Abstract
To guide vaccine design, we assessed whether human monoclonal antibodies (MAbs) b12 and b6 against the CD4 binding site (CD4bs) on HIV-1 gp120 and F240 against an immundominant epitope on gp41 could prevent vaginal transmission of simian HIV (SHIV)-162P4 to macaques. The two anti-gp120 MAbs have similar monomeric gp120-binding properties, measured in vitro, but b12 is strongly neutralizing and b6 is not. F240 is nonneutralizing. Applied vaginally at a high dose, the strongly neutralizing MAb b12 provided sterilizing immunity in seven of seven animals, b6 in zero of five animals, and F240 in two of five animals. Compared with control animals, the protection by b12 achieved statistical significance, whereas that caused by F240 did not. For two of three unprotected F240-treated animals there was a trend toward lowered viremia. The potential protective effect of F240 may relate to the relatively strong ability of this antibody to capture infectious virions. Additional passive transfer experiments also indicated that the ability of the administered anti-gp120 MAbs to neutralize the challenge virus was a critical influence on protection. Furthermore, when data from all of the experiments were combined, there was a significant increase in the number of founder viruses establishing infection in animals receiving MAb b6, compared with other nonprotected macaques. Thus, a gp120-binding, weakly neutralizing MAb to the CD4bs was, at best, completely ineffective at protection. A nonneutralizing antibody to gp41 may have a limited capacity to protect, but the results suggest that the central focus of HIV-1 vaccine research should be on the induction of potently neutralizing antibodies.
Keywords: binding antibodies, passive immunization
Developing an effective vaccine would reduce the global spread of HIV type 1 (HIV-1); however, progress has been limited. Large-scale trials have yielded negative or ambiguous results, several vaccines provided no protection at all, and the outcome of the RV144 trial was marginal (1, 2). A long-standing goal in vaccine design has been to induce broadly neutralizing antibodies (NAbs) active against a wide range of viruses via binding to their functional, trimeric envelope glycoprotein (Env) complexes (3–5). Several NAbs with appropriate properties have been isolated from infected people but not recipients of Env-based vaccines, which generally elicit antibodies (Abs) that can bind only the immunogen or inactive fragments of the Env complex but not to native Env spikes (4, 6). These “binding antibodies” cannot neutralize most primary viruses to a meaningful extent, and until recently they have not been considered particularly useful to vaccine design. However, it has been argued that any protection achieved in the RV144 trial could have been attributable to binding Abs, on the basis that the vaccine induced no other measurable immunologic component consistently (2, 7). The RV144 vaccine was a recombinant canarypox vector expressing several HIV-1 antigens, including gp120 tethered to a membrane, followed by a boost with monomeric gp120 proteins. Anti–gp120-binding Abs but no anti-gp41 Abs were induced, as no immunogenic gp41 regions were included in the vaccine (8).
To address what humoral responses are most relevant to vaccine design, we have conducted passive transfer experiments comparing the abilities of NAbs and nonneutralizing Abs (non-NAbs) to protect macaques from vaginal challenge. In these studies, we used human monoclonal antibodies (MAbs) b12 and b6 against the CD4 binding site (CD4bs) on HIV-1 gp120 (9, 10). These MAbs bind gp120 to comparable extents in vitro, but b12 is >100-fold more potent in neutralization assays against the challenge viruses used. When administered vaginally at the same concentrations, only b12 protected macaques from vaginal simian-HIV (SHIV)-162P4 transmission. F240, a non-NAb against gp41, reduced the number of infected animals compared with control, but not to a statistically significant extent. In the unprotected F240-treated animals, there was a trend toward lowered viremia. The potential protective effect of F240 may relate to the relatively strong ability of this antibody to capture infectious virions. Additional passive transfer experiments using SHIV-162P3 suggested that neutralization of the challenge virus is a critical influence on protection. We also observed that the number of founder viruses infecting the challenged macaques was increased in b6 recipients, compared with other groups. These various findings should be considered in the design of HIV-1 vaccines.
Results
In Vitro Properties of the Test MAbs.
