Abstract
MicroRNA (miRNA) species (miR) regulate mRNA translation and are implicated as mediators of disease pathology via coordinated regulation of molecular effector pathways. Unraveling miR disease-related activities will facilitate future therapeutic interventions. miR-155 recently has been identified with critical immune regulatory functions. Although detected in articular tissues, the functional role of miR-155 in inflammatory arthritis has not been defined. We report here that miR-155 is up-regulated in synovial membrane and synovial fluid (SF) macrophages from patients with rheumatoid arthritis (RA). The increased expression of miR-155 in SF CD14+ cells was associated with lower expression of the miR-155 target, Src homology 2-containing inositol phosphatase-1 (SHIP-1), an inhibitor of inflammation. Similarly, SHIP-1 expression was decreased in CD68+ cells in the synovial lining layer in RA patients as compared with osteoarthritis patients. Overexpression of miR-155 in PB CD14+ cells led to down-regulation of SHIP-1 and an increase in the production of proinflammatory cytokines. Conversely, inhibition of miR-155 in RA synovial CD14+ cells reduced TNF-α production. Finally, miR-155–deficient mice are resistant to collagen-induced arthritis, with profound suppression of antigen-specific Th17 cell and autoantibody responses and markedly reduced articular inflammation. Our data therefore identify a role of miR-155 in clinical and experimental arthritis and suggest that miR-155 may be an intriguing therapeutic target.
Rheumatoid arthritis (RA) is a chronic autoimmune disease involving synovial inflammation and adjacent cartilage and bone destruction. RA causes progressive disability associated with early mortality primarily reflecting vascular comorbidity. RA is driven by dysregulated adaptive and innate immune pathways (1) that offer an increasingly rich therapeutic resource. Thus, cytokine inhibitors (e.g., TNF, IL-6 receptor), B-cell depletion, and T-cell costimulatory blockade are components of current standard of care. Partial, transient, or nonresponse is common, however, and clinical or radiographic remission is rarely sustained; significant unmet clinical need remains.
The prospect of targeting multiple pathways simultaneously is attractive to optimize the neutralization of complex effector immune pathways. A class of posttranscriptional regulators termed “microRNAs” (miRNAs) has been identified that appears to be critical for fine-tuning many biological processes and offers the prospect of multiply targeting RA (2, 3). miRNAs are noncoding 22- or 23-nucleotide RNAs that act via formation of an miRNA-induced silencing complex (miRISC). miRNAs block target mRNA translation or induce mRNA cleavage upon binding to miRNA recognition elements within the 3′ UTRs of target mRNA (4).
Emerging data suggest that single miRNA species (miRs) can profoundly alter the phenotype and outcome of immune responses (5–7). For example, increased lymphocyte expression of miR17-92 promotes lymphoproliferation and occurrence of autoimmunity manifest in anti-DNA autoreactivity (5). Moreover, miRNA dysregulation has been reported in a number of pathologic conditions (8). RA peripheral blood mononuclear cells (PBMC) express elevated levels of miR-146a, miR-155, miR-132, and miR-16, with miR-146a and miR-16 particularly associated with disease activity (9). In addition, miR-146a expression in RA CD4+ cells is positively correlated with the levels of TNF-α in both peripheral blood (PB) and synovial fluid (SF) (10). Also, miR-155 and miR-146a are up-regulated, whereas miR-124a and miR-15a are down-regulated in synovial membrane in clinical and experimental arthritis (11–14). MiR-124a regulates the cell cycle (13), and miR-155 modulates the production of metalloproteinases (11) of RA synovial fibroblasts; manipulation of miR-15a expression triggers apoptosis of these cells (14). However, few reports thus far have addressed the presence and functional impact of miRNAs in inflammatory arthritis. Because of the aberrant expression of miR-155 in RA patients (9, 11), we sought to focus on the functional contribution of miR-155 in clinical and experimental arthritis models. We report here that miR-155 is crucial for the proinflammatory activation of human myeloid cells and antigen-driven inflammatory arthritis. These data provide a powerful proof-of-concept for miR-155–based therapeutic approaches that could modulate the aberrant innate and adaptive autoimmunity associated with RA.
