Abstract
Nitrogen bisphosphonates (NBPs) are commonly prescribed for osteoporosis but have also been found to induce inflammatory reactions and to delay the progression of breast cancer. The inflammatory and anticancer effects of the NBPs might be associated with an ability to modulate innate immune signaling. In mice, intraperitoneal NBP administration causes a rapid influx of neutrophils and monocytes that is dependent on the myeloid differentiation primary response gene 88 (MyD88) mediator of Toll-like receptor (TLR) and IL-1 signaling. Bone marrow chimeras demonstrate that this inflammatory response is partially dependent on TLR4 expression by hematopoietic cells and the IL-1 receptor on radioresistant cells. In vitro, NBPs directly stimulate neither murine bone marrow-derived mononuclear cells nor human peripheral blood mononuclear cells, but rather prime them to produce increased amounts of cytokines when exposed to IL-1 or TLR ligands. This potentiation is mediated by a reduction in IL-1 receptor-associated kinase-M, a negative regulator of MyD88-dependent signaling. In vivo, this property renders the NBPs as effective adjuvants that enhance both cellular and antibody responses to antigens.
Keywords: inflammation, vaccination, costimulation
The nitrogen-containing bisphosphonates (NBPs) alendronate (ALD), zoledronate (ZLD), pamidronate, risedronate, and ibandorate are clinically effective treatments for osteoporosis and cancer-related bone disorders. Bisphosphonates bind to the bone surface, where they prevent osteoclast-mediated bone degradation through inhibition of the mevalonate pathway, which leads to altered protein prenylation (1). Several types of inflammatory reactions have been observed in patients treated with NBPs, including flu-like symptoms, esophageal irritation, and osteonecrosis of the jaw (2). These side effects might be attributable to the immune-modulating effects of NBPs (3). Inhibition of the mevalonate pathway by NBPs causes isopentenyl-5-pyrophosphate accumulation in monocytes, augmenting γδ T-cell activation in vitro (4), but additional effects likely contribute to the in vivo inflammatory responses to NBPs (1). Pretreatment of mice with NBPs increases IL-1 production in response to bacterial LPS (5), a Toll-like receptor 4 (TLR4) ligand. In some murine models, the inflammatory properties of NBPs are dependent upon IL-1 signaling (6). However, the mechanisms of in vivo NBP immune modulation remain elusive, in large part because of the sporadic inflammatory effects of the drugs in humans.
Recently, NBPs were found to have direct anticancer activity in animal models (7), to extend disease-free survival (8), and potentially to decrease relapse rates (9) in breast cancer patients. Controlled clinical trials are underway to examine the efficacy of NBPs in cancer therapy (10). However, there is a lack of biological markers to guide the dosing of NBPs in these studies, because direct inhibition of tumor cells at physiologically relevant concentrations of NBPs has not been observed (11). The in vivo effects of NBPs on tumor–host interactions, including immune system modulation, have recently begun to be explored (9). The goal of the studies presented here was to elucidate how NBPs variably modulate immune cell activation by TLR ligands and IL-1. The results of our experiments indicate that the NBPs do not directly activate immune or proinflammatory cellular receptors, but rather prime both hematopoietic and other cells to low concentrations of TLR ligands and IL-1, by down-regulating the concentrations of interleukin receptor-associated kinase-M (IRAK-M).
Results
In Vivo Inflammatory Actions of NBPs Are Dependent on IL-1R, TLR4, and MyD88.
