Abstract
In many phototrophic microorganisms and plants, chloroplasts change their positions relative to the incident light to achieve optimal photosynthesis. In the case of motile green algae, cells change their swimming direction by switching between positive and negative phototaxis, i.e., swimming toward or away from the light source, depending on environmental and internal conditions. However, little is known about the molecular signals that determine the phototactic direction. Using the green alga Chlamydomonas reinhardtii, we found that cellular reduction-oxidation (redox) poise plays a key role: Cells always exhibited positive phototaxis after treatment with reactive oxygen species (ROS) and always displayed negative phototaxis after treatment with ROS quenchers. The redox-dependent switching of the sign of phototaxis may contribute in turn to the maintenance of cellular redox homeostasis.
Phototaxis is a behavior in which organisms move toward or away from directional light (“positive” or “negative” phototaxis, respectively). For motile phototrophic organisms such as green algae, switching between positive and negative phototaxis is crucial for maintaining the optimal light exposure for photosynthesis: They need to harvest light energy, but when light intensity is far above the saturation point of photosynthesis, they suffer from high-light stress (1). Thus, the direction or “sign” of phototaxis should be tightly controlled. The biflagellate unicellular green alga Chlamydomonas reinhardtii provides useful advantages for the study of the phototactic sign. Cells quickly change their swimming direction following light perception by channelrhodopsin (2). Many phototaxis-deficient mutants have been isolated, including a strain that exhibits only negative phototaxis [agg1 (phototactic aggregation), as discussed below].
Several extracellular factors have been shown to affect the sign of phototaxis in Chlamydomonas (3). Most importantly, light intensity controls the sign, which is positive under low light and negative under high light (4). Extracellular calcium concentration also affects the sign, an effect that is consistent with the calcium-dependent regulation of the beating balance between the two flagella (5, 6). However, even under light and medium conditions that are known to change the phototactic sign, there almost always is a population of cells that shows phototaxis of the opposite sign. In addition, after prolonged light stimulation, cells exhibit adaptation and become less sensitive to the light signal. Thus, the effect of the extracellular factors must vary depending on the internal condition of each cell. Factors that have been thought to affect the internal conditions include circadian rhythm and photosynthetic activity (7–9). Circadian rhythm dynamically affects the magnitude of phototaxis, but whether it also affects the sign of phototaxis is not known (7). In contrast, photosynthetic activity clearly affects the sign: Cells of a certain wild-type Chlamydomonas that show negative phototaxis under normal light conditions show positive phototaxis when treated with an inhibitor of photosynthesis, 3-(3′,4′-dichlorophenyl)-l,l-dimethylurea (DCMU) (8). The molecular mechanism underlying this phenomenon is unknown.
Eukaryotic cells possess multiple systems for maintaining the cytoplasm in a moderately reduced state called “reduction-oxidation (redox) homeostasis” (10). Because photosynthesis is known to change the cytoplasmic redox poise greatly (11, 12), we surmised that redox balance might play a role in switching the sign of phototaxis. To test this hypothesis, we examined phototaxis in Chlamydomonas after artificially changing the cellular redox poise. We report here that the sign of phototaxis is, in fact, determined by the cellular redox poise.
Results
To change the Chlamydomonas cellular redox poise, we used two groups of membrane-permeable redox reagents: reactive oxygen species (ROS), which cause oxidative stress (group 1), and reagents that quench ROS and reduce oxidative stress (group 2). We used H2O2, tertiary butyl hydroperoxide (t-BOOH), and Neutral Red as group 1 and 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine 1-oxyl (TEMPOL), dimethylthiourea (DMTU), and l-histidine as group 2. Cells were treated with these reagents at concentrations sufficient to cause oxidative stress (group 1) (13) or reductive stress (group 2) (14). Following reagent treatment, phototactic behavior was observed by positioning a green light-emitting diode (LED) (λ = 525 nm, ∼50 μmol photons m−2 s−1) adjacent to one side of the culture dish.
We observed both a wild-type and a negatively phototactic strain, agg1 (15). First, to observe the net movement of cells, cell suspensions were illuminated with directional green light. Wild-type cells showed positive phototaxis, whereas agg1 cells exhibited negative phototaxis (Fig. 1). However, after treatment with an ROS (H2O2), cells of both strains came to display positive phototaxis. Conversely, both strains came to show negative phototaxis after treatment with an ROS quencher (TEMPOL) (Fig. 1).
