Abstract
Predator–prey relationships provide a classic paradigm for the study of innate animal behavior. Odors from carnivores elicit stereotyped fear and avoidance responses in rodents, although sensory mechanisms involved are largely unknown. Here, we identified a chemical produced by predators that activates a mouse olfactory receptor and produces an innate behavioral response. We purified this predator cue from bobcat urine and identified it to be a biogenic amine, 2-phenylethylamine. Quantitative HPLC analysis across 38 mammalian species indicates enriched 2-phenylethylamine production by numerous carnivores, with some producing >3,000-fold more than herbivores examined. Calcium imaging of neuronal responses in mouse olfactory tissue slices identified dispersed carnivore odor-selective sensory neurons that also responded to 2-phenylethylamine. Two prey species, rat and mouse, avoid a 2-phenylethylamine odor source, and loss-of-function studies involving enzymatic depletion of 2-phenylethylamine from a carnivore odor indicate it to be required for full avoidance behavior. Thus, rodent olfactory sensory neurons and chemosensory receptors have the capacity for recognizing interspecies odors. One such cue, carnivore-derived 2-phenylethylamine, is a key component of a predator odor blend that triggers hard-wired aversion circuits in the rodent brain. These data show how a single, volatile chemical detected in the environment can drive an elaborate danger-associated behavioral response in mammals.
Keywords: kairomone, olfaction, pheromone, trace amine-associated receptors, G protein-coupled receptor
Predator–prey relationships provide a classic paradigm for understanding the molecular basis of complex behavior (1). Predator-derived visual, auditory, and olfactory cues induce hard-wired defensive responses in prey that are sculpted by strong evolutionary pressure and are critical for survival. For example, odors from felines, canines, and other predators elicit innate reactions in rodents, including stereotyped avoidance behaviors and stimulation of the hypothalamic-pituitary-adrenal axis that coordinates sympathetic stress responses (1). Aversive reactions to odors can function in reverse as well, as skunk thiols facilitate prey escape by repelling predator species (2).
Predator odors contain a class of ecological chemosignals termed kairomones, cues transmitted between species that benefit the detecting organism. Predator odor-derived kairomones that elicit defensive responses in rodents are largely unknown and can be found in fur, dander, saliva, urine, or feces of divergent predator species. One volatile chemical produced by foxes, 2,5-dihydro-2,4,5-trimethylthiazole (TMT), and two nonvolatile lipocalins produced by cats and rats elicit fear-like or aversive behavior in mice, enabling remote or contact-based detection of predator cues (3–5). Each of these chemicals is not broadly produced by predators, raising the possibility that rodents detect a multitude of species-specific predator signals, each of which triggers a hard-wired defensive response. Alternatively, or in addition, prey species could detect predators through common metabolites derived from shared metabolic pathways or a carnivorous diet (6). Although common predator metabolites could, in principle, provide a generalizable mechanism for rodents to avoid many predators, even those not previously encountered during the history of an individual or species, no such kairomones have been identified.
Predator odors are thought to activate sensory receptors in both the olfactory epithelium and vomeronasal organ of rodents (1, 4, 5), but particular rodent sensory receptors that selectively respond to predator odors have not been identified. Some crude predator odor sources, such as cat fur and saliva, activate neural circuitry associated with the accessory olfactory system and are thus likely detected by vomeronasal receptors (1, 7). Furthermore, predator-derived lipocalins activate mouse vomeronasal sensory neurons and do not trigger defensive behavior in animals lacking TrpC2 (5), a key signal transduction channel in vomeronasal neurons (8, 9). Other predator odors, however, elicit powerful aversion responses through the main olfactory system. Mice lacking sensory receptors in a broad dorsal domain of the main olfactory epithelium do not avoid TMT or leopard urine, and instead ignore or are attracted to them (4). Thus, multiple olfactory subsystems detect different predator odors and enact appropriate defensive responses. Olfactory receptors that selectively respond to predator odors, whether expressed in the main olfactory epithelium, vomeronasal organ, or other olfactory substructure, could provide a strong evolutionary advantage for rodents.
Here, we identify 2-phenylethylamine to be a natural product with enriched production by numerous carnivores. This chemical activates HEK293 cells expressing a mouse olfactory receptor and elicits calcium responses in mouse olfactory sensory neurons. 2-phenylethylamine also evokes physiological and behavioral responses in two prey species, as it repels mice and rats, and induces an associated corticosterone surge in rats. Innate avoidance responses were maintained in mice lacking TrpC2, suggesting that vomeronasal signaling is not required. Furthermore, depletion of 2-phenylethylamine from one carnivore odor, lion urine, alters rat response behavior. Together, these data indicate that 2-phenylethylamine is a predator odor-derived kairomone detected and avoided by prey.