Results
MiR-155 Is Up-Regulated in RA Synovial Macrophages and Monocytes and Promotes Production of Proinflammatory Cytokines.
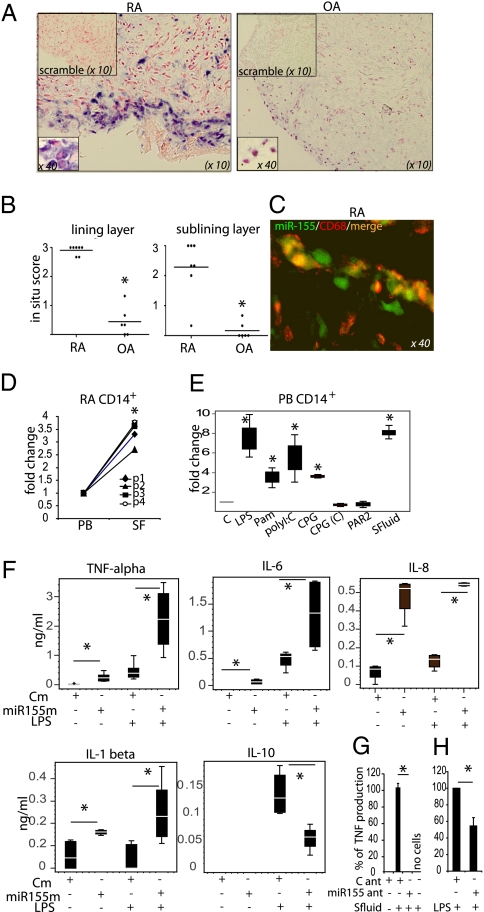
Macrophages/monocytes and dendritic cells are a critical source of proinflammatory cytokines in RA synovium (1). However, the mechanism that underlies their chronic activation is poorly understood. miR-155 has emerged recently as an important regulator of myeloid cell biology (15). In situ hybridization revealed that miR-155 is strongly expressed in RA but not OA synovial biopsies, mainly in the lining layer and to a lesser extent in the sublining layer (Fig. 1 A and B). Double immunofluorescence staining showed that miR-155 is expressed in a majority of CD68+ macrophages in the RA membrane-lining layer (Fig. 1C). In addition, miR-155 is significantly up-regulated in RA SF-derived CD14+ cells compared with those obtained from PB CD14+ cells (Fig. 1D). These observations suggest that the inflammatory microenvironment present in the RA synovial compartment may trigger the expression of miR-155 in synovial monocytes/macrophages. Indeed, exposure of PB CD14+ cells to 25% cell-free RA SF to mimic this milieu strongly up-regulated the expression of miR-155 (Fig. 1E). To examine possible factors responsible for this regulation, PB CD14+ cells next were incubated with candidate stimuli previously implicated in enhanced cytokine production in RA synovium, such as Toll-like receptor (TLR) ligands (16) and protease-activated receptor 2 (PAR2) agonists (17, 18). Selective TLR but not PAR2 stimulation increased the expression of miR-155 in PB CD14+ cells (Fig. 1E).
Fig. 1.
MiR-155 is up-regulated in RA synovial macrophages and monocytes and promotes production of proinflammatory cytokines. (A and B) Expression of miR-155 in RA and OA biopsies. (A) Representative staining. (B) Quantitative evaluation of miR-155 expression, *P < 0.05 RA vs. OA. (C) Representative photograph of double staining for miR-155 (green) and macrophage marker CD68 (red) in RA lining layer. Double-positive cells are orange or yellow. (D) Expression of miR-155 in paired PB and SF CD14+ cells from RA patients (n = 4), *P < 0.05 PB vs. SF. (E) TLR ligands and RA SF up-regulate miR-155 expression in PB CD14+ cells (n = 3). Cells were stimulated with TLR ligands or PAR2 agonist or 25% SF for 24 h as described in Materials and Methods. *P < 0.05 stimulated vs nonstimulated. (F) PB CD14+ cells overexpressing miR-155 produce proinflammatory cytokines. CD14+ cells were transfected with control or miR-155 mimic (20 nM). After 24 h, LPS (100 ng/mL) was added to some wells for a further 16 h (n = 5). (G and H) PB CD14+ cells and RA SF CD14+ cells (n = 2 and 3, respectively) were transfected with control or miR-155 antagomir. After 24 h cells were exposed to SF (F) or LPS (G) for a further 24 h. *P < 0.05 as indicated. Data are means ± SEM or box-and-whisker diagrams. Cm, control mimic; miR155ant, miR-155 antagomir; miR155m, miR-155 mimic, C ant, control antagomir; PAR2, PAR2 agonist, (SLIGKV-NH2). The in situ scoring system is described in SI Materials and Methods.