Fig. 1.
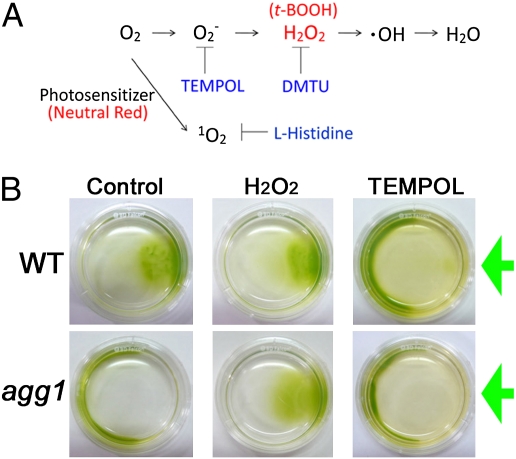
The redox reagents changed the Chlamydomonas swimming directions. (A) Typical ROS generated from oxygen and ROS-quenching reagents. Various ROS are produced by partial reduction of oxygen: a superoxide anion (O2−), H2O2, and a hydroxyl radical (·OH). t-BOOH has an effect similar to that of H2O2. Singlet oxygen, 1O2, is generated by photosensitizers such as chlorophylls in vivo. Membrane-permeable ROS and ROS quenchers used in this study are shown in red and blue, respectively. (B) Dish phototaxis assay. Cell suspensions, with or without redox reagents, were photographed after illumination from the right side for 15 min (green arrows). Without redox reagents, wild-type and agg1 cells showed positive and negative phototaxis, respectively. Both strains exhibited positive phototaxis after treatment with 25 μM H2O2, and both showed negative phototaxis after treatment with 50 mM TEMPOL.
We next examined whether individual cells orient themselves to the direction of light, to ensure that the photo-accumulation was brought about by the cells’ phototaxis rather than by a photophobic response. For this purpose, we measured the angle (θ) between individual cell's swimming direction and the light direction 15 s after the onset of stimulation with directional light (Fig. 2A). After treatment with two kinds of ROS, both wild-type and agg1 cells exhibited positive phototaxis; in contrast, after treatment with the two kinds of ROS quenchers, both strains exhibited negative phototaxis (Fig. 2B and Movies S1, S2, S3, and S4). The threshold concentrations of the ROS and ROS quenchers needed for the sign switching varied slightly from one experiment to another, presumably depending on the culture conditions. However, the difference in the threshold concentrations was always within ∼50% of the concentrations indicated in Fig. 2B. Under all conditions, cells of oda1 (outer dynein arm), a mutant that lacks the redox-sensitive outer arm dynein (14, 16, 17), displayed almost the same flagellar beat frequency as the control cells (Table S1), indicating that the ROS or ROS quenchers did not cause a significant change in cellular ATP concentration. An inducer (Neutral Red) and a reducer (l-histidine) of singlet oxygen did not affect the sign of phototaxis (Fig. 2C) (18, 19). This result indicates that only specific types of ROS affect the sign of phototaxis (Discussion). For a quantification of the sign of phototaxis after different treatments, the average values of cosθ (Fig. 2 B and C) were measured as phototactic indexes for individual cells (Fig. 3). The phototactic indexes were reversed when the wild-type cells were treated with TEMPOL or DMTU and when the agg1 cells were treated with H2O2 or t-BOOH. These data suggest that the sign of phototaxis is regulated by certain kinds of ROS.
Fig. 2.