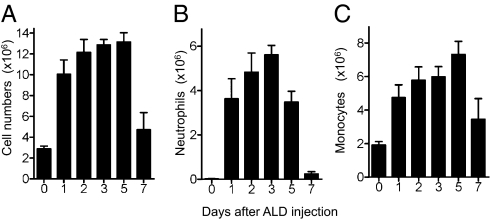
ALD is a widely used NBP that can induce inflammatory side effects and has been used in previous murine models (5, 6). Injection of ALD into the peritoneal cavity of WT mice caused a massive leukocyte infiltration that began within 24 h and persisted for up to 5 d (Fig. 1). The infiltrating leukocytes were primarily neutrophils and monocytes, with numbers peaking at 3 and 5 d, respectively (Fig. 1). Mice deficient in TLR4 or myeloid differentiation primary response gene 88 (MyD88) (but not TLR7 or TLR9) had reduced leukocyte recruitment in response to ALD (Tables 1 and 2), indicating that TLR4 and MyD88 signaling are involved in these effects. Notably, the neutrophil infiltration in Myd88−/− mice did not significantly increase with ALD treatment (Table 1), although it did in Tlr4−/−mice. These results indicated that MyD88 may contribute to NBP induced inflammation through another signaling pathway that depends on this adapter molecule, such as the IL-1 receptor (IL-1R) (5, 6). Indeed, Il1r−/− mice had significantly reduced neutrophil infiltration in response to ALD, although total leukocyte counts in the peritoneum were similar to those in WT mice (Table 1). Taken together, the results showed that TLR4/MyD88 signaling contributed to leukocyte recruitment, and IL-1R signaling was involved in neutrophil recruitment by ALD.
Fig. 1.
Time course of alendronate-induced peritonitis C57BL/6 mice were injected intraperitoneally with vehicle or 0.8 mg per mouse ALD. Six- to 8-wk-old female WT mice (n = 5 mice per group) were killed on the indicated days. Total number of peritoneal cells (A), neutrophils (B), and monocytes (C) were quantified. Data shown are means + SEM pooled from two independent experiments.
Table 1.
Alendronate-induced peritonitis is dependent upon IL-1R and MyD88
| PBS |
ALD |
|||||||
| Treatment Strain | N | Total cell (×106) | Neutrophils (×105) | Monocytes (×106) | N | Total cell (×106) | Neutrophils (×105) | Monocytes (×106) |
| WT | 10 | 2.62 ± 0.18 | 0.20 ± 0.05 | 1.96 ± 0.12 | 12 | 22.1 ± 3.8 | 89.3 ± 19.8 | 11.5 ± 1.9 |
| Tlr7−/− | 9 | 4.74 ± 0.65 | 0.58 ± 0.23 | 3.61 ± 0.53 | 9 | 17.9 ± 1.4 | 78.5 ± 9.4 | 9.2 ± 0.6 |
| Tlr9−/− | 9 | 3.06 ± 0.41 | 0.41 ± 0.2 | 2.38 ± 0.33 | 9 | 17.8 ± 2.6 | 78.6 ± 17.3 | 8.5 ± 1.1 |
| Myd88−/− | 6 | 1.98 ± 0.54 | 0.24 ± 0.14 | 1.48 ± 0.38 | 9 | 5.1 ± 1.3* | 1.4 ± 0.6* | 3.0 ± 0.7* |
| Il1r−/− | 6 | 3.59 ± 0.22 | 1.31 ± 0.15 | 2.65 ± 0.14 | 7 | 14.6 ± 2.3 | 24.4 ± 6.4* | 9.9 ± 1.7 |
Data shown are mean ± SEM pooled from two to three independent experiments. N: number of mice. Values in bold letters in ALD group are P < 0.02 compared with corresponding PBS group by Mann-Whitney test. *P < 0.001 compared with ALD treated WT group by one-way ANOVA with Dunnett's post hoc test.
Table 2.
Alendronate-induced peritonitis is dependent upon TLR4 signaling
| PBS |
ALD |
|||||||
| Treatment Strain | N | Total cell (×106) | Neutrophils (×105) | Monocytes (×106) | N | Total cell (×106) | Neutrophils (×105) | Monocytes (×106) |
| WT | 7 | 1.79 ± 0.18 | 0.14 ± 0.06 | 1.79 ± 0.18 | 8 | 7.3 ± 0.5 | 64.6 ± 6.8 | 7.3 ± 0.5 |
| Tlr4−/− | 7 | 0.75 ± 0.15 | 0.03 ± 0.02 | 0.75 ± 0.15 | 10 | 5.6 ± 0.6* | 27.3 ± 2.6* | 5.6 ± 0.6* |
| C3H HeOuJ | 5 | 4.08 ± 0.95 | 0.45 ± 0.15 | 3.21 ± 0.75 | 7 | 22.5 ± 2.2 | 109.3 ± 9.1 | 11.5 ± 1.2 |
| C3H HeJ | 5 | 4.04 ± 0.46 | 0.28 ± 0.18 | 2.98 ± 0.39 | 8 | 9.7 ± 2.6* | 23.1 ± 7.4* | 6.8 ± 1.8* |
Data shown are mean ± SEM pooled from two to three independent experiments. N: number of mice. Values in bold letters in ALD group are P < 0.005 compared with corresponding PBS group by Mann-Whitney test. *P < 0.001 compared with ALD treated WT group (WT vs. TLR4−/−, and C3H HeOuJ vs. C3H HeJ, respectively) by one-way ANOVA with Dunnett's post hoc test.
