the vanilloid receptors is relatively large in length, containing > 300 residues or nearly half of the whole channel subunit, a significant portion of which is involved in formation of ankyrin repeats, a well-conserved structural motif in all vanilloid receptors and other subfamilies of TRP channels (1). The C terminus comprises 100–150 residues, of which the S6-linker region contains the TRP box, a signature sequence conserved among TRP channels, whereas a considerable portion of its distal end (approximately 88 residues in rTRPV1) is not required for temperature gating (20). Taking these features into consideration, we constructed chimeras involving exchanges of subregions in both termini (Fig. 2A).
For the N terminus, we divided it between the ankyrin repeat domain (ARD; 1–357) (13) and the remaining membrane-proximal domain (MPD; 358–434). (All residue numbers refer to rat rTRPV1.) We exchanged the two parts separately between rTRPV1 and rTRPV2. Two products were found to be functional, one involving TRPV1-MPD in TRPV2, namely, TRPV2/V1(358–434), and the other with TRPV2-ARD in TRPV1 [i.e., TRPV1/V2(1–357)] (Fig. 2A; other reverse chimeras are not functional). In combination, the two chimeras cover the whole N terminus. The transfer of TRPV1-MPD fundamentally transformed the activation behavior of TRPV2, rendering its time course more akin to that of TRPV1 (Fig. 2C). Importantly, the enthalpy of the chimera was dramatically reduced (ΔH ∼ 66 kcal/mol, or a nearly 70% decrease from the wild-type TRPV2; Fig. 2E). Evidently, the substitution of the MPD alone sufficed to account for the large enthalpy difference between TRPV1 and TRPV2. The replacement also affected the temperature-independent kinetics of TRPV2 presumably because the exchanged domain introduced new rate-limiting steps into the chimera or altered the coupling between sensor movement and downstream gating causing changes in temperature-independent gating steps.
The replacement of the distal N terminus (1–357) in TRPV1 also altered the activation of TRPV1, bringing about a fast opening component as occurred in TRPV2 (Fig. 2C). The temperature response curve of the chimera showed both an increased activation threshold (> 48 °C) and a steeper slope at the midpoint (Fig. 2C). The fitting of the current traces by a three-state model resulted in ΔH ∼ 120 kcal/mol, which was approximately 30 kcal/mol larger than that of wild-type TRPV1, though relative to the marked change from the replacement of the MPD, the difference was minor.
To further delineate what portion of the ARD contributed to this change, we constructed chimeras containing a partial replacement of the ARD. In particular, we found that the exchange of the distal N terminus up to the first four ankyrin repeats from TRPV2 into TRPV1 led to a functional channel [TRPV1/V2(1–283)]. This chimera showed an activation enthalpy of ΔH ∼ 88 kcal/mol, similar to the wild-type TRPV1. It also retained the wild-type activation time course and temperature response curve (Fig. 2C). Thus, the transfer of the first four ankyrin repeats did not perturb the temperature dependence of the channel, suggesting that the last two ankyrin repeats (ANK5–6), which are adjacent to the MPD, were responsible for the enthalpy change when the whole ARD was exchanged. Interestingly, such a result fits well with the structural data that the first four ankyrin repeats (ANK1–4) of these channels maintain the canonical fold of ankyrin repeats, whereas the last two ankyrin repeats (ANK5–6) are atypical for their unusually long helices and finger loops as well as distorted orientations. In view of the profound impact of the MPD, the relatively minor effect of ANK5–6 could have occurred because of its inevitable interactions with the MPD.
We similarly divided the C terminus of the channels into halves and exchanged them separately between TRPV1 and TRPV2. Here, we found that the substitutions in TRPV2 by the cognate parts of TRPV1 resulted in functional channels. Fig. 2D shows the responses of such a chimeric set, constructed with a breakpoint at position 734, which is approximately 50 residues from S6 and approximately 100 residues from the distal end. Both chimeras attained the activation profiles of wild-type TRPV2, including the time course, the temperature threshold, and the slope sensitivity. Fitting their activation time courses by a three-state model resulted in ΔH ≈ 190 kcal/mol for TRPV2/V1(S6–734) and approximately 210 kcal/mol for TRPV2/V1(734–end), both of which were comparable to the enthalpy of TRPV2. Thus, neither exchange altered the enthalpy of the parental channel. Chimeras constructed at other breakpoints produced comparable results, suggesting that the attainment of the thermal sensitivity was independent of the choices of the exchanged regions.
Membrane-Proximal N Terminus Confers Gain of Function.