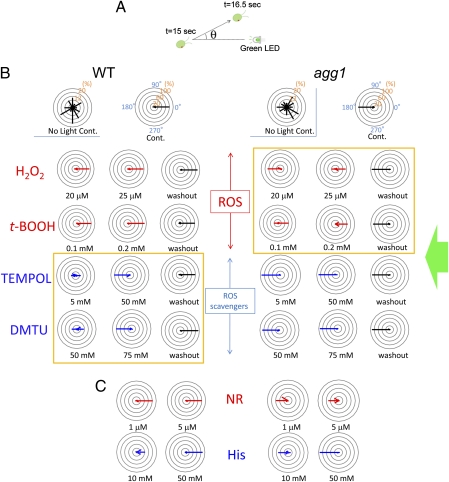
The effect of redox reagents on the phototactic sign was dose dependent and reversible. (A) Cell swimming direction was measured for 1.5 s following 15 s illumination with a green LED to avoid the effects of photophobic response occurring immediately after the onset of the LED illumination. (B and C) Polar histograms depicting the percentage of cells moving in a particular direction relative to light illuminated from the right, with or without treatment with redox reagents (12 bins of 30°; n = 50 cells per condition). Experiments were done at least three times per condition, and representative results are shown. (B) Both strains showed random swimming when not illuminated with a green LED. Wild-type cells exhibited positive phototaxis, and agg1 cells exhibited negative phototaxis without treatment with any reagents. Note that wild-type cells exhibited negative phototaxis after treatment with ROS scavengers, whereas agg1 cells showed positive phototaxis after treatment with ROS (histograms in yellow boxes). After washout of the reagents, each strain reverted to the same phototaxis direction as control cells, suggesting that the effects of the reagents are reversible. (C) Neutral Red (NR) and l-histidine (His), an inducer and a scavenger of singlet oxygen (a type of ROS), respectively, did not affect the sign of phototaxis.
Fig. 3.
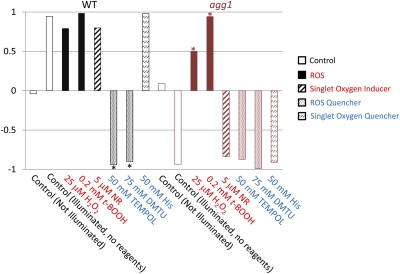
Phototactic indexes change significantly after treatment with certain types of redox reagents. The phototactic Index (± SD) was calculated as an average value of cosθ in Fig. 2 (n = 50). When cells are not illuminated and swim in random directions, the phototactic index should be ∼0. When 100% of cells show clear positive or negative phototaxis, the phototactic index is 1 or −1, respectively. Asterisks indicate a significant differences from control (illuminated, no reagents) conditions (P < 1.0 × 10−16, t test). His, l-histidine; NR. Neutral Red.
As mentioned earlier, a previous study has shown that the sign of phototaxis varies depending on the cell's photosynthetic activity. To determine whether the redox-dependent change in phototactic sign observed in this study depends on some change in photosynthetic activity, we examined dark-grown cells of y1a [a mutant lacking y1 (yellow when grown in the dark) gene], cells in which chlorophyll synthesis is blocked (20). As shown in Fig. 4, the dark-grown y1a cells showed the same change in the phototactic sign after treatment with the redox reagents as wild-type and agg1 cells. These findings suggest that ROS affected the sign of phototaxis without involving a change in the photosynthetic activity. Under control conditions, the dark-grown y1a cells showed strong negative phototaxis. Because y1a cells also showed strong negative phototaxis when the cells were grown under light and displayed photosynthesis (Fig. S1), it is likely that y1a carries a mutation that produces negative phototaxis.
Fig. 4.
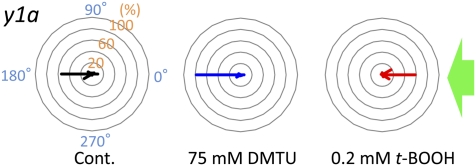
The redox reagents changed the phototactic sign of a chlorophyll-deficient mutant. The y1a strain has a defect in chlorophyll synthesis when grown in the dark. Dark-grown y1a cells showed strong negative phototaxis without treatment with redox reagents. Treatment of y1a cells with redox reagents changed the phototactic sign in the same manner as in agg1 cells. These data suggest that the phototactic sign was changed directly by the cytoplasmic redox poise, not by a change in photosynthetic activity.