Overexpression of miR-155 in RA SF CD14+ cells suggested that it could be involved in posttranscriptional control of the inflammatory pathways in these cells. To test this possibility, PB CD14+ cells were transfected with control or miR-155 mimics (confirmation of expression is shown in Fig. S1). Overexpression of miR-155 triggered the production of cytokines and chemokines strongly implicated in RA synovitis, namely TNF-α, IL-6, IL-1β, and IL-8. Furthermore, miR-155 significantly increased LPS-induced proinflammatory cytokine production but decreased IL-10 production compared with control mimic (Fig. 1F). Moreover, miR-155 overexpression triggered TNF-α and IL-6 production by CD14+-derived macrophage colony-stimulating factor (M-CSF) mature macrophages (Fig. S2). Next, CD14+ cells were transfected with either a control or miR-155 inhibitor (antagomirs) and were incubated with RA SF to mimic again the synovial microenvironment; then cytokine production was measured. RA SF triggered TNF-α production in cells transfected with control antagomir (Fig. 1G), but this effect was abrogated in cells transfected with miR-155 antagomir. Finally, to confirm that target tissue monocytes endogenously matured in vivo could be regulated by miR-155, RA synovial CD14+ cells were isolated, immediately transfected with either control or miR-155 antagomir, and then were reactivated by LPS stimulation. Crucially, miR-155 antagomir inhibited the production of TNF-α (Fig. 1H). Together these data indicate that the chronic proinflammatory phenotype of CD14+ myeloid cells in RA synovium could be regulated by aberrant expression of miR-155.
miR-155 Targets Src Homology 2-Containing Inositol Phosphatase-1 in RA SF CD14+ Cells.
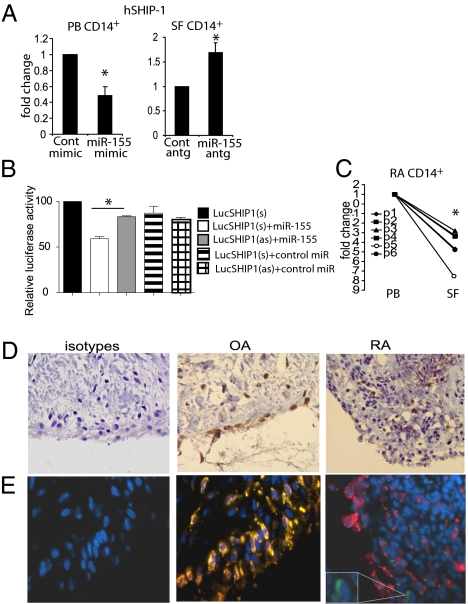
To investigate the posttranscriptional mechanisms whereby miR-155 uniquely operates in synovial monocytes, we focused on Src homology 2-containing inositol phosphatase-1 (SHIP-1) because it has been reported to be a target of miR-155 in murine myeloid cells (19), is a potent inhibitor of many inflammatory pathways, and served as a plausible candidate pathway (20). MiR-155 overexpression in PB CD14+ cells markedly decreased SHIP-1 mRNA expression, whereas inhibition of miR-155 significantly increased the expression of SHIP-1 mRNA in RA SF CD14+ cells (Fig. 2A). To verify that human SHIP-1 is targeted directly by miR-155, the 3′ UTR of SHIP-1 was cloned into a pMir luciferase system. Cells transfected with pMir-SHIP alone exhibited no change in luciferase expression. Transfection of pMir with sense (s) or antisense (as) SHIP in the presence of control mimic revealed only marginal effects on luciferase expression. In contrast, in the presence of miR-155 mimic, cells transfected with pMir-SHIP(s) but not pMir-SHIP(as) showed diminished expression of luciferase (Fig. 2B). These results demonstrate that miR-155 can directly target human SHIP-1 for degradation.