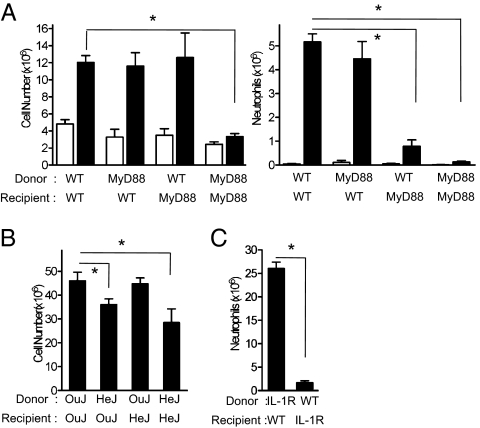
TLR4 in Hematopoietic Cells and IL-1R in Nonhematopoietic Cells Are Necessary for ALD-Induced Peritonitis.
Bone marrow chimera mice were produced to elucidate whether TLR4 and IL-1 in hematopoietic (radiosensitive) or nonhematopoietic (radioresistant) cells were involved in NBP-induced inflammation. In irradiated WT mice reconstituted with Myd88−/− bone marrow, ALD-induced peritoneal neutrophil infiltration was diminished; however, total leukocyte infiltration in response to ALD was not impaired (Fig. 2A). Conversely, there was no significant difference in neutrophil recruitment in WT mice reconstituted with Tlr4−/− bone marrow (neutrophils ×106 ± SEM: OuJ→OuJ = 22.1 ± 1.7 and HeJ→OuJ = 16.4 ± 1.6; P > 0.05 by one-way ANOVA with Dunnett's post hoc test), although total cell accumulation was significantly inhibited (Fig. 2B). These results indicate that TLR4 on radio-sensitive bone marrow-derived cells is critical for monocyte infiltration into the peritoneum. The lack of attenuation in monocyte infiltration in WT mice that received Myd88−/− bone marrow may be because of compensation by TIR-domain–containing adapter-inducing IFN-β, which can transduce TLR4 signaling in the absence of MyD88 (12). In contrast, IL-1R expressed by radioresistant cells is largely responsible for neutrophil infiltration in response to intraperitoneal NBP administration (Fig. 2C).
Fig. 2.
ALD-induced peritonitis in chimeras is codependent on IL-1R/MyD88 in radioresistant tissue and TLR4 in radiosensitive tissue. The chimera mice, MyD88/WT (A), C3H HeJ/HeOuJ (B), and IL-1R/WT (C) were injected intraperitoneally with vehicle (WT→WT n = 5, MyD88→WT n = 6, WT→MyD88 n = 6, and MyD88→MyD88 n = 4) or ALD (WT→WT n = 6, MyD88→WT n = 9, WT→MyD88 n = 11, MyD88→MyD88 n = 5, OuJ→OuJ n = 10, HeJ→HeJ n = 6, OuJ→HeJ n = 7, HeJ→OuJ n = 9, IL-1R→WT n = 5, and WT→IL-1R n = 8). The peritoneal cells were harvested, counted, and neutrophils were quantified 3 d after ALD injection. Data are means + SEM pooled from at least two independent experiments. *P < 0.05 by one-way ANOVA with Dunnett's post hoc test (A and B) or Mann-Whitney test (C).
Effects of NBPs on TLR and IL-1 Signaling.
