Abstract
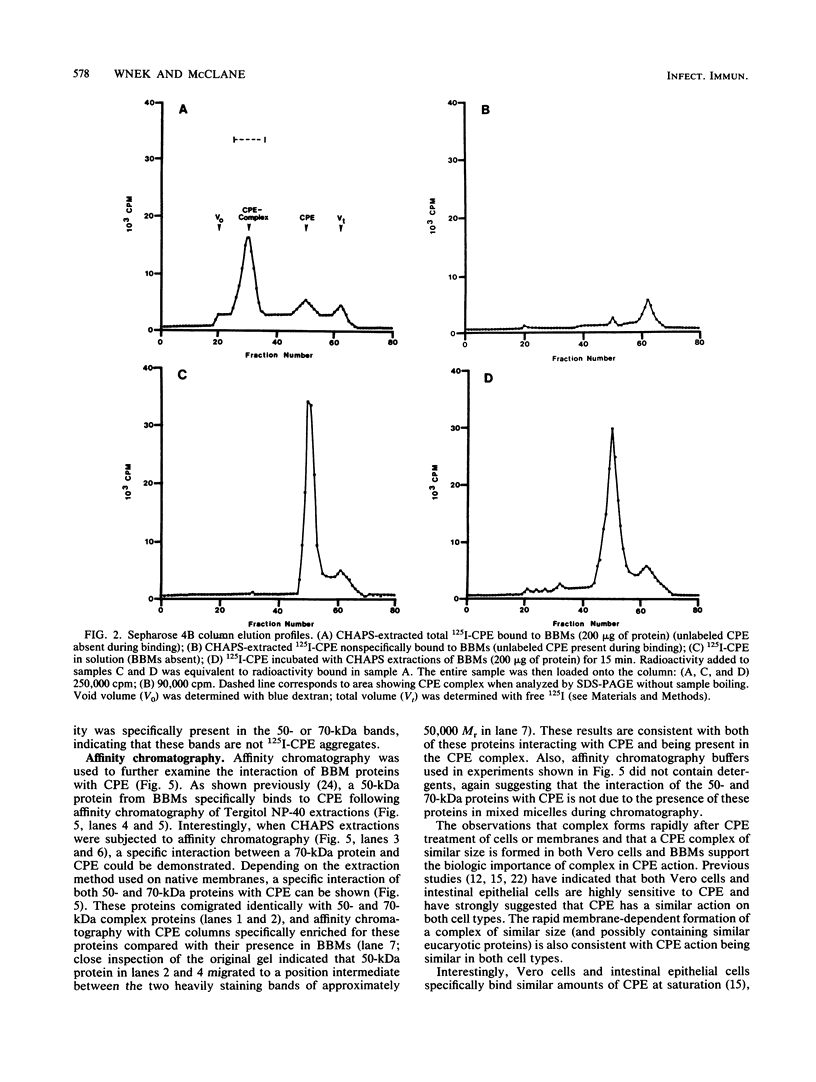
Clostridium perfringens type A 125I-enterotoxin (125I-CPE) was bound to rabbit intestinal brush border membranes (BBMs) or Vero cells and then solubilized with 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate (CHAPS). Solubilized radioactivity was analyzed by gel filtration chromatography on a Sepharose 4B column or by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) without sample boiling and autoradiography. Specifically bound 125I-CPE extracted from either BBMs or Vero cells was primarily associated with a complex of approximately 160,000 Mr. The CPE complex was partially purified by gel filtration or SDS-PAGE without sample boiling. SDS-PAGE analysis with sample boiling of the partially purified 125I-CPE complex from Vero cells or BBMs suggested that CPE complex contains both a 50,000-Mr protein and a 70,000-Mr protein in approximately equimolar amounts. This result is supported by affinity chromatography with CPE immobilized on Sepharose 4B, which showed the specific interaction of similar size proteins with CPE. The simplest explanation for these results is that CPE (Mr 35,000) interacts with 50,000-Mr and 70,000-Mr eucaryotic proteins to form a membrane-dependent complex of approximately 160,000 Mr. These results suggest that the receptor or target site(s) or both for CPE are similar in both BBMs and Vero cells. The significance of these findings in terms of CPE binding, insertion, and biologic action is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreyfus L. A., Robertson D. C. Solubilization and partial characterization of the intestinal receptor for Escherichia coli heat-stable enterotoxin. Infect Immun. 1984 Nov;46(2):537–543. doi: 10.1128/iai.46.2.537-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. L., Jr, Duncan C. L. Anomalous aggregation of Clostridium perfringens enterotoxin under dissociating conditions. Can J Microbiol. 1976 Sep;22(9):1410–1414. doi: 10.1139/m76-209. [DOI] [PubMed] [Google Scholar]
- Giger O., Pariza M. W. Mechanism of action of Clostridium perfringens enterotoxin. Effects on membrane permeability and amino acid transport in primary cultures of adult rat hepatocytes. Biochim Biophys Acta. 1980 Jan 25;595(2):264–276. doi: 10.1016/0005-2736(80)90089-9. [DOI] [PubMed] [Google Scholar]
- Granum P. E. Inhibition of protein synthesis by a tryptic polypeptide of Clostridium perfringens type A enterotoxin. Biochim Biophys Acta. 1982 Oct 20;708(1):6–11. doi: 10.1016/0167-4838(82)90196-0. [DOI] [PubMed] [Google Scholar]
- Granum P. E. The effect of Ca++ and Mg++ on the action of Clostridium perfringens enterotoxin on Vero cells. Acta Pathol Microbiol Immunol Scand B. 1985 Feb;93(1):41–48. doi: 10.1111/j.1699-0463.1985.tb02849.x. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y., Uemura T., Kozaki S., Sakaguchi G. Effects of Ca2+ and other cations on the action of Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1986 Oct 31;889(1):65–71. doi: 10.1016/0167-4889(86)90009-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Ozutsumi K., Iwahashi H., Sugimoto N. Primary action of Clostridium perfringens type A enterotoxin on HeLa and Vero cells in the absence of extracellular calcium: rapid and characteristic changes in membrane permeability. Biochem Biophys Res Commun. 1986 Dec 15;141(2):704–710. doi: 10.1016/s0006-291x(86)80229-7. [DOI] [PubMed] [Google Scholar]
- McClane B. A., Hanna P. C., Wnek A. P. Clostridium perfringens enterotoxin. Microb Pathog. 1988 May;4(5):317–323. doi: 10.1016/0882-4010(88)90059-9. [DOI] [PubMed] [Google Scholar]