MAbs b6 and b12 both recognize broadly similar epitopes that overlap the CD4bs on gp120, but b12 binds to trimeric Env on the virion surface and neutralizes primary isolates much more efficiently than b6 (9, 10). MAb F240 binds a Cluster 1 epitope on gp41 and does not significantly neutralize various test viruses (11, 12). These properties were confirmed in neutralization assays against SHIV-162P4 and SHIV-162P3, which are genetically related, CCR5-using, macaque challenge viruses derived from the HIV-1 SF162 primary isolate (13–15). SHIV-162P4 is considered a neutralization-sensitive Tier 1 virus, but SHIV-162P3 is generally more neutralization-resistant at Tier 2.
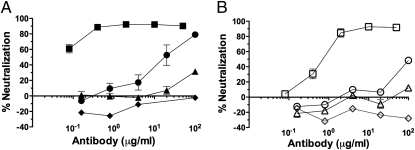
The IC50 values for b12 against SHIV-162P4 were 0.03 μg/mL in a rhesus peripheral blood mononuclear cell (PBMC) assay and 1.3 μg/mL in a TZM-bl assay, but the corresponding values for b6 were 20 and 300 μg/mL (i.e., 670- and 230-fold higher), respectively. For F240 and DEN3 (an anti-Dengue NS1 human IgG1 antibody), a control human MAb to the Dengue virus antigen NS1, the IC50 values were >100 μg/mL in both assays (Fig. 1 and Table 1). SHIV-162P3 is more neutralization-resistant than SHIV-162P4 in a PBMC assay in that only b12 neutralized it with an IC50 < 100 μg/mL, although in a TZM-bl assay SHIV-162P3 was the more sensitive of the two viruses to b12 neutralization (Fig. 1 and Table 1).
Fig. 1.
Sensitivity of the challenge viruses to the test MAbs in a rhesus PBMC neutralization assay. (A) SHIV-162P4 or (B) SHIV-162P3 was incubated with the indicated concentrations of MAbs b12 (■, □), b6 (●, ○), F240 (▲, △), or DEN3 (◆, ◇) before infection of rhesus macaque PBMCs. Virus replication on day 9 was measured using a p27 antigen assay. Inhibition of virus replication was calculated as percentage neutralization (no MAb = 0% neutralization; no replication = 100% neutralization). The data depicted were derived from a single assay that is representative of three of similar design.
Table 1.
Sensitivity of the challenge viruses to the test MAbs in different neutralization assays
| SHIV-162P4 |
SHIV-162P3 |
|||||
| IC50 (μg/mL) |
||||||
| Assay | b12 | b6 | F240 | b12 | b6 | F240 |
| Env-pseudovirus, TZM-bl | ND | ND | ND | 0.06 | >100 | >100 |
| Virus, TZM-bl | 1.26 | >100 | >100 | 0.07 | >100 | >100 |
| Virus, rhesus PBMC | 0.03 | 19.6 | >100 | 0.59 | >100 | >100 |
ND, not done.
In a gp120-binding ELISA, MAbs b12 and b6 had very similar apparent affinities for gp120 proteins derived from both SHIV-162P3 and SHIV-162P4. In contrast, and as expected, neither F240 nor DEN3 bound detectably to gp120 from either virus (Fig. 2 A and B). We also assessed the ability of immobilized MAbs to capture infectious SHIV-162P4 virions from solution. Compared with the assay background level (control MAb DEN3), MAbs b12 and b6 captured quantifiable and comparable amounts of infectious virions, but ∼20-fold greater quantities were captured by the anti-gp41 MAb F240 (Fig. 2C). The ability of nonneutralizing antibodies to bind infectious virions has been attributed to the presence of nonfunctional Env species on the virus surface (16). F240, like some MAbs to V3 and CD4-induced gp120 epitopes (16), appears to be much more effective at virus capture than ones directed against the CD4bs.
Fig. 2.
The gp120- and virus-binding properties of the test MAbs. Detergent lysates of (A) SHIV-162P4 or (B) SHIV-162P3 were used as the antigen source for a gp120-capture assay. MAbs b12 (■, □), b6 (●, ○), F240 (▲, △), or DEN3 (◆, ◇) were added to the test wells at the indicated concentrations and the amount of bound antibody was determined as an A405 value. (C) SHIV-162P4 virions were used as the antigen source for a virus-capture ELISA involving the same MAbs (∇, no MAb). Virion capture was quantified using a TZM-bl cell infection assay, with a luciferase (RLU) endpoint.
Macaque Passive Transfer and Challenge Studies.