Fig. 2.
The miR-155 target SHIP-1 is down-regulated in RA SF CD14+ cells and in RA synovial tissue macrophages. (A) miR-155 regulates expression of SHIP-1 in RA PB and SF CD14+ cells. Cells were transfected with miR-155 mimic or antagomir or with appropriate controls as described in Fig. 1. Quantitative analysis of SHIP-1 mRNA expression is shown (n = 3). (B) miR-155 binds directly to the 3′ UTR of human SHIP-1 mRNA. pMir-hSHIP1 (sense) and pMir-hSHIP1 (antisense) 3′ UTR luciferase plasmids were cotransfected with control or miR-155 mimic (40 nM) in HEK293 cells. Luciferase activity was analyzed at 24 h. (C) Expression of SHIP-1 in paired PB and SF CD14+ cells from RA patients (n = 6). Data are means ± SEM. *P < 0.05. (D and E) Synovial specimens from RA (n = 5) and OA (n = 5) patients stained with anti-human SHIP-1 (D, brown) and counterstained with anti-CD68 antibody (E). Representative staining of one of five specimens is shown; blue, DAPI; red, CD68+; green, SHIP-1+; yellow, CD68+/SHIP-1+. (Magnification: 63×.) Enlargements in E show a SHIP-1+ cell (green). Cont, control mimic; hSHIP-1, human SHIP-1; miR155antg, miR-155 antagomir; miR155m, miR-155 mimic; Cont antg, control antagomir. Relative luciferase activity is the maximal activity in the presence of pMiR plasmid alone.
SHIP-1 Expression Is Down-Regulated in RA SF CD14+ Cells and RA Synovial Tissue Macrophages.
Given the direct interaction of miR-155 and SHIP-1 in CD14+ cells, we next examined the expression of SHIP-1 in RA patients. Consistent with the high expression of miR-155 in RA synovial CD14+ cells (Fig. 1A), the expression of SHIP-1 mRNA in these cells was markedly down-regulated compared with that in matching PB CD14+ cells (Fig. 2C). Moreover, consistent with the distribution of miR-155 in RA synovium, SHIP-1 protein was not expressed in cells of the superficial lining layer of RA patients. This absence of expression was in a sharp contrast to cells in the lining layer of OA synovium (Fig. 2D). Double immunofluorescent staining revealed that such SHIP-1 expression was present in CD68+ macrophages in OA but not in RA synovium (Fig. 2E). In contrast, SHIP-1 was expressed in RA CD3 and CD20 lymphocyte subsets, suggesting some lineage specificity in the regulation of SHIP-1 (Fig. S3). These data indicate that a functional proinflammatory miR-155/SHIP-1 pathway may be operational in synovial cells in RA patients.
miR-155 Is Crucial for the Development of Collagen-Induced Arthritis.