What is the target of redox regulation in the phototaxis signaling processes? Several steps have been postulated in the phototaxis pathway: (i) photoreception of light at the eyespot; (ii) cation influx through channelrhodopsins and local depolarization of the plasma membrane; (iii) Ca2+ influx (>10−7 M) into the flagella via flagellar voltage-dependent Ca2+ channels; (iv) activation of some Ca2+-sensitive flagellar/axonemal components; and (v) change in the beating balance between the two flagella, which causes the phototactic turning (21). The fifth step may occur because the sensitivity to Ca2+ differs in the flagellum closer to the eyespot (the cis-flagellum) and the flagellum farther from the eyespot (the trans-flagellum). Previous studies showed that the cis-flagellar axoneme beats more strongly than the trans-axoneme when demembranated cells are reactivated with ATP at <10−8 M Ca2+, whereas the trans-axoneme beats more strongly than the cis-axoneme at 10−7 M to 10−6 M Ca2+ (6, 22). Because this dominance switching may well reflect the mechanism of phototactic turning, we examined whether it is affected by redox. However, we found that the redox reagents did not change the flagellar dominance in either wild-type or agg1 cells (Fig. S2). This result suggests that redox might affect some process or processes upstream of the flagellar dominance control (Discussion).
Discussion
We have demonstrated clear switching of the phototactic sign in Chlamydomonas with ROS and their quenchers. These data show that cytoplasmic redox poise that is affected by certain kinds of ROS (H2O2 and O2−) regulates the sign of phototaxis at both cell-population and single-cell levels. Previously, photosynthetic activity has been shown to affect the sign of phototaxis (8); however, how photosynthesis affects the sign was unclear. The results of experiments using various redox modulators and experiments using a photosynthesis-deficient strain suggest that the redox poise, which is affected by photosynthetic activity, changes the phototactic sign of Chlamydomonas. However, singlet oxygen did not affect the sign of phototaxis, although it is generated by photosynthesis (23). Singlet oxygen in plants has been shown to function not as a toxin but as a signal to up-regulate genes that induce stress responses (24). We surmise that only ROS that function as a toxin may affect the phototactic sign.
What redox-sensitive factor or cellular process switches the phototactic sign? Gene expression does not appear to be involved in the switching, because cells change the phototactic sign quite quickly (<1 min) after the treatment with ROS or ROS quenchers or after the washing out of these reagents. On the other hand, the cytoplasmic redox state may have widespread effects on cellular metabolism, although the ROS and ROS quenchers used in this study apparently did not change the cellular ATP level significantly as estimated from the motility of oda1 (Table S1). Thus, we cannot yet tell whether a redox signaling pathway changes the phototactic sign directly or changes it indirectly as a result of simultaneous redox-dependent reactions.
Nevertheless, we can ask whether the redox poise affects the phototactic sign by acting on flagella or on some process in the signal transduction pathway. If redox poise acts on the flagellar activity control, two previously reported proteins are worth considering as possible targets. First, a flavodoxin family protein, Agg3p, present in the flagellar membrane/matrix, has been suggested to be involved in the phototactic sign reversal (25). This possibility has arisen from the observation that Agg3p RNAi strains showed strong negative phototaxis, like the mutant agg1 used in this study. Flavodoxin acts as a reducer; that is, its oxidation is coupled with the reduction of other protein(s). If we assume that the oxidized state of Agg3p triggers the negative-to-positive switching of the phototactic sign, the behavior of the Agg3 RNAi strain is consistent with our finding that negative phototaxis occurs under reduced cytoplasm conditions. The second protein to consider is Agg2p, a membrane protein implicated in the regulation of the phototactic sign and localized at the base of flagella (25). Although we have shown that the properties of the Ca2+-dependent dominance change between the cis- and trans-flagella apparently are not influenced by the redox poise, some detergent-soluble components could regulate the flagellar dominance and phototaxis in a redox-sensitive manner. It is interesting that NAD- and NADP-dependent malate dehydrogenases are present in the flagellar membrane/matrix from the Chlamydomonas flagellar proteome database (26). Malate is a compound used indirectly to transport excess reducing equivalents from the chloroplast to the cytosol (27). Photosynthetic activity may change the redox poise of flagellar cytosol through the functions of those enzymes.