We previously showed that b12 provides dose-dependent protection when given to macaques vaginally as a single bolus before vaginal challenge with a single high dose of SHIV-162P4 (17). In this model, the animals are first treated with progesterone 30 d before experiments, to increase their susceptibility to vaginal infection (17, 18). We now gave the animals the same amount (5 mg in 4 mL of isotonic saline) of b6, b12, F240, or DEN3 and challenged each of them 30 min later with SHIV-162P4. All five animals receiving DEN3 were infected, as determined by plasma viremia over the subsequent 10 wk, which confirms the infectivity of the challenge stock (Fig. 3). All seven recipients of b12 were protected (DEN3 vs. b12, P = 0.0013), but in marked contrast all five animals given b6 became infected (Fig. 3). Thus, a strongly neutralizing MAb to the CD4bs on gp120 provides 100% protection, although the same dose of a weakly neutralizing MAb to an overlapping epitope gives 0% protection under identical conditions; the difference is highly significant (b6 vs. b12, P = 0.0013). An intermediate result was seen with F240, in that three of the five test animals became infected (Fig. 3). This anti-gp41 non-NAb may have protected two animals, although the infection rates in the F240 and DEN3 groups were not significantly different (P = 0.22).
Fig. 3.
Plasma viremia in macaques infected with SHIV-162P4 in the presence of test MAbs. The test MAbs were administered vaginally in saline (5 mg in 5 mL), 30 min before addition of SHIV-162P4. Plasma viremia (viral RNA) was measured weekly for 10 wk postchallenge. The mean AUC values for the infected animals in each group are recorded on each panel. The assay sensitivity limit was 125 RNA copies/mL (A) b12, zero of seven infected; (B) b6, five of five infected; (C) F240, three of five infected; (D) DEN3, five of five infected. The infection rates in the b6 and b12 groups were significantly different (P = 0.0013).
We analyzed the plasma viremia levels in the 14 infected animals over a 10-wk period postchallenge (Fig. 3). The area under the curve (AUC) of the viral load profiles for the three infected F240-recipients (3.7 × 105 days × RNA copies/mL) was reduced compared with the DEN3-treated animals (1.5 × 108 days × RNA copies/mL), to an extent that approached statistical significance (P = 0.071). The median AUC value for the infected b6 recipients (3.7 × 106 days × RNA copies/mL) was also marginally less than for those given DEN3, but not to a statistically significant extent (P = 0.11).
A similar experiment was carried out using the more PBMC neutralization-resistant SHIV-162P3 virus and the same amounts of the same MAbs, again delivered vaginally 30 min before challenge. Three of four macaques in each of the b12, b6, and F240 groups became infected, as did both the animals given DEN3. Thus, the same amount of b12 does not have the same protective effect against the more PBMC neutralization-resistant virus, despite having comparable gp120- and virion-capture properties when tested against both challenge viruses in vitro (Fig. 2). There were no significant differences between the AUC values for the viral load profiles among the various groups [(in days × RNA copies/mL) b12, 6.6 × 107; b6, 3.5 × 108; F240, 6.1 × 107; DEN3, 8.4 × 107].
In a final experiment, we delivered b12, b6, or DEN3 systemically to macaques, via intravenous infusion, at 25 mg/kg, and challenged the animals vaginally with SHIV-162P3. One of the two DEN3 recipients remained uninfected, the infection-failure probably being a chance event. The challenge dose is calibrated to have an average infection rate of 90% for control animals, which is our general experience when using this stock in unrelated studies (19). Only one of the four animals given b12, but all four receiving b6, became infected. Because of the small group sizes, the difference between the b6 and b12 groups approached but did not reach statistical significance (P = 0.071). However, the trend again suggests that a weakly neutralizing MAb against gp120 is less protective than a strongly neutralizing one, even when both are present systemically in high concentrations at the time of vaginal challenge.
Analysis of Founder Viruses in Infected Animals.