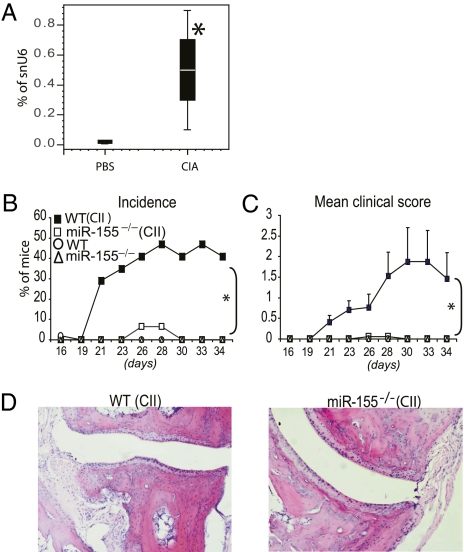
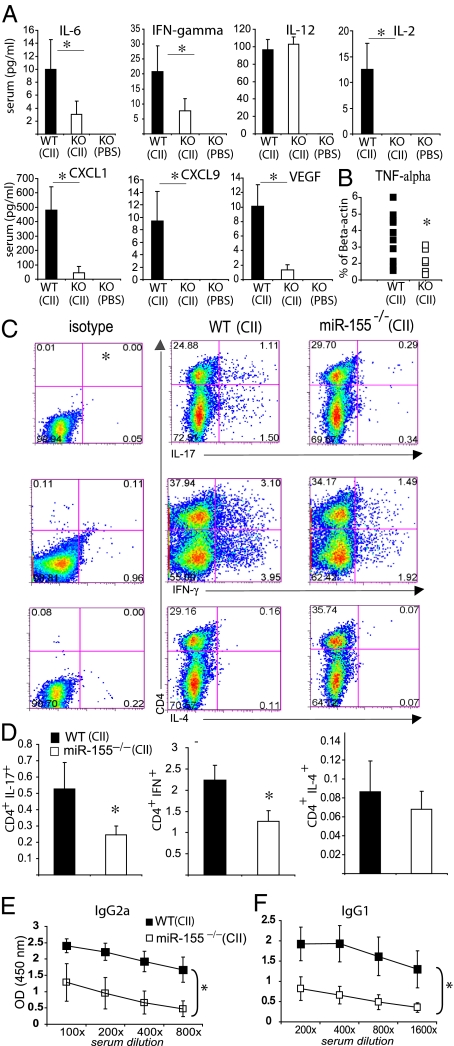
We next examined the contribution of miR-155–regulated pathways to the development and progression of arthritis in collagen-induced arthritis (CIA) in mice. WT or miR-155−/− C57BL/6 mice were immunized with type II collagen in complete Freund's adjuvant and challenged 21 d later to provoke arthritis. Commensurate with our observations in human synovium, miR-155 expression was elevated in arthritic joints in WT mice by day 34 (Fig. 3A). Although ∼50% of WT littermates developed arthritis, commensurate with the numbers expected on the B6 background (21), miR-155−/− collagen-immunized mice did not develop clinical evidence of arthritis (Fig. 3 B and C). Furthermore, in contrast to WT controls, miR-155−/− mice developed neither synovial inflammation nor cartilage and bone destruction (Fig. 3D). Control WT and miR-155−/− mice administered PBS developed no symptoms of arthritis. Serum IL-6, VEGF, IL-2, and IFN-γ and the chemokines CXCL1 and CXCL9 were decreased significantly in miR-155−/− mice compared with WT mice (Fig. 4A). Other cytokines either were unchanged [IL-12, FGF-β, IL-10, IL-1α, IL-1β, monocyte chemotactic protein 1, and chemokine (C-C motif) ligand 3] or were below the limit of detection (TNF-α, GM-CSF, IL-4, IL-13, IP-10, and IL-17). Synovial TNF-α expression was significantly reduced in miR-155−/− mice compared with WT mice (Fig. 4B). This reduction was associated with higher expression of SHIP-1 and lower expression of some proinflammatory mediators, including TNF-α and IL-1β in miR-155−/− bone marrow-derived macrophages (Fig S4). To investigate further the mechanism of disease suppression, we measured the development of antigen-specific Th17, Th1, and Th2 cells in draining lymph nodes (DLN) as well as the production of collagen-specific autoantibodies in the serum of WT and miR-155−/− mice. CD4+ cells from miR-155−/− DLNs produced significantly less IL-17 and IFN-γ than did CD4+ cells from WT DLN (Fig. 4 C and D). Moreover, CD4− cells from miR-155–deficient mice produced significantly less IL-17 and IFN-γ than did CD4− cells from WT mice. There was no significant difference in the production of IL-4 in either group, suggesting that the suppression of disease is not mediated by exaggeration of a compensatory Th2 response. WT mice immunized with collagen produced significantly high titers of collagen-specific IgG2a and IgG1 than did miR-155−/− mice. As expected, unimmunized control WT and miR-155−/− mice had no detectable collagen antibodies (Fig. 4E). Finally, to distinguish the contribution of miR-155 to autoantibody production from autoantibody-mediated effector function in experimental arthritis, the collagen antibody-induced arthritis (CAIA) (22) model was used. miR-155−/− and WT mice that received passive transfer of collagen antibodies developed arthritis with similar incidence and clinical severity (Fig. S5 A–D). Moreover production of TNF-α by miR-155−/− and WT macrophages stimulated with immunocomplexes in vitro was similar (Fig. S5E). Together these data clearly show that miR-155 is crucial for the development of arthritis by supporting production of proinflammatory cytokines and the differentiation of antigen-specific T cells and antigen-specific antibodies but is not required for autoantibody-mediated proinflammatory effector functions.