Our above analyses implicated the MPD of the N terminus as a prominent structural component mediating the thermal sensitivity of TRPV1 and TRPV2. To further elucidate the significance of the region, we examined other vanilloid receptor homologues including the temperature-insensitive human isoform hTRPV2 (21). We asked whether the abnormality of hTRPV2 is due to alteration of its MPD. To address the question, we transferred the region from a functional channel, rTRPV1, into hTRPV2 (Fig. 3A). Remarkably, the transfer conferred large temperature responses to the chimera (Fig. 3 B and E). Whereas the wild-type hTRPV2 at -60 mV showed no detectable activity up to 60 °C (approximately -27 pA/pF), the chimera reached approximately -300 pA/pF at 60 °C; leading to a > 10-fold increase in current density.
Fig. 3.
The membrane-proximal N terminus reveals a conserved mechanism of temperature gating in vanilloid receptors. (A) Sequence alignment of the membrane-proximal N terminus. Gray indicates conserved residues. (B–D) Whole-cell currents evoked by temperature for wild-type channels (hTRPV2, mTRPV3, and mTRPV4) and respective chimeras containing rTRPV1-MPD. The exchange of the region restored heat responses in hTRPV2 and mTRPV4 and significantly altered activation properties of mTRPV3. (E) Temperature response curves for chimeras versus wild types. For hTRPV2 and mTRPV4, the current density (normalized by membrane capacitance and then scaled to the mean maximum) is plotted. For mTRPV3, the normalized current is shown. Mean maximum current density (pA/pF): 27 ± 9 (n = 4) for hTRPV2 (WT), 302 ± 55 (n = 6) for hTRPV2/rV1(358–434), 33 ± 4 (n = 3) for mTRPV4 (WT), and 116 ± 20 (n = 3) for mTRPV3/rV1(358–434).
Similar chimeric substitutions were also effective in mTRPV4 and mTRPV3. The thermal sensitivity of TRPV4 has been controversial (22–26), and under our experimental conditions we were able to detect only a small current from mTRPV4 above approximately 55 °C (Fig. 3 C and E). The replacement of its MPD by rTRPV1-MPD led to robust temperature responses starting at 40 °C, giving rise to an increase in current density by about 4-fold at approximately 55 °C and a reduction in threshold by > 10 °C. The replacement in TRPV3, on the other hand, profoundly altered the activation properties of the channel (Fig. 3 D and E). When directly exposed to temperature jumps without prior sensitization by 2-APB, wild-type mTRPV3 exhibited a high activation threshold (> 50 °C) and steep temperature dependence (Fig. 3E), both of which became markedly reduced in the chimeric channel. The MPD of the N terminus therefore mediates the temperature response of all four heat-sensitive homologues, supporting that its function is conserved.
Membrane-Proximal N Terminus Controls Temperature Dependence of TRPV1.
If the MPD contributes to temperature sensing, mutations in the region might be expected to also alter the temperature dependence of TRPV1. To test the hypothesis and also to delineate a minimal functional region, we constructed several rTRPV1 mutants on different sites across the domain. Considering that single residue changes generally cannot account for large enthalpy changes, we made contiguous mutations of multiple residues at each site. Fig. 4A illustrates the sites that were probed, each of which was replaced by the counter residues of rTRPV2. Except at the C-terminal end (430–434), all other four mutants were responsive to temperature, showing a time course grossly similar to that of wild-type TRPV1. However, the activation enthalpy of their responses was significantly reduced (Fig. 4B), leading to ΔH ∼ 54–69 kcal/mol as compared to ΔH ∼ 90 kcal/mol for wild-type TRPV1 (Fig. 4 C and D). Thus, local perturbations in the MPD could indeed alter the slope sensitivity of TRPV1. The substitutions caused a decrease rather than an increase in enthalpy presumably because the residues are involved in interactions with other residues in TRPV2 that are different in TRPV1. It is noteworthy that the mutations also changed the threshold of activation, and such changes occurred in an interesting pattern where the residues toward the C-terminal half tended to be more effective than the N-terminal half. Because an increased threshold is correlated with a reduced entropy, this increasing effectiveness in threshold changes would suggest that the MPD tends to be more disordered toward its C-terminal end (i.e., near the membrane).
Fig. 4.
Mutations within the membrane-proximal N terminus alter temperature dependence of TRPV1. (A) Amino acid sequence of the membrane-proximal N terminus of rat TRPV1. Five subregions (colored in gray) were mutated, each replaced by the cognate part of TRPV2. The first four replacements resulted in functional channels, whereas the last one was nonfunctional. (B) Representative current traces of mutant channels and their fits by a two-state model. (C) Temperature response curves of mutant channels. Mutations toward the C-terminal half also changed the activation threshold in addition to the slope of the curve. (D) Comparison of activation enthalpy of mutant channels with the wild-type.