We used plasma samples from the various SHIV-162P3 and SHIV-16P4–infected animals described above to quantify the number of viral lineages that expanded in each of them (Fig. 4) (20, 21). Four additional animals that became infected with SHIV-162P3 after receiving a DC-SIGN-Fc fusion protein vaginally before challenge were also included in the analysis. From 33 infected animals, 605 env sequences were generated using the single-genome amplification method. Sequences from six additional macaques challenged and infected with SHIV-162P3 after receiving a control microbicide gel (hydroxyethylcellulose, HEC) were kindly provided by Athe Tsibris (Massachusetts General Hospital, Boston) and Steven Wolinsky (Northwestern University, Chicago). Sequences for founder virus enumeration were obtained 2 to 4 wk postchallenge, at or just past peak viremia. Both challenge virus stocks had a similar degree of maximum diversity (0.7%), which was sufficient for enumerating the founder variants. Founder-virus lineage numbers were determined as described in ref. 20. Too few sequences were obtained from one infected F240 recipient to clearly identify the number of founder viruses. For all other animals, each founder was identified as a distinct, low-diversity group of sequences contained within the genetic diversity of the inoculum. Before immune selection, viral diversification follows a pattern of random mutations, which accumulate in each expanding founder population; the number and distribution of changes can be used to estimate the most recent common ancestor (22). Excluding hypermutated sequences, each low-diversity lineage contained sequences that were either identical to the consensus of that lineage or differed by one to three nucleotides distributed over the ∼2,600-bp gene. Compiling all of the changes from each identified founder sequence, we found that 54% of all sequences were identical to their own consensus sequence, 35% had one change, 10% had two changes, and only 1% had three changes. This distribution and the overall homogeneity within each phylogenetically distinct lineage are consistent with the short duration of infection, and with previous reports of maximum diversity during acute infection following mucosal transmission (20, 21, 23).
Fig. 4.
Number of founder viruses infecting macaques challenged with SHIV-162P4 or SHIV-162P3 in the presence and absence of MAbs. The number of founder viruses measured in each infected animal is shown on the y axis. The animals are grouped on the y axis according to the treatment (MAb, or control agent) they received before challenge. SHIV-162P3 and SHIV-162P4 infections are depicted by different symbols (see labels in figure), as is whether the MAbs were administered intravenously or vaginally before challenge. Four animals received a DC-SIGN-Fc fusion protein (5 mg in 4 mL) vaginally before SHIV-162P3 challenge; each became infected. The data for the gel control group were generated by Athe Tsibris and Steven Wolinsky. For the b6 group vs. DEN3 plus HEC control groups, the difference is statistically significant (P = 0.016).
Most SHIV-162P3 and SHIV-162P4 infections were established in control animals by either a single, or less often two, founder viruses (Fig. 4). The median number of founder viruses in DEN3 and HEC gel recipients, combined, was 1, irrespective of the challenge virus (mean ± SEM, 1.6 ± 0.34). Thus, even though progesterone was used to thin the vaginal epithelium and facilitate transmission, the number of founder viruses is similar to those seen in sexually infected humans or in macaques challenged mucosally in the absence of progesterone (20, 21, 23).
The numbers of founder viruses establishing infections in b12, F240, and DC-SIGN-Fc recipients were similar to those measured in control animals, the median values being 1 for each of the three groups (Fig. 4). In marked contrast, however, a significantly greater number of founder viruses established infections when the nonneutralizing anti-gp120 MAb b6 was present at the time of challenge. Thus, the median value for all infections in b6 recipients was 3. Compared with control infections, the difference was significant (P3 and P4 viruses, systemic and vaginal MAb delivery; P = 0.016 for b6 vs. DEN3 plus HEC gel controls). There was no correlation between plasma viral load in the infected animals (measured as AUC over time) and the number of founder viruses establishing the infection (r = −0.19, P = 0.29, Spearman rank correlation), which is consistent with other reports (21, 24).
Discussion
Here, we report that a weakly neutralizing, gp120-binding antibody to a CD4bs epitope, b6, fails to protect macaques from vaginal infection under conditions in which a strongly neutralizing antibody, b12, to an overlapping epitope on gp120 is highly protective. Thus, topical administration of a high dose (5 mg) of b6 immediately before vaginal challenge with SHIV-162P4 protected zero of five animals, whereas the same amount of b12 provided sterilizing immunity to seven of seven animals. In another experiment, intravenous administration of b6 at a high dose (25 mg/kg) protected zero of four macaques vaginally challenged with a related virus, SHIV-162P3, whereas b12 protected three of four animals. The mere presence of a gp120-binding antibody in vivo, either in the plasma or the vaginal lumen, is not, therefore, sufficient to protect against vaginal challenge. We also showed that the same amount (5 mg) of vaginally delivered b12 was more effective at protecting against vaginal challenge with SHIV-162P4 than the more PBMC neutralization-resistant SHIV-162P3. These different outcomes occurred despite b12 and b6 binding comparably to gp120s from the two viruses. Hence, gp120-binding activity is not a correlate of protection, at least for MAbs to the CD4bs. Our results are consistent with the failure of passively administered polyclonal preparations containing nonneutralizing anti-Env antibodies to protect macaques against challenge with different SHIVs (25, 26), and with the failure of recombinant monomeric gp120 vaccines to protect humans in phase III trials despite inducing nonneutralizing, gp120-binding Abs consistently (3–7). In a previous study, vaginally applied b12 conferred dose-dependent protection against SHIV-162P4 vaginal challenge (17). Thus, protection is a function both of the concentration of NAb present and the sensitivity of the challenge virus to that antibody, which may have implications for vaccine design.