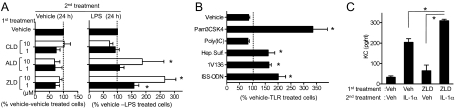
In purified populations of mouse bone marrow-derived macrophages (BMDM), treatment with NBPs alone did not induce the production of IL-6 (Fig. 3A). However, BMDM that were preincubated with NBPs, but not with clodronate (CLD, a noninflammatory nonnitrogen bisphosphonate), had enhanced cytokine production in response to LPS, a TLR4 ligand (Fig. 3A), and multiple other TLR activators (Fig. 3B). ZLD was the most potent compound among the NBPs tested (Fig. 3A). Because Tlr4−/− mice had attenuated cell infiltration compared with WT mice, whereas NBPs were not directly immunostimulatory to BMDM in vitro, we reasoned that NBPs might sensitize hematopoietic cells to low concentrations of TLR4 ligands derived from endogenous tissues or from gut bacteria, which are ubiquitously present in the peritoneal cavity (13). Supporting this contention, NBPs sensitized BMDMs not only to LPS, but also to heparan sulfate, an endogenous TLR4 ligand derived from extracellular matrix (Fig. 3B).
Fig. 3.
Nitrogen bisphosphonates synergize with MyD88-dependent signaling. (A) BMDM were pretreated (first treatment) for 24 h with vehicle or the indicated doses of CLD, ALD, or ZLD. The cells were then treated for an additional 24 h with vehicle or 5 ng/mL LPS (second treatment). The supernatants were assayed for IL-6. The values were normalized to the levels in the vehicle-vehicle treated cells (46.3 ± 26.3 pg/mL) (Left) and the vehicle-LPS treated cells (1,055 ± 384 pg/mL) (Right). (B) BMDM pretreated with 10 μM ZLD for 24 h, then treated with the indicated TLR ligands [100 ng/mL Pam3CSK4 (TLR2), 25 μg/mL poly I:C (TLR3), 100 μg/mL heparin sulfate (Hep. Sulf., TLR4.), 1 μM 1V136 (TLR7), or 50 μg/mL immunostimulatory oligonucleotide (ISS-ODN) (TLR9)] for an additional 24 h. IL-6 secretion in the media was quantified by ELISA. The values were normalized to the percent of IL-6 produced by cell pretreated with vehicle and then each TLR ligand, respectively (vehicle; 23.0 ± 13.9 pg/mL, Pam3CSK4; 979 ± 356 pg/mL, 1V136;291 ± 72.7 pg/mL, ISS-ODN;144 ± 38.7 pg/mL, poly I:C;56.3 ± 17.3 pg/mL). Experiments were performed in triplicates in A and B. Data shown are mean ± SEM of three to four independent experiments. *P < 0.05 by one-way ANOVA with Dunnett's test compared with vehicle-treated cells. (C) KC secretion by primary murine peritoneal mesothelial cells pretreated with vehicle or 10 μM ZLD followed by 10 pg/mL murine IL-1α for an additional 24 h. Data shown are mean ± SEM pooled from two independent experiments. *P < 0.05 with Mann-Whitney test.
Although NBP pretreatment of BMDMs primed the cells for increased cytokine production in response to TLRs that signal via MyD88, the drugs did not significantly alter TLR3-induced cytokine release (Fig. 3B). These data suggested that NBP synergy was related to alterations in MyD88 signaling because TLR2, -4, -7, and -9, but not TLR3, are dependent upon this adapter protein (12). IL-1R also requires MyD88 activity for inflammatory signal transduction. Accordingly, primary mesothelial cells [IL-1–sensitive peritoneal cells (14)] primed with ZLD were sensitized to low concentrations of IL-1α (Fig. 3C). Taken together, the in vitro and in vivo results indicated that MyD88 signaling is modulated by NBPs.
Suppression of IRAK-M Levels by NBPs.