Fig. 3.
miR-155 is crucial for the development of CIA. On day 0, miR-155−/− and WT mice (n = 15–17 per group) were injected intradermally with either a type II chicken collagen/Freund's complete adjuvant emulsion (CII/CFA) (200 μg) or PBS. On day 21, type II chicken collagen in PBS (200 μg) was injected i.p. Mice were killed on day 34. (A) miR-155 is up-regulated in the articular tissue of WT mice with CIA. (B and C) The incidence (B) and mean clinical score (C) are shown. In contrast to WT littermates, miR-155−/− mice do not develop signs of CIA. (D) In contrast to WT mice, miR-155−/− mice did not develop articular inflammation and degradation of cartilage and bone. (Magnification: ×10.) Data are means ± SEM or box-and-whisker diagrams. (n = 15–17); *P < 0.05, miR-155−/− vs. WT mice (CIA protocol).
Fig. 4.
miR-155−/− mice exhibit reduced development of T- and B-cell responses during CIA. Groups are as in Fig. 3. CII/miR-155−/− mice show (A) reduced systemic cytokine and chemokine concentrations and (B) reduced expression of TNF-α mRNA in articular tissue. (C and D) CII-miR-155−/− mice show a reduced differentiation of Th17 and Th1 cells in DLN. Representative staining (C) and quantitative evaluation (D) are shown. (E and F) miR-155 is required for the production of collagen-specific antibodies. Quantitative evaluation of antibody activity in isotypes IgG2a (E) and IgG1 (F) is shown. Data are means ± SEM, n = 15–17; *P < 0.05, miR-155−/− vs. WT mice (CIA protocol). CII, type II chicken collagen.
Discussion
miR-155 has been implicated in the differentiation and activation of cells of both the innate and the adaptive immune systems (3). It regulates myeloid cell differentiation and, putatively, their response to TLR ligands in mice (15, 23–26). It mediates a proposed regulatory role in T-cell homeostasis (27) and in the development of B cells in germinal centers (6, 28). Recently, expression of miR-155 has been demonstrated in the context of several autoimmune diseases, including RA. Up-regulated expression of miR-155 in PBMC (9) and synovial membrane cells (11) of RA patients has been observed. However, given the redundant and highly cell-specific effects mediated by microRNA species, the precise functional implications of miR-155 expression in the immunopathogenesis of arthritis remained obscure.
We now demonstrate that miR-155 is up-regulated significantly in the RA synovial compartment, particularly in CD68+ macrophages in the membrane-lining layer and in SF CD14+ cells and that this up-regulation is associated with the lower expression of the miR-155 target, an inhibitor of inflammation, namely SHIP-1, both in SF CD14+ cells and in macrophages in RA synovial tissue. It coincides with the recent report showing that synovial tissue from RA patients is highly enriched in miR-155 compared with synovia from patients with OA (11), although the latter did not provide cellular specificity. Moreover, CD14+ monocytes and macrophages overexpressing miR-155 exhibit decreased SHIP-1 expression that may lead to increased production of proinflammatory cytokines, including IL-6, which is an inducer for the development of the autoreactive Th17 cells, and TNF-α, an established mediator of chronic inflammation. Both IL-6 and TNF are validated clinical therapeutic targets in RA. Recent studies show that SHIP-1 is essential for the suppressor activity of macrophages on inflammatory and IL-17–driven responses (29). Interestingly, SHIP-1 was readily detectable in T and B cells in RA synovium (Fig. S3), possibly reflecting a differential role of SHIP-1 in the regulation of macrophage and lymphocyte activation (29). Thus, the miR-155/SHIP pathway may be partially responsible for excessive proinflammatory activation of myeloid cells in inflammatory arthritis. We cannot, however, exclude a role for other miR-155–regulated targets (3) in the modulation of macrophage cytokine responses, and future studies will be important to define the range of pathways that might mediate such effects; candidate pathways include at least suppressor of cytokine signaling 1 (26).