Vaginal administration of a high dose (5 mg) of the nonneutralizing anti-gp41 antibody F240 did, however, have some effects against SHIV-162P4 vaginal challenge. Thus, two of five animals were uninfected and two of the three infected animals had lower viral loads during primary infection. Taken together, these findings suggest that F240 can exert some kind of antiviral action during the earliest stages of vaginal transmission, although the underlying mechanisms remain unclear. One in vitro observation of possible relevance is that immobilized F240 was much more effective than the two CD4bs MAbs at capturing infectious SHIV-162P4 virions from solution. At the MAb concentrations applied in vivo, perhaps F240 binds to functionally inactive gp41 stumps on infectious virions, targeting them to Fc receptor-bearing phagocytes or driving other events that lead, overall, to a reduction in the infectivity of the inoculum. Further studies with Fc-engineered versions of F240 and different challenge protocols will be required to confirm and extend these findings (27, 28). A recent report suggests that vaccine-induced, vaginal anti-gp41 antibodies may protect against SHIV-162P3 vaginal challenge (29). However, as the vaccine construct used in that study did not include the F240 epitope, antibodies with that specificity cannot be responsible for any protection observed (29). We further note that the RV144 vaccine trial did not involve gp41 immunogens, which means the results we describe are not directly relevant to ongoing attempts to understand the outcome of that study (8).
Caveats should be expressed concerning the interpretation of our experiments and the conclusions that can be drawn. We treated the macaques with progesterone 30 d before challenge, a procedure that thins the vaginal epithelium and facilitates infection. We also used a single vaginal inoculation of a challenge virus at a dose higher than occurs during sexual transmission to women (17–19). Hence, this macaque model is stringent. Progesterone treatment of macaques might mimic an important physiological process in women, the natural cycle of menses (30–32). In the absence of progesterone, infection of macaques is more likely to occur in the luteal phase of the menstrual cycle, when the vaginal epithelium is at its thinnest, and it is possible that similar, natural variations in the risk of HIV-1 infection also arise in women (30). Concerns that progesterone treatment might suppress immune responses and enhance infection appear to have been substantially resolved (31, 32). However, the reduced amount of vaginal mucus present in progesterone-treated animals at the time of vaginal MAb addition might have an impact on the outcome of the study, if mucus and MAbs act cooperatively to prevent infection. Of considerable relevance is that it is now apparent that vaginal infection of macaques under these conditions does closely mirror what happens in HIV-1–infected women; in both species only one, or a few, mucosally transmitted viruses expand in the new host (Fig. 4) (20, 21, 23).
The increased number of founder viruses transmitted to the macaques given MAb b6 is intriguing. The virus inoculum was set at a level that infects almost all of the control macaques, so an increase in the number of founder viruses is inconsequential to the infection status of an animal. However, under more limiting conditions involving a smaller inoculum, any agent that elevates the number of infecting events may increase the probability of infection. Although this point is highly speculative, it may have potential implications for the design of vaccines strategies that involve the induction of nonneutralizing or poorly neutralizing Abs, or very low levels of neutralizing Abs. Additional macaque experiments to further explore our observations with b6 seem justified. We do not yet know how b6 increased the number of founder viruses. We assume that whatever happened is a direct consequence of a MAb–virus interaction followed by a potentiating event. Two types of interaction can be envisaged. First, the very high concentrations of the weakly neutralizing Ab b6 might allow some degree of occupancy of functional spikes on the virion surface in vivo; although such binding is generally thought to lead to neutralization at relatively high levels of NAb occupancy (33–35), low-level occupancy can lead to enhanced infection (35, 36). In principle, subneutralizing concentrations of a Nab, such as b12, may also yield a low level of binding and a similar outcome. The second possibility is that b6 may interact with nonfunctional Env present on infectious virions (16, 36). Such an event could promote virion cross-linking or the uptake of virions by Fc receptor-bearing cells. However, these mechanisms would also apply to the anti-gp41 MAb F240, which did not increase the number of founder viruses transmitted and indeed may have had a partially protective effect overall. Why b6 and F240 differ in this regard is not yet clear. Additional studies with the same and different MAbs, perhaps using different challenge formats, will be required to address these questions.