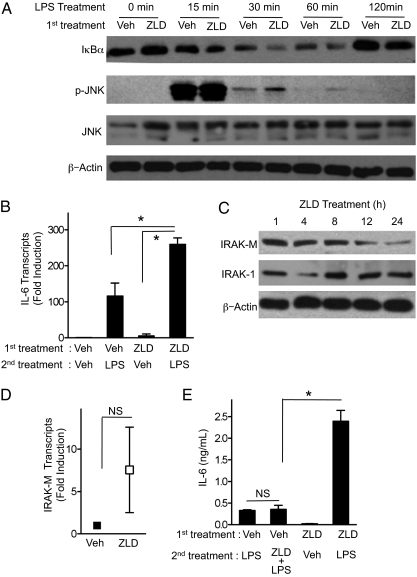
Activation of either TLR/MyD88 or IL-1/MyD88 receptor complexes stimulate the activity of the IRAKs, with subsequent induction of NF-κB–dependent genes, including cytokines, chemokines, and cell-surface receptors (15). Indeed, ZLD pretreatment sensitized BMDM to subsequent LPS treatment with an increase in IκBα degradation (a surrogate for NF-κB activation), an increase in JNK activation, and an increase in IL-6 mRNA transcription compared with control cells (Fig. 4 A and B). However, some NF-κB–induced genes encode proximal inhibitors of IRAK activity, which serve to restrain the IRAK immunostimulatory pathway. Prominent among the inducible inhibitors is IRAK-M, which interferes with IRAK-1 and IRAK-4 activation after TLR or IL-1R stimulation (16). Incubation of BMDM with ZLD caused a time-dependent reduction in IRAK-M levels, without altering IRAK-1 expression (Fig. 4C). The IRAK-M mRNA levels were not decreased by NBP treatment (Fig. 4D), indicating a posttranscriptional mechanism. The gradual decrease in IRAK-M over time was consistent with lack of synergy observed when BMDM were treated with a TLR ligand and NBP at the same time (Fig. 4E).
Fig. 4.
ZLD potentiates LPS stimulation by reducing protein levels of IRAK-M. (A) BMDM were pretreated (first treatment) with 10 μM ZLD or vehicle for 24 h and then treated for the indicated times with 5 ng/mL LPS. Cell lysates were immunoblotted with the indicated antibodies. (B) IL-6 transcript levels were assayed by quantitative PCR from BMDM that were pretreated 10 μM ZLD or vehicle for 24 h and then treated for 4 h with 5 ng/mL LPS. *P < 0.05 by Mann-Whitney test. (C) BMDM were treated with 10 μM ZLD or vehicle then harvested at the indicated times. The lysates were immunoblotted for IRAK-M, IRAK-1, and β-actin. (D) IRAK-M transcripts in BMDM were assessed by quantitative PCR 24 h after treatment with vehicle or 10 μM ZLD. There was no significant difference (NS) between the means + SEM shown by Mann-Whitney test. (E) BMDM were pretreated with 10 μM ZLD or vehicle for 24 h. The cells were then treated for an additional 24 h with vehicle (Veh), 5 ng/mL LPS, or 5 ng/mL LPS plus 10 μM ZLD. *P < 0.05 by ANOVA with Bonferroni post hoc testing. For B, D, and E, data are means ± SEM from three independent experiments.
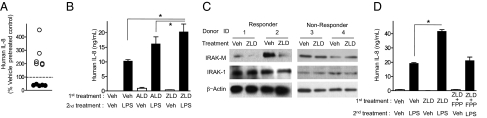
IRAK-M levels are normally very low in unmanipulated cells, unless recently stimulated with a TLR ligand or with IL-1 (16). Therefore, the cellular response to IRAK-M modulation would be expected to vary among individuals, with variable exposure to immune stimuli in the environment. Consistent with this expectation, pretreatment of human peripheral blood mononuclear cells (PBMCs) with NBP increased cytokine response to LPS in approximately half of random blood donors, but did not in the other half (Fig. 5 A and B). Similarly, IRAK-M levels were reduced by NBP treatment in the responsive PBMCs, but not in cells from the nonresponsive donors (Fig. 5C). NBPs are established inhibitors of protein prenylation because they inhibit the synthesis of farnesyl pyrophosphate (FPP) (1). In the NBP-responsive PBMC populations, addition of high concentrations of FPP, together with ZLD, abrogated the priming effect of the NBP (Fig. 5D). These results are consistent with the assumption that protein prenylation inhibition plays a role in NBP-dependent sensitization of immune cells to MyD88 signaling.
Fig. 5.