It also is possible, however, that the redox poise affects photoreception by modulating the eyespot or the subsequent signal transduction process that leads to a change in flagellar activity. As stated above, the phototactic behavior of a Chlamydomonas cell most likely is based on the turning of its swimming path, caused by a transient imbalance between the propulsive forces produced by the two flagella (28–30). Because the cell rotates around its long axis at a frequency of ∼2 Hz while swimming, the two flagella alternate their positions periodically. Thus, the turning direction should reverse if the onset of the transient beating imbalance advances or delays by ∼0.25 s. Such a delay or advance could be produced at any of the stages of signal transduction, which include photoreception, membrane depolarization, Ca2+ entry, and a change in the activity of the two flagella. A change in the photoreception step actually has been shown to cause a reversal of the steering direction (31). Thus, the photoreceptor may well be the target of the redox control. Detailed comparison of the movement and photoreception properties between positively phototactic cells (28) and negatively phototactic cells after treatment with redox reagents may provide some clues to the mechanism of the reversal of phototactic sign.
In conclusion, we have shown that the sign of phototaxis in Chlamydomonas is switched by the cytoplasmic redox poise that is changed by certain kinds of ROS. This redox-controlled switching of phototaxis direction may help maintain the redox homeostasis in the cytoplasm (but not in the chloroplast) via the regulation of photosynthesis. When the cytoplasm is oxidized, cells migrate toward light and increase photosynthesis activity, and thus the cytoplasm is reduced. When the cytoplasm becomes overly reduced, cells switch to negative phototaxis, the photosynthesis decreases, and the cytoplasm becomes oxidized. Such regulation may function as a negative-feedback mechanism that maintains the moderately reduced state of the cytoplasm.
Materials and Methods
Cell Culture and Strains.
Chlamydomonas reinhardtii strains CC124 (nit1− (nitrate reductase), nit2−, agg1−, mt−(mating type)) (15), CC125 (nit1−, nit2−, mt+) CC1168 (y1−, mt+) (20), and CC2228 (oda1−, mt+) (16) were used and are referred to as “agg1,” “wild type,” “y1a,” and “oda1,” respectively. Cells were grown in tris-acetate phosphate medium (32) with aeration at 20 °C on a 12-h/12-h light/dark cycle. The y1a cells were grown in 24-h darkness.
Reagents.
DMTU, Neutral Red, Igepal CA-630, and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich. ATP, creatine kinase, and creatine phosphate were purchased from Roche Diagnostics. All other chemicals were purchased from Wako Pure Chemical Industries.
Phototaxis Assay.
The phototactic behaviors of Chlamydomonas cells were quantified by recording and analyzing their behavior under continuous unidirectional illumination using a green LED (λ = 25 nm, ∼50 μmol photons m−2 s−1). The photon flux density was measured with an LI-1000 Data Logger (LI-COR Inc.). The wavelength of the light source was selected to be close to the maximum absorption wavelength (500 nm) of Chlamydomonas channelrhodopsin 1, the photoreceptor functioning in phototaxis (33–35). Cells were washed with an experimental solution [5 mM Hepes (pH 7.4), 0.2 mM EGTA, 1 mM KCl, 0.3 mM CaCl2] (22), shaken gently under white light for 1 h, and kept under dim red light for 15 min before the phototaxis assays.
For dish assays, cell suspensions (∼107 cells/mL) were put in petri dishes (30 mm in diameter, 10 mm thick), illuminated with a green LED from one side for 15 min, and photographed (DMC-LX2; Panasonic).
For single-cell analysis, petri dishes were placed on the stage of an inverted microscope (IX70; Olympus) and illuminated with a green LED. The behavior of the cells was observed with dim red light (λ >600 nm, ∼5 μmol photons m−2 s−1) and was video recorded using a CCD camera (XC-75; Sony). The angle (θ) between the light direction and the swimming direction was measured during 1.5 s following illumination with a green LED for 15 s. Images of swimming cells were auto-tracked using Image Hyper software (Science Eye). The angles were calculated from the cell trajectories.
Beat Frequency Analysis.
Flagellar beat frequency was determined from the power spectra of cell body vibration signals in microscope images of a population of cells (36). Median frequency was obtained from the power spectra averaged for 20 s.
Reactivation of Demembranated Cell Models.