It is hard to quantify the local concentrations of Abs present at the site of encounter with the challenge virus after vaginal addition of a bolus (5 mg in 4 mL), but they may be higher than would arise in Env-vaccinated humans. We also note that we used a high-dose viral inoculum, and more modest b12 serum concentrations can protect against a lower dose (27). Overall, although the possible protection provided by the non-NAb F240 is of interest, the solid protection provided by the b12 NAb underpins our belief that the emphasis should remain on designing HIV-1 vaccines able to induce broadly active and potent neutralizing antibodies.
Materials and Methods
Monoclonal Antibodies.
IgG1 b12 and IgG1 b6 are human antibodies (IgG1, κ) that recognize epitopes overlapping the gp120 CD4bs (9, 10, 37). IgG1 (κ) F240 hybridoma was created from B cells isolated from an HIV-1–infected individual and recognizes a nonhelical hydrophobic region, positions 598 to 604, that forms a disulfide loop in the six-helix bundle configuration of gp41 (11, 12). DEN3, an anti-Dengue NS1 human IgG1 antibody, served as an isotype control MAb (27). Recombinant b6, b12, and DEN3 were expressed in Chinese hamster ovary (CHO-K1) cells, as described elsewhere (38). F240 was purified from spent supernatant of hybridoma cells, as previously described (11, 39). Endotoxin contamination was monitored using a quantitative chromagenic Limulus Amoebecyte Lysate assay (Cambrex), according to the manufacturer's instructions. MAb preparations contained <3 IU of endotoxin per milliliter.
Challenge Viruses.
R5 viruses SHIV-162 Passages 3 and 4 were derived from the HIV-1 SF162 primary isolate as described elsewhere (13–15). SHIV-162P3, propagated in phytohemagglutin-activated rhesus macaque PBMCs, was obtained through the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Cat. No. 6526; Contributors: Janet Harouse, Cecilia Cheng-Mayer, and Ranajit Pal, Aaron Diamond AIDS Research Center, New York). The SHIV-162P4 stock was donated by Leo Stamatatos (Seattle Biomedical Research Institute, Seattle) (40).
Neutralization Assays.
Three assays used were as follows: (i) Infectious Env-pseudoviruses of SHIV-162P3 were generated in 293T cells for use in a single-cycle assay with TZM-bl target cells (41). (ii) TZM-bl cell-based neutralization assays using replication competent SHIV-162P3 and SHIV-162P4 were modified from previously described methods (42, 43). In both TZM-bl cell formats, the extent of viral entry was determined as a percentage reduction of infectivity (measured as relative light units; RLU) compared with control (no MAb). (iii) Neutralization assays using rhesus PBMCs were conducted as previously described (38). All experiments were performed in triplicate and repeated at least twice with similar results. A nonlinear regression curve (Prism software) was used to determine the IC50 (50%) neutralization titer.
Anti-gp120 Binding Antibody ELISA.
MAbs were tested for binding activity to SHIV-162P3 and SHIV-162P4 gp120s using a detergent-treated lysate of each virus, as described elsewhere (10).
Virus Capture Assay.
A concentrated stock of SHIV-162P4 (TCID50 > 2 × 105, as titered on TZM-bl cells) was used in a virus capture assay, as described previously (16), with the following minor modifications: After MAbs, and then viruses, were added to the ELISA plate wells, all washes (a maximum of five) were performed using PBS. TZM-bl cells (5 × 103 in 200 μL of medium per well) were used to quantify the captured viruses, with luciferase activity (RLU) measured after 72 h. This method is superior to quantifying the p24 antigen content of captured viruses, as it avoids artifacts associated with noninfectious particles (16).
Macaque Challenge Studies.