- McClane B. A., McDonel J. L. The effects of Clostridium perfringens enterotoxin on morphology, viability, and macromolecular synthesis in Vero cells. J Cell Physiol. 1979 May;99(2):191–200. doi: 10.1002/jcp.1040990205. [DOI] [PubMed] [Google Scholar]
- McClane B. A. Osmotic stabilizers differentially inhibit permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1984 Oct 17;777(1):99–106. doi: 10.1016/0005-2736(84)90501-7. [DOI] [PubMed] [Google Scholar]
- McClane B. A., Wnek A. P., Hulkower K. I., Hanna P. C. Divalent cation involvement in the action of Clostridium perfringens type A enterotoxin. Early events in enterotoxin action are divalent cation-independent. J Biol Chem. 1988 Feb 15;263(5):2423–2435. [PubMed] [Google Scholar]
- McClane B. A., Wnek A. P., Whitaker-Dowling P. Interferon pretreatment enhances the sensitivity of Vero cells to Clostridium perfringens type A enterotoxin. Microb Pathog. 1987 Sep;3(3):195–206. doi: 10.1016/0882-4010(87)90096-9. [DOI] [PubMed] [Google Scholar]
- McDonel J. L. Binding of Clostridium perfringens [125I]enterotoxin to rabbit intestinal cells. Biochemistry. 1980 Oct 14;19(21):4801–4807. doi: 10.1021/bi00562a014. [DOI] [PubMed] [Google Scholar]
- McDonel J. L., McClane B. A. Binding versus biological activity of Clostridium perfringens enterotoxin in Vero cells. Biochem Biophys Res Commun. 1979 Mar 30;87(2):497–504. doi: 10.1016/0006-291x(79)91823-0. [DOI] [PubMed] [Google Scholar]
- McDonel J. L., McClane B. A. Production, purification, and assay of Clostridium perfringens enterotoxin. Methods Enzymol. 1988;165:94–103. doi: 10.1016/s0076-6879(88)65018-x. [DOI] [PubMed] [Google Scholar]
- Petrie H. T., McDonel J. L., Schlegel R. A. Intracellular antibody against Clostridium perfringens enterotoxin fails to counteract toxin-induced damage. Cell Biol Int Rep. 1982 Jul;6(7):705–711. doi: 10.1016/0309-1651(82)90140-0. [DOI] [PubMed] [Google Scholar]
- Shandera W. X., Tacket C. O., Blake P. A. Food poisoning due to Clostridium perfringens in the United States. J Infect Dis. 1983 Jan;147(1):167–170. doi: 10.1093/infdis/147.1.167. [DOI] [PubMed] [Google Scholar]
- Sigrist H., Ronner P., Semenza G. A hydrophobic form of the small-intestinal sucrase-isomaltase complex. Biochim Biophys Acta. 1975 Oct 17;406(3):433–446. doi: 10.1016/0005-2736(75)90022-x. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Skjelkvåle R., Berg T. Quantitation of binding and subcellular distribution of Clostridium perfringens enterotoxin in rat liver cells. Infect Immun. 1982 Aug;37(2):486–491. doi: 10.1128/iai.37.2.486-491.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnek A. P., McClane B. A. Comparison of receptors for Clostridium perfringens type A and cholera enterotoxins in isolated rabbit intestinal brush border membranes. Microb Pathog. 1986 Feb;1(1):89–100. doi: 10.1016/0882-4010(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Wnek A. P., McClane B. A. Identification of a 50,000 Mr protein from rabbit brush border membranes that binds Clostridium perfringens enterotoxin. Biochem Biophys Res Commun. 1983 May 16;112(3):1099–1105. doi: 10.1016/0006-291x(83)91731-x. [DOI] [PubMed] [Google Scholar]
- Wnek A. P., Strouse R. J., McClane B. A. Production and characterization of monoclonal antibodies against Clostridium perfringens type A enterotoxin. Infect Immun. 1985 Nov;50(2):442–448. doi: 10.1128/iai.50.2.442-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]