Discussion
To understand the molecular basis of the strong temperature dependence of thermal TRP channels, we have undertaken a chimeric analysis on a group of heat-sensitive homologous channels. Our data converged to a fragment of approximately 80 residues on the N terminus of the channels that connects the ankyrin repeats to the first TM domain. Alterations of this region profoundly altered the enthalpy or the slope sensitivity of all heat-sensitive vanilloid receptors (TRPV1–4), whereas its replacement in the temperature-insensitive homologues conferred gain-of-function. On the other hand, the exchanges of the rest parts of the channels including the TM core, the C terminus, and the ankyrin repeats (ANK1–4) affected only activation kinetics and thresholds but not the slope sensitivity. Thus, the MPD of the N terminus emerged as the only component capable of perturbing the enthalpy of the channel. It is interesting that the region maps to the missing segment in the nonfunctional TRPV1b splice variant (21, 27). The effectiveness of the region throughout different channels suggests that its function is conserved.
The function of a structural domain in the overall gating of a channel may be broadly divided into two categories: the detection of stimulus and the downstream gating (e.g., allosteric coupling and gate opening). Whereas all our chimeric data can be explained by a function in temperature sensing, several aspects of the data appear to be difficult to reconcile with downstream gating. First, our model-based analysis already took into account downstream gating (e.g., TRPV2), but the modeling still incurred changes in the activation enthalpy for temperature sensing. If we only allowed for changes in the downstream gating steps, the model was unable to fit the change of the slope sensitivity (Fig. S1). Second, our negative results on the TM domains, the C terminus, and the distal N terminus are also informative on the issue. Regions such as the C terminus and the TM domains are more proximal to the gate (28) and thus are more likely involved in allosteric coupling or gate opening. The inability of these regions to alter the enthalpy of gating suggests that the enthalpic difference between TRPV1 and TRPV2 is not due to downstream gating. Third, the chimeras involving the exchange of the MPD often exhibited simultaneous decreases in both activation threshold and slope sensitivity. Such correlated changes were atypical of changes in allosteric coupling. For example, if the difference between TRPV1 and TRPV2 arose from allosteric coupling, the high activation threshold of TRPV2 would imply that its allosteric coupling is weaker than that of TRPV1, which would in turn imply a shallower slope sensitivity for TRPV2 than TRPV1, but the data were the opposite, thereby arguing again that the difference in TRPV1 and TRPV2 does not stem from downstream gating. Fourth, the lack of an effect of the TM domains on the enthalpy of gating suggests that the chimeric effects of the MPD are not due to the voltage sensors of the channels. Finally, our FRET measurements reveal that the MPD fragment, when isolated from the full channels, was assembled into multimers that could undergo temperature-dependent structural changes (Fig. S2).
If the N terminus of the channels is responsible for temperature sensing, the other previously identified sites would be involved in other aspects of gating. For example, the outer pore mutants of TRPV3 show a selective loss of the temperature response (14). If the outer pore of the TRPV3 channel mediates downstream gating, the mutants ought to remain thermally sensitive. To test the hypothesis, we examined the temperature response of the mutants in the presence of 2-APB, a chemical agonist of the channel. Fig. S3 shows that these mutants indeed remained strongly responsive to temperature changes when a low concentration of 2-APB (10 μM) was added to background. Yang et al. (15) also show that the pore turret region is important for temperature gating of TRPV1. We previously demonstrated that TRPV1 lacking this region remains activated by temperature (29). Here, we have further characterized the full temperature dependence of the deletion mutant (Fig. S3), which we found remained similar to the wild-type TRPV1. The retained temperature dependence in these mutant channels suggests that they still contain intact thermal sensors, which are presumably located outside the pore domain. Our present results are therefore not contradictory with the previous ones; instead they reflect the different aspects of the gating.
The C terminus of TRPV1 and TRPM8 has also been implicated in temperature gating because its exchange reverses the hot and cold sensitivity of the two channels (16). Several mechanistic roles have been considered for the C terminus to explain this chimeric effect (16). One is that it directly mediates temperature sensing. Alternatively, it is involved in allosteric coupling between temperature sensing and gate opening. Such a role in allosteric coupling appears to be less intuitive, but is nevertheless possible as evident from the “flipped” voltage-dependent gating of HCN channels, which comprise positively charged voltage sensors but are activated by hyperpolarization (30). Thus, allosteric linkage is capable of determining the polarity of stimulus sensitivity. In our present experiments, we observed that the exchange of the C-terminal domains between TRPV1 and TRPV2 did not produce a significant change in the temperature dependence of the channels. Also, the C-terminal chimeras of TRPV1 and TRPM8 displayed considerably lower temperature dependence than wild-type channels [e.g., Q10 < 3 for voltage gating (16)], suggesting that additional components such as the N terminus may be necessary for the strong temperature dependence of the wild-type channels. Together, these observations led us to hypothesize that the C terminus of the channels interacts with the MPD of the N terminus to mediate transduction of thermal energy acquired by the N terminus.