A single intramuscular injection of Depo-Provera (progesterone) was given to female Indian rhesus macaques 30 d before challenge, to synchronize the menstrual cycle, thin the vaginal epithelium, and facilitate virus transmission (17–19). Virus challenge and antibody delivery protocols are more fully described elsewhere (17, 19, 27, 38). On the day of challenge, 4 mL of MAbs formulated in isotonic saline at 1 mg/mL were applied atraumatically to the vagina, 30 min before SHIV-162P3 or SHIV-162P4 was added in a 1-mL volume containing 500 TCID50. In one experiment, the MAbs were administered by intravenous infusion at 25 mg/kg the day before SHIV-162P3 challenge. Infection status was determined by measuring plasma viral load at 7, 14, 21, 28, 42, 56, and 70 d postchallenge, using a commercially available branched DNA assay with a sensitivity limit of 125 RNA copies per milliliter (Siemens Diagnostics). All protocols were approved by the Tulane University Institutional Animal Care and Use Committee. The animals were housed in accordance with the American Association for Accreditation of Laboratory Animal Care Standards. At the start of all experiments, all animals were experimentally naive and were negative for antibodies against HIV-1, SIV, and type D retrovirus.
Founder Virus Measurements.
The entire env gene was sequenced using a limiting dilution PCR so only one amplifiable molecule was present in each reaction. Viral RNA was isolated using the QIAamp Viral RNA Mini Kit (Qiagen) and immediately reverse-transcribed into single-stranded cDNA using SuperScript III (Invitrogen) with the env-specific primer SIVEnvR1 5′-TGTAATAAATCCCTTCCAGTCCCCCC-3′. Each cDNA synthesis reaction included 1× reaction buffer, 0.5 mM of each deoxynucleoside triphosphate (dNTP), 5 mM DTT, 2 U/mL RNaseOUT (RNase inhibitor), 10 U/mL of Super-Script III reverse-transcription mix, and 0.25 mM antisense primer. cDNA was serially diluted and distributed among independent PCR reactions to identify a dilution where amplification occurred in <30% of the total number of reactions. PCR amplification was performed in the presence of 1× buffer, 2 mM MgSO4, 0.2 mM of each dNTP, 0.2 μM of each primer, and 0.025 U/μL Platinum Taq High Fidelity polymerase (Invitrogen) in a 20-μL reaction. First-round PCR was performed with sense primer SIVEnvF1 5′-CCTCCCCCTCCAGGACTAGC-3′ and antisense primer SIVEnvR1 5′-TGTAATAAATCCCTTCCAGTCCCCCC-3′ under the following conditions: 1 cycle of 94 °C for 2 min, 35 cycles at 94 °C for 15 s, 55 °C for 30 s, and 68 °C for 4 min, followed by a final extension of 68 °C for 10 min. Next, 1 μL of the first-round PCR product was added to a second-round PCR that included the sense primer SIVEnvF2 5′-TATAATAGACATGGAGACACCCTTGAGGGAGC-3′ and antisense primer SIVEnvR2 5′-ATGAGACATRTCTATTGCCAATTTGTA-3′, performed under the same conditions used for first-round PCR, but with a total of 45 cycles. Correct sized amplicons were identified by agarose gel eletrophoresis and directly sequenced with second round PCR primers and six HIV-1 specific primers. Sequences were deposited in GenBank with accession numbers JN010802--JN011447.
Statistical Analysis.
Proportions were compared by Fisher's exact test and median viral loads over time (AUC) by Mann-Whitney U test, both one-tailed. The α-level was set to P < 0.05. Spearman rank correlation r-values were calculated and evaluated by two-tailed tests. Analyses were performed in Prism (Graphpad).
Acknowledgments
We thank Athe Tsibris and Steven Wolinsky for contributing founder virus data for the gel control group (Fig. 4); Leo Stamatatos for the SHIV-162P4 challenge stock; and Kelsi Rasmussen and Janell LeBlanc for their technical support. The animal studies were funded by the International AIDS Vaccine Initiative, with additional support by National Institutes of Health Cooperative Agreement U19 AI76982 (to J.P.M.); in vitro studies were also supported by National Institutes of Health Grants R37 AI36082 (to J.P.M.) and AI33292 and AI1055332 (to D.R.B.); sequencing and founder virus analysis was performed with federal funds from the National Cancer Institute, National Institutes of Health, under Contract HHSN266200400088C. B.M. was supported by the Alfred Benzon Foundation.