Cell models were prepared and analyzed by the method of Kamiya and Witman (6), with modifications. Calcium buffers for reactivation were prepared according to Wakabayashi et al. (37), with modifications. In brief, after cells were washed with a buffer containing 10 mM Hepes (pH 7.4) and 4% sucrose, cells were demembranated with HMKP buffer [30 mM Hepes, 5 mM MgSO4, 50 mM K-acetate, 1% polyethylene glycol (Mr 20,000)] containing 0.1% Igepal CA-630. Cell models were reactivated in four different reactivation buffers with either Ca2+-free or Ca2+ buffer and with either reducing or oxidizing buffer. All buffers were made in the HMKP buffer containing 1 mM ATP, 70 U/mL creatine kinase, and 5 mM creatine phosphate. To change the concentration of free Ca2+, either 1 mM EGTA or 5.2 mM EGTA plus 3.2 mM CaCl2 [which results in a final free-Ca2+ concentration of ∼3.9 × 10−8 M (∼pCa7.4)] was added. These buffers are referred to as “Ca2+-free buffer” and “+Ca2+ buffer,” respectively. To change the redox state, either 1 mM DTT or 10 μM DTNB (17) was added. These buffers are referred to as “reducing buffer” and “oxidizing buffer,” respectively. Cell models reactivated in either buffer solution were observed under a dark-field microscope. Cells that were rotating in small areas by predominantly beating one of the two flagella were chosen, and the dominance of the cis- or trans-flagellum (the flagellum closer to the eyespot or farther from the eyespot) over the other was determined by visual inspection (6).
Supplementary Material
Acknowledgments
We thank Benjamin D. Engel for his critical reading of the manuscript, Takahiro Ide and Yuta Mitani for assistance with the phototaxis assays, and Drs. Ichiro Terashima and Ko Noguchi for the measurement of photosynthetic activity of Chlamydomonas cells and for helpful discussions. This work was supported by grants from the Japan Society for Promotion of Sciences and the Asahi Glass Foundation (to K.W.) and a by grant from the Ministry of Education, Culture, Sports, Science and Technology (to K.W. and R.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100592108/-/DCSupplemental.
References
- 1.Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- 2.Nultsch W. The photocontrol of movement of Chlamydomonas. Symp Soc Exp Biol. 1983;36:521–539. [PubMed] [Google Scholar]
- 3.Hegemann P, Berthold P. In: The Chlamydomonas Sourcebook. 2nd Ed. Witman GB, editor. Vol. 3. San Diego: Academic; 2009. pp. 395–430. [Google Scholar]
- 4.Feinleib MEH, Curry GM. The relationship between stimulus intensity and oriented phototactic response (topotaxis) in Chlamydomonas. Physiol Plant. 1971;25:346–352. [Google Scholar]
- 5.Morel-Laurens N. Calcium control of phototactic orientation in Chlamydomonas reinhardtii: Sign and strength of response. Photochem Photobiol. 1987;45:119–128. doi: 10.1111/j.1751-1097.1987.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984;98:97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo T, Johnson CH, Hastings JW. Action spectrum for resetting the circadian phototaxis rhythm in the CW15 strain of Chlamydomonas 1: Cells in darkness. Plant Physiol. 1991;95:197–205. doi: 10.1104/pp.95.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T, Watanabe M. Photosynthesis modulates the sign of phototaxis of wild-type Chlamydomonas reinhardtii. Effects of red background illumination and 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea. FEBS Lett. 1993;336:516–520. doi: 10.1016/0014-5793(93)80867-t. [DOI] [PubMed] [Google Scholar]
- 9.Boonyareth M, Saranak J, Pinthong D, Sanvarinda Y, Foster KW. Roles of cyclic AMP in regulation of phototaxis in Chlamydomonas reinhardtii. Biologia. 2009;64:1058–1065. [Google Scholar]
- 10.Wheeler GL, Grant CM. Regulation of redox homeostasis in the yeast Saccharomyces cerevisiae. Physiol Plant. 2004;120:12–20. doi: 10.1111/j.0031-9317.2004.0193.x. [DOI] [PubMed] [Google Scholar]
- 11.Heineke D, et al. Redox transfer across the inner chloroplast envelope membrane. Plant Physiol. 1991;95:1131–1137. doi: 10.1104/pp.95.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heber U. Metabolite exchange between chloroplasts and cytoplasm. Annu Rev Plant Physiol. 1974;25:393–421. [Google Scholar]