Our data also shed light on whether membrane lipids are essential to temperature gating of TRP channels. Lipids are of potential interest to a thermosensitive process because they can undergo temperature-dependent phase transitions. Previous studies indicate that the heat sensitivity of TRPV1 remains after perturbations of membrane fluidity (4) whereas the cold sensitivity of TRPM8 is reconstituted in artificial lipid bilayers (8). These experiments provide strong evidences that the bulk-phase lipids of membranes are not responsible as a major energy source for temperature gating. But the involvement of boundary lipids around the channels has been less clear. Here, we found that TRPV1 and TRPV2 have drastically different temperature dependence, suggesting that lipids alone, even if they are involved, are inadequate—the channels must be also involved to confer the homologue-specific phenotypes. For such a model, alterations of TM domains would be expected to profoundly change the temperature dependence of the channels. To the contrary, the exchange of the whole TM core between TRPV1 and TRPV2 was ineffectual. Thus, in addition to affirming an insignificant role for the bulk-phase lipids, our data provide evidences for further ruling out the boundary lipids around the channels in driving temperature gating.
In conclusion, we demonstrate that temperature-gated channels possess localized structural components for detection of temperature changes. The identification of thermal sensor domains, which are analogous to discovering the S4 region for voltage-gated channels, will help unravel the mechanisms of temperature-dependent gating of thermal TRP channels and the physical basis for their large enthalpy.
Materials and Methods
Temperature Jumps.
Temperature jumps were produced by laser irradiation as previously described (7). Constant temperature steps were generated by irradiating the tip of an open pipette and using the current of the electrode as a readout for feedback control. The sequence of the modulation pulses was stored and subsequently played back to apply temperature jumps to whole-cells or membrane patches. Temperature was calibrated off-line from the pipette current using the temperature dependence of electrolyte conductivity.
Data Analysis.
The time-resolved current traces at different temperatures were fit globally with appropriate kinetic models (C1-O2 or C1-C2-O3). The current of the channel was predicted from the occupancy probability of the model, which was evaluated over the entire experimental time (both within the temperature pulses and in-between the pulses). The fitting was performed in Matlab (The MathWorks) by least-squares optimization.
Other Materials and Methods.
Details for the methods described above and for cell culture, expression, and electrophysiology are provided in SI Material and Methods.
Supplementary Material
Acknowledgments.
The authors thank Huai-hu Chuang for sharing a TRPV1 chimera cDNA clone (31) and Charles Bowman and Fred Sachs for reading the manuscript. This work was supported by grants from the National Institutes of Health (R01-GM65994/84891) and the National Science Foundation of China (31028006).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105196108/-/DCSupplemental.
References
- 1.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 2.Gracheva EO, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Hui K, Qin F. Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophys J. 2003;85:2988–3006. doi: 10.1016/S0006-3495(03)74719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voets T, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 6.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci USA. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J, Liu BL, Qin F. Kinetic and energetic analysis of thermally activated TRPV1 channels. Biophys J. 2010;99:1743–1753. doi: 10.1016/j.bpj.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 10.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol. 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandl J, et al. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat Neurosci. 2010;13:708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci USA. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Grandl J, et al. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci. 2008;11:1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci USA. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brauchi S, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA. 2007;104:10246–10251. doi: 10.1073/pnas.0703420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Ma W, Ryu S, Qin F. Inhibitory modulation of distal C-terminal on protein kinase C-dependent phospho-regulation of rat TRPV1 receptors. J Physiol. 2004;560:627–638. doi: 10.1113/jphysiol.2004.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeper MP, et al. Activation properties of heterologously expressed mammalian TRPV2: Evidence for species dependence. J Biol Chem. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- 22.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 24.Delany NS, et al. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- 25.Guler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 27.Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol. 2005;67:1119–1127. doi: 10.1124/mol.104.009852. [DOI] [PubMed] [Google Scholar]
- 28.Salazar H, et al. Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol. 2009;16:704–710. doi: 10.1038/nsmb.1633. [DOI] [PubMed] [Google Scholar]
- 29.Yao J, Liu BL, Qin F. Pore turret of thermal TRP channels is not essential for temperature sensing. Proc Natl Acad Sci USA. 2010;107:E125. doi: 10.1073/pnas.1008272107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannikko R, Elinder F, Larsson HP. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature. 2002;419:837–841. doi: 10.1038/nature01038. [DOI] [PubMed] [Google Scholar]
- 31.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci USA. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.