Footnotes
References
- 1.Barouch DH, Korber B. HIV-1 vaccine development after STEP. Annu Rev Med. 2010;61:153–167. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: Lessons learned and the way forward. Curr Opin HIV AIDS. 2010;5:428–434. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LM, Burton DR. Rational antibody-based HIV-1 vaccine design: Current approaches and future directions. Curr Opin Immunol. 2010;22:358–366. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 5.Tomaras GD, Haynes BF. Strategies for eliciting HIV-1 inhibitory antibodies. Curr Opin HIV AIDS. 2010;5:421–427. doi: 10.1097/COH.0b013e32833d2d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton DR, Weiss RA. AIDS/HIV. A boost for HIV vaccine design. Science. 2010;329:770–773. doi: 10.1126/science.1194693. [DOI] [PubMed] [Google Scholar]
- 7.Corey L, McElrath MJ. HIV vaccines: Mosaic approach to virus diversity. Nat Med. 2010;16:268–270. doi: 10.1038/nm0310-268. [DOI] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 10.Pantophlet R, et al. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol. 2003;77:642–658. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavacini LA, Duval M, Robinson J, Posner MR. Interactions of human antibodies, epitope exposure, antibody binding and neutralization of primary isolate HIV-1 virions. AIDS. 2002;16:2409–2417. doi: 10.1097/00002030-200212060-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cavacini LA, et al. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res Hum Retroviruses. 1998;14:1271–1280. doi: 10.1089/aid.1998.14.1271. [DOI] [PubMed] [Google Scholar]
- 13.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 14.Harouse JM, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan RC, Harouse JM, Gettie A, Cheng-Mayer C. In vivo adaptation of SHIV(SF162): Chimeric virus expressing a NSI, CCR5-specific envelope protein. J Med Primatol. 1999;28:164–168. doi: 10.1111/j.1600-0684.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 16.Poignard P, et al. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J Virol. 2003;77:353–365. doi: 10.1128/JVI.77.1.353-365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veazey RS, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 18.Marx PA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 19.Veazey RS, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keele BF, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, et al. Low dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorgi EE, et al. Estimating time since infection in early homogeneous HIV-1 samples using a poisson model. BMC Bioinformatics. 2010;11:532. doi: 10.1186/1471-2105-11-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone M, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novitsky VR, et al. Transmission of single and multiple viral variants in primary HIV-1 subtype C infection. PLoS ONE. 2011;6:e16714. doi: 10.1371/journal.pone.0016714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola JR, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 27.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 29.Bomsel M, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Quinn TC, Overbaugh J. HIV/AIDS in women: An expanding epidemic. Science. 2005;308:1582–1583. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 31.Genescà M, McChesney MB, Miller CJ. Depo-Provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus. PLoS ONE. 2010;5:e9814. doi: 10.1371/journal.pone.0009814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders-Beer B, et al. Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques. AIDS Res Hum Retroviruses. 2010;26:433–443. doi: 10.1089/aid.2009.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klasse PJ. Modeling how many envelope glycoprotein trimers per virion participate in human immunodeficiency virus infectivity and its neutralization by antibody. Virology. 2007;369:245–262. doi: 10.1016/j.virol.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parren PW, et al. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan N, et al. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binley JM, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda LR, et al. The neutralization properties of a HIV-specific antibody are markedly altered by glycosylation events outside the antigen-binding domain. J Immunol. 2007;178:7132–7138. doi: 10.4049/jimmunol.178.11.7132. [DOI] [PubMed] [Google Scholar]
- 40.Kraft Z, et al. Macaques infected with a CCR5-tropic simian/human immunodeficiency virus (SHIV) develop broadly reactive anti-HIV neutralizing antibodies. J Virol. 2007;81:6402–6411. doi: 10.1128/JVI.00424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwick MB, et al. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J Virol. 2001;75:12198–12208. doi: 10.1128/JVI.75.24.12198-12208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willey R, Nason MC, Nishimura Y, Follmann DA, Martin MA. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res Hum Retroviruses. 2010;26:89–98. doi: 10.1089/aid.2009.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascola JR, et al. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J Virol. 2002;76:4810–4821. doi: 10.1128/JVI.76.10.4810-4821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]