Consistent with the foregoing discussion, we report here that miR-155 deficiency is protective against development of CIA. At a mechanistic level, reduced disease was associated with impaired production of proinflammatory cytokines, development of the autoreactive Th17 cells, production of anti-collagen II antibodies, and lack of articular inflammation. The generation of autoantibodies is prerequisite to the progression of CIA. B-cell–deficient mice are protected against the development of CIA (30). We have unraveled an unequivocal role for miR-155 in the generation of collagen II-specific IgGs. These data reflect a role for miR-155 in antibody class switching and B-cell differentiation into plasma cells, as was demonstrated recently (6, 28). However, it should be noted that miR-155 is dispensable for induction of arthritis following passive transfer of collagen-specific autoantibodies (Fig. S5). In line with this observation, we detected no role for miR-155 in regulating cytokine production in response to immune complexes in vitro; these data suggest that the effects of miR-155 in myeloid lineage activation may be somewhat stimulus dependent.
Disruption of Th17 pathway components (31, 32) and myeloid-cell activation (33) protects mice against several rodent models of arthritis. Our data suggest that miR-155 seems to be indispensable for the development of antigen-dependent induction of arthritis and subsequent cytokine-driven articular inflammation. Here we postulate that a modified cytokine milieu produced by myeloid cells may be one of the potential mechanisms responsible for impaired development of collagen-specific Th17 cells during arthritis in miR155-deficient mice, particularly given the potent stimulatory effect of miR-155 on human myeloid cells to produce IL-6 and IL-1β, whose involvement in Th17 differentiation is established (Fig. 1F). In line with this reasoning, we noted a decrease in systemic IL-6 concentrations in miR-155−/− mice. In agreement with our data, O'Connell et al. (34) recently reported that miR-155 plays a role in the development of Th17 cells during experimental autoimmune encephalomyelitis.
The role of IFNγ-producing Th1 cells in the development of CIA (1) is unclear, with net protective effects postulated on the basis of IFNγR−/− and IL-12p35−/− models (32, 35). Nevertheless, IFN-γ plays an important role in proinflammatory macrophage activation (36). miR-155−/− mice elaborated significantly less IFN-γ–producing cells than the WT mice during CIA. Serum levels of IL-2, a known Th1 expansion factor, were decreased in miR-155−/− mice and possibly could be responsible for an impaired differentiation of Th1 cells in miR-155–deficient mice. Although a relative increase in Th2 differentiation caused by v-maf musculoaponeurotic fibrosarcoma oncogene (c-maf) activation was established in miR-155–deficient mice under steady-state conditions (7), we did not observe any change in IL-4 production in our experimental model. This result could be explained by strong skewing properties of the adjuvant used in experimental arthritis, which promotes Th17/Th1 differentiation. Also, it has been suggested that miR-155 is required for homeostatic proliferation of regulatory T cells, and miR-155–deficient mice show reduced numbers of forkhead box P3-positive cells (27). However, as reported here and elsewhere (34), this deficit did not render miR-155−/− mice susceptible to the development of autoimmunity as occurs in CIA and experimental autoimmune encephalitis.
Consistent with a crucial role of miR-155 in the regulation of proinflammatory cytokine production by human synovial myeloid cells, miR-155−/− mice showed reduced expression of articular TNF-α, reduced levels of VEGF and chemokines [including CXCL1 (IL-8 homolog) and CXCL9, which play critical role in articular inflammation], neoangiogenesis of hyperplastic synovium, and recruitment of inflammatory cells into the joint cavity. The central role of cytokines in the effector biology of RA is shown unequivocally in the successful clinical application of biologic inhibitor agents (e.g, agents that neutralize TNF and IL-6 receptor).