ZLD reduces IRAK-M in human PBMCs and enhances LPS induced responses. (A) Human PBMC from 11 independent donors were pretreated (first treatment) with 10 μM ZLD and then 100 pg/mL LPS for an additional 24 h. The supernatants were tested for IL-8 by ELISA. The values were normalized by the value of cells treated with vehicle and then treated with LPS (9.19 ± 2.08 ng/mL). Nonresponders are shown in black and dashed line represents 100%. (B) Triplicate wells of PBMC from a single responsive donor were pretreated with vehicle, 10 μM ALD, or 10 μM ZLD for 24 h and then treated with vehicle or 1 ng/mL LPS for 24 h. IL-8 in the supernatant was measured by ELISA. *P < 0.05 by one-way ANOVA with Dunnett's post hoc test. (C) Responder PBMCs (donors 1 and 2) and nonresponder PBMCs (donors 3 and 4) were treated with 10 μM ZLD or vehicle for 24 h. The lysates were immunoblotted and probed for IRAK-M, IRAK-1, and β-actin, as shown. (D) PBMCs from a representative responsive donor were pretreated with vehicle (Veh), 10 μM ZLD, or 10 μM ZLD and 80 μg/mL farnesyl pyrophosphate (ZLD+FPP) for 24 h, then treated with 1 ng/mL LPS or vehicle for 24 h in triplicate. *P < 0.05 by one-way ANOVA with Dunnetts post hoc test.
ZLD Enhances the Adaptive Immune Response to Antigens.
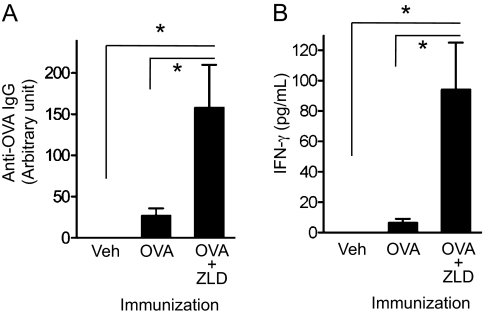
The NF-κB dependent activation of antigen-presenting cells is an essential element in the induction of immune responses following immunization. A drug that enhances or prolongs the activation might therefore function as an adjuvant. To test this possibility, 10 μg ZLD was injected subcutaneously along with a model antigen (30 μg ovalbumin, OVA) into mice and repeated 1 wk later. By the sixth week after the initial injection, the amounts of anti-OVA IgG antibodies were significantly higher in mice that received ZLD as an adjuvant, compared with the animals that received antigen alone (Fig. 6A). To determine if the NBP also promoted cellular immune responses, the vaccinated animals were euthanized at 6 wk and the isolated splenocytes were restimulated in vitro with antigen. The release of IFN-γ in response to OVA rechallenge was significantly higher in mice that received ZLD as an adjuvant compared with animals that received antigen without this NBP (Fig. 6B). There were no overt signs of inflammation at the ZLD subcutaneous injection sites, in contrast to the inflammatory effects of NBPs in the peritoneal cavity.
Fig. 6.
ZLD potentiates adaptive immunity as an adjuvant. WT mice (n = 8–9 per group) were immunized twice, 1 wk apart, subcutaneously with vehicle (Veh), 30 μg OVA, or OVA plus 10 μg ZLD (OVA+ZLD). (A) Total serum anti-OVA IgG 6 wk after the initial vaccination was measured by ELISA. (B) Antigen-specific IFN-γ release by splenocytes. The splenocytes were restimulated with OVA (100 μg/mL) in vitro for 3 d. Data shown are pooled from two independent experiments. *P < 0.05 by one-way ANOVA with Dunnett's post hoc test.
Discussion
The results of these experiments indicate that NBPs do not have direct immune stimulatory or proinflammatory activity. Rather, preincubation with NBPs augments the activity of TLR ligands, IL-1, and exogenous antigens. The priming effects of NBPs require the adapter protein MyD88, and are primarily exerted on both radiation-sensitive bone marrow-derived cells shown in vitro and radiation-resistant mesothelial cells (or similar). The ability of NBPs to sensitize bone marrow-derived cells to MyD88 signaling requires several hours of exposure to the drugs. During this time, the intracellular level of IRAK-M, an inhibitor of TLR and IL-1 signaling, decreases progressively.