- 13.Leisinger U, et al. The glutathione peroxidase homologous gene from Chlamydomonas reinhardtii is transcriptionally up-regulated by singlet oxygen. Plant Mol Biol. 2001;46:395–408. doi: 10.1023/a:1010601424452. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi K, King SM. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. J Cell Biol. 2006;173:743–754. doi: 10.1083/jcb.200603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyth RD, Ebersold WT. Genetic investigation of a negatively phototactic strain of Chlamydomonas reinhardtii. Genet Res. 1985;46:133–148. doi: 10.1017/s001667230002262x. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J Cell Biol. 1988;107:2253–2258. doi: 10.1083/jcb.107.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison A, et al. Redox-based control of the gamma heavy chain ATPase from Chlamydomonas outer arm dynein. Cell Motil Cytoskeleton. 2002;52:131–143. doi: 10.1002/cm.10044. [DOI] [PubMed] [Google Scholar]
- 18.Fischer BB, Krieger-Liszkay A, Eggen RL. Photosensitizers neutral red (type I) and rose bengal (type II) cause light-dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ Sci Technol. 2004;38:6307–6313. doi: 10.1021/es049673y. [DOI] [PubMed] [Google Scholar]
- 19.Egorov SYu, Kurella EG, Boldyrev AA, Krasnovsky AA., Jr Quenching of singlet molecular oxygen by carnosine and related antioxidants. Monitoring 1270-nm phosphorescence in aqueous media. Biochem Mol Biol Int. 1997;41:687–694. doi: 10.1080/15216549700201731. [DOI] [PubMed] [Google Scholar]
- 20.Wang W-Y, Boynton JE, Gillham NW. Genetic control of chlorophyll biosynthesis: Effect of increased δ-aminolevulinic acid synthesis on the phenotype of the y-1 mutant of Chlamydomonas. Mol Gen Genet. 1977;152:7–12. [Google Scholar]
- 21.Harris EH. In: The Chlamydomonas Sourcebook. 2nd Ed. Harris EH, editor. Vol. 1. San Diego: Academic; 2009. pp. 89–117. [Google Scholar]
- 22.Okita N, Isogai N, Hirono M, Kamiya R, Yoshimura K. Phototactic activity in Chlamydomonas ‘non-phototactic’ mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein. J Cell Sci. 2005;118:529–537. doi: 10.1242/jcs.01633. [DOI] [PubMed] [Google Scholar]
- 23.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 24.op den Camp RG, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iomini C, Li L, Mo W, Dutcher SK, Piperno G. Two flagellar genes, AGG2 and AGG3, mediate orientation to light in Chlamydomonas. Curr Biol. 2006;16:1147–1153. doi: 10.1016/j.cub.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheibe R. Malate valves to balance cellular energy supply. Physiol Plant. 2004;120:21–26. doi: 10.1111/j.0031-9317.2004.0222.x. [DOI] [PubMed] [Google Scholar]
- 28.Isogai N, Kamiya R, Yoshimura K. Dominance between the two flagella during phototactic turning in Chlamydomonas. Zoolog Sci. 2000;17:1261–1266. [Google Scholar]
- 29.Horst CJ, Witman GB. ptx1, a nonphototactic mutant of Chlamydomonas, lacks control of flagellar dominance. J Cell Biol. 1993;120:733–741. doi: 10.1083/jcb.120.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josef K, Saranak J, Foster KW. Ciliary behavior of a negatively phototactic Chlamydomonas reinhardtii. Cell Motil Cytoskeleton. 2005;61:97–111. doi: 10.1002/cm.20069. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, et al. Diversion of the sign of phototaxis in a Chlamydomonas reinhardtii mutant incorporated with retinal and its analogs. FEBS Lett. 1992;314:275–279. doi: 10.1016/0014-5793(92)81488-8. [DOI] [PubMed] [Google Scholar]
- 32.Gorman DS, Levine RP. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sineshchekov OA, Jung K-H, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardti. Proc Natl Acad Sci USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthold P, et al. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell. 2008;20:1665–1677. doi: 10.1105/tpc.108.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagel G, et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya R. Analysis of cell vibration for assessing axonemal motility in Chlamydomonas. Methods. 2000;22:383–387. doi: 10.1006/meth.2000.1090. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi K, Yagi T, Kamiya R. Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central-pair/radial spoke system. Cell Motil Cytoskeleton. 1997;38:22–28. doi: 10.1002/(SICI)1097-0169(1997)38:1<22::AID-CM3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.