The miRNA network is emerging as an important contributory factor in the pathophysiology of RA. The potential of a single miRNA species to modulate many distinct disease-regulatory pathways simultaneously renders miRNAs particularly attractive candidate targets. Therefore, a better understanding of the complex nature of miRNA regulatory interactions with multiple inflammatory pathways is bound to prove important for identifying future targets and developing therapeutic strategies. In this report, we identify a clinically relevant, functional miR-155/SHIP-1 pathway that may be responsible for some of the excessive inflammatory response observed in articular tissues in RA patients.
Materials and Methods
Cell Culture.
CD14+ cells from SF or PB of RA patients and CD14+ cells from PB of healthy control subjects were purified using CD14 MACS MicroBeads (Miltenyi Biotech). Cells were stimulated with LPS (100 ng/mL), CpG, CpG control (both 5 μg/mL), Pam3CSK4 (300 ng/mL), or poly I:C (50 μg/mL) (all from InvivoGen) for 24 h. Also, PB and SF CD14+ cells were transfected with either miR-155 mimic or miR-155 antagomir (each at 20 or 40 nM) or, as controls, with scrambled mimic and antagomir (20 or 40 nM) (Thermo Scientific Dharmacon), using the N-TER nanoparticle siRNA transfection system (Sigma). Cells and supernatant were collected after 48 h. LPS (100 ng/mL) was added to some cultures 24 h after transfection for a further 16 h of stimulation.
CIA.
CIA was induced in B6 WT or miR-155−/− male littermates at 14 wk of age according to a previously described method (21). Further information is given in SI Materials and Methods.
Note Added in Proof.
While this manuscript was in revision, Bluml et al. (37) showed similar resistance of miR-155–deficient mice to antigen-induced arthritis commensurate with our own observations.
Supplementary Material
Acknowledgments
We thank Shauna Kerr, Lynn Crawford, Majid Al Salmani, and Lisa Jolly for their technical assistance and Dr. Carl Goodyear for preparing immunocomplexes. This study was supported by a Career Development Grant from Arthritis Research United Kingdom (to M.K.-S.) and by grants from the Wellcome Trust, the Medical Research Council United Kingdom; and the NovoNordisk AMC/University of Glasgow Fellowship Programme. I.B.M. and S.G. were supported by FP7 Masterswitch.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019536108/-/DCSupplemental.
References
- 1.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 4.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Xiao C, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thai TH, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez A, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 9.Pauley KM, et al. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101–R111. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, et al. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R81–R93. doi: 10.1186/ar3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanczyk J, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 12.Nakasa T, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58:1284–1292. doi: 10.1002/art.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamachi Y, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60:1294–1304. doi: 10.1002/art.24475. [DOI] [PubMed] [Google Scholar]
- 14.Nagata Y, et al. Induction of apoptosis in the synovium of mice with autoantibody-mediated arthritis by the intraarticular injection of double-stranded MicroRNA-15a. Arthritis Rheum. 2009;60:2677–2683. doi: 10.1002/art.24762. [DOI] [PubMed] [Google Scholar]
- 15.O'Connell RM, et al. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ospelt C, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58:3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 17.Kelso EB, et al. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: Ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 2007;56:765–771. doi: 10.1002/art.22423. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell WR, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106:7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An H, et al. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood. 2005;105:4685–4692. doi: 10.1182/blood-2005-01-0191. [DOI] [PubMed] [Google Scholar]
- 21.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 22.Nandakumar KS, Holmdahl R. Collagen antibody induced arthritis. Methods Mol Med. 2007;136:215–223. doi: 10.1007/978-1-59745-402-5_16. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell RM, et al. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc Natl Acad Sci USA. 2010;107:14235–14240. doi: 10.1073/pnas.1009798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceppi M, et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci USA. 2009;106:2735–2740. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Androulidaki A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu LF, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vigorito E, et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung WH, Tarasenko T, Bolland S. Differential roles for the inositol phosphatase SHIP in the regulation of macrophages and lymphocytes. Immunol Res. 2009;43:243–251. doi: 10.1007/s12026-008-8078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell IK, et al. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- 34.O'Connell RM, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manoury-Schwartz B, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 36.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 37.Bluml S, et al. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthrit Rheum. 2011;63:1281–1288. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.