The NBPs are established inhibitors of the mevalonate biosynthetic pathway, which controls the formation of FPP, and the prenylation of multiple proteins (1). Inhibition of prenylation most likely is required for the immune stimulatory effects of NBPs, as the addition of exogenous FPP to cultured mononuclear leukocytes abrogates the increase in cytokine production caused by NBP pretreatment. Moreover, CLD, which is not an FPP inhibitor, did not enhance the inflammatory effects of TLR ligands or IL-1. Although statins can also block the mevalonate pathway, these agents do not share the proinflammatory effects of NBPs in humans. Thus, it is likely that additional effects of NBPs, besides inhibition of prenylation, mediate their immune stimulatory actions. In this regard, bisphosphonates have been reported to inhibit various cellular phosphatases, such as protein tyrosine phosphatase 1B, which negatively regulate the kinase cascades necessary for the activation of immune cells (17).
Activating ligands for TLRs include both exogenous pathogen-associated molecular patterns and endogenous damage-associated molecular patterns. The association of NBP therapy with occasional osteonecrosis of the jaw, following dental procedures, may be explained by the interaction of the drugs with cells that are subsequently exposed to pathogen-associated molecular patterns derived from oral bacteria (18). Similarly, NBP-induced peritonitis in mice may be attributable to potentiation of TLR4 activation by low levels of endotoxins released by gut bacteria. Consistent with this notion, NBP injection into subcutaneous tissues of the mouse did not induce detectable inflammation.
Exactly how antigens in a vaccine without adjuvant stimulate dendritic and other antigen-presenting cells is not clear. Uptake of the exogenous antigens may activate the inflammasome, leading to small amounts of IL-1 production, and also may promote the release of TLR activating damage-associated molecular patterns. The ability of NBPs to enhance both TLR and IL-1 signaling would promote the activation of antigen-presenting cells by either mechanism.
Alternatively, the adjuvant activity of NBPs could be through its effects on IRAK-M. The synthesis of the IRAK-M molecule is normally induced through the NF-κB pathway (16). The accumulation of IRAK-M progressively inhibits IRAK enzyme activity, thus restraining excessive NF-κB activation via a feedback-inhibition process (16). Recent results have demonstrated that a reduction of IRAK-M synthesis with siRNA significantly strengthens the cellular immune response to tumor antigens (19). The adjuvant effects of NBPs, documented in the present studies, are thus consistent with the siRNA experiments.
The exposure of macrophages, dendritic cells, and other cell types to some TLR activators leads to a refractory state called TLR tolerance (20). The persistent elevation of IRAK-M levels, as well as increases in the levels of suppressors of cytokine signaling proteins and various phosphatases, have all been implicated in the TLR tolerance phenomenon (20). The role of classic TLR tolerance in the immune response to vaccination is not clear. However, the fact that NBPs both down-regulate IRAK-M, and potentiate the cellular and humoral immune responses to exogenous antigens, points to a potential relationship between TLR tolerance and efficient antigen presentation, which might be exploited in the design of improved vaccines against poorly immunogenic antigens. In addition, the down-regulation of IRAK-M by NBPs in tumor-associated immune cells may allow for tumor recognition and contribute to the anticancer affects of NBPs observed in vivo. To the best of our knowledge, the NBPs are the only clinically used agents that can inhibit IRAK-M accumulation in antigen-presenting cells without altering IRAK or other stimulatory components of the NF-κB pathway. Moreover, the subcutaneous or intramuscular administration of NBPs during vaccination should achieve the concentrations that are necessary to inhibit IRAK-M locally, without causing systemic inflammation. As such, the NBPs may represent a unique and easily deployable adjuvant for human studies.
Materials and Methods
Detailed protocols are given in SI Materials and Methods.
Mice.
C57BL/6 were purchased from Charles River Laboratory. C3H/HeJ, C3H/HeOuJ, and Il1r1−/− mice were obtained from The Jackson Laboratories. Tlr4−/−, Tlr7−/−, Tlr9−/−, and Myd88−/− mice were gifts from S. Akira (Osaka University, Japan) and were maintained on a C57BL/6 background at the University of California, San Diego. All animal protocols received prior approval by the Institutional Animal Care and Use Committee at the University of California at San Diego.
Reagents.
ZLD (AK Scientific), ALD, and CLD (Sigma) were endotoxin-free. In vitro studies were performed with LPS (Sigma), heparan sulfate (Sigma), IL-1α (eBioscience), TLR7 ligand (1V136), Pam3CSK4 (Invivogen), poly(I:C) (Invivogen) and single-stranded phosphorothioate immunostimulatory oligonucleotide (Tri-Link Biotechnology).
Statistics.
The statistical differences were analyzed by Mann-Whitney tests, one-way ANOVA with Dunnett's or Bonferroni post hoc tests, as indicated, using Prism software (version 5.0, GraphPad Software, Inc.). A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank S. Yao, C. Gray, L. Ronacher, and R. Mathewson for technical assistance. This work was supported by National Cancer Institute Grant T32CA121938, the Arthritis Foundation, Grant CA23100 from the National Institutes of Health/National Cancer Institute, and Grant HHSN272200900034C from the Division of Extramural Activities/National Institutes of Health/National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107899108/-/DCSupplemental.
References
- 1.Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 2.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 3.Lesclous P, et al. Bisphosphonate-associated osteonecrosis of the jaw: A key role of inflammation? Bone. 2009;45:843–852. doi: 10.1016/j.bone.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Thompson K, et al. Activation of gammadelta T cells by bisphosphonates. Adv Exp Med Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Motegi K, Iwakura Y, Endo Y. Involvement of interleukin-1 in the inflammatory actions of aminobisphosphonates in mice. Br J Pharmacol. 2000;130:1646–1654. doi: 10.1038/sj.bjp.0703460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng X, et al. Mutual augmentation of the induction of the histamine-forming enzyme, histidine decarboxylase, between alendronate and immuno-stimulants (IL-1, TNF, and LPS), and its prevention by clodronate. Toxicol Appl Pharmacol. 2006;213:64–73. doi: 10.1016/j.taap.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Gnant M, et al. ABCSG-12 Trial Investigators. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 8.Rack B, et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30:1807–1813. [PubMed] [Google Scholar]
- 9.Clézardin P. Bisphosphonates’ antitumor activity: An unravelled side of a multifaceted drug class. Bone. 2011;48:71–79. doi: 10.1016/j.bone.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Gnant M. Bisphosphonates in the prevention of disease recurrence: Current results and ongoing trials. Curr Cancer Drug Targets. 2009;9:824–833. doi: 10.2174/156800909789760267. [DOI] [PubMed] [Google Scholar]
- 11.Brown HK, Holen I. Anti-tumour effects of bisphosphonates—What have we learned from in vivo models? Curr Cancer Drug Targets. 2009;9:807–823. doi: 10.2174/156800909789760339. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Mohan C. Toll-like receptor signaling pathways—Therapeutic opportunities. Mediators Inflamm. 2010 doi: 10.1155/2010/781235. 10.1155/2010/781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. FASEB J. 2005;19:872–874. doi: 10.1096/fj.04-3211fje. [DOI] [PubMed] [Google Scholar]
- 14.Eigenbrod T, Park JH, Harder J, Iwakura Y, Núñez G. Cutting edge: Critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: Critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–1991. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard LL, Moore BB. IRAK-M regulation and function in host defense and immune homeostasis. Infect Dis Rep. 2010;2:pii, e9. doi: 10.4081/idr.2010.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoncic PD, McGlade CJ, Tremblay ML. PTP1B and TC-PTP: Novel roles in immune-cell signaling. Can J Physiol Pharmacol. 2006;84:667–675. doi: 10.1139/y06-012. [DOI] [PubMed] [Google Scholar]
- 18.Kumar SK, et al. The role of microbial biofilms in osteonecrosis of the jaw associated with bisphosphonate therapy. Curr Osteoporos Rep. 2010;8:40–48. doi: 10.1007/s11914-010-0008-1. [DOI] [PubMed] [Google Scholar]
- 19.Turnis ME, et al. IRAK-M removal counteracts dendritic cell vaccine deficits in migration and longevity. J Immunol. 2010;185:4223–4232. doi: 10.4049/jimmunol.0903507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.