Results
Identification of a Predator Odor.
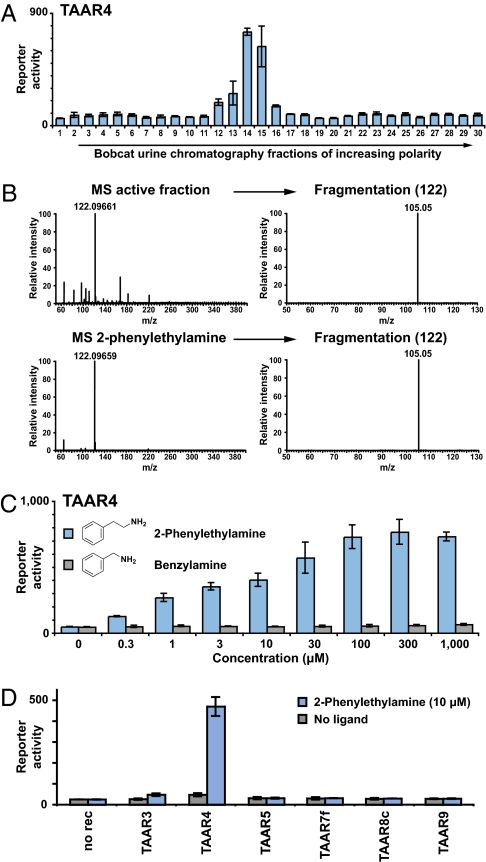
In the course of identifying natural and synthetic ligands for olfactory trace amine-associated receptors (TAARs) (10), we found that mouse TAAR4 selectively detects the urine of several carnivore species (Fig. 1A). HEK293 cells were cotransfected with TAAR expression plasmids and a cAMP-dependent reporter gene encoding secreted alkaline phosphatase (CRE-SEAP). Transfected cells were incubated with urine extracts from different mammalian species, and phosphatase activity was quantified with a fluorescent substrate as a reporter for TAAR activation. Urine extracts (10-fold dilution) from bobcat and mountain lion activated TAAR4, whereas rodent and human urine extracts did not. Responses to predator odors were not observed in control cells transfected with reporter gene alone. Three other TAARs—TAAR7f, TAAR8c, and TAAR9—detected natural products common to urine of numerous mammalian species, including mouse, rat, human, and carnivores (Fig. 1B and Fig. S1). However, these receptors detect carnivore and noncarnivore urines with similar sensitivity. We reasoned that TAAR4 detected a specific chemical enriched in predator urine, and that this cue might function as a kairomone.
Fig. 1.
TAAR4 detects predator odors. (A) HEK293 cells were transfected with TAAR4 and reporter plasmids, incubated with urine extracts of species indicated, and assayed for reporter activity (triplicates ± SD). TAAR4 was activated by urine extracts (10-fold dilution) of two rodent predators, bobcat and mountain lion, but not of mouse, rat, or human. No responses were observed to animal odors in control cells transfected with reporter plasmid alone. TAAR4 was expressed as a fusion protein with an N-terminal sequence of bovine rhodopsin, which provided enhanced signal (24). (B) Rat TAAR9, rat TAAR8c, and mouse TAAR7f detected urine of multiple species, including mouse, rat, and human. Urine (diluted 300- or 100-fold) or urine extracts (diluted 30-, 10-, or 3-fold) of species indicated were tested (triplicates ± SD).
We used a chemical fractionation approach to purify and characterize the predator urine-enriched activator. Basic dichloromethane extracts of bobcat urine were separated by silica gel chromatography, and fractions were analyzed with the reporter gene assay (Fig. 2A). Several chromatography fractions containing the TAAR4 activator were obtained and analyzed by mass spectrometry (Fig. 2B). A constituent was detected with exactly the same mass (m/z = 122) as ionized 2-phenylethylamine. Furthermore, fragmentation of this constituent and detection by tandem mass spectrometry identified a daughter ion (m/z = 105) corresponding to neutral loss of ammonia, an identical fragmentation pattern to that observed with 2-phenylethylamine. Commercially available 2-phenylethylamine was a potent activator of TAAR4 (EC50 ≈ 2 μM), whereas related amines with small perturbations in structure, such as benzylamine, did not similarly activate TAAR4 (Fig. 2C). A panel of other structurally related chemicals and phenylalanine metabolites also did not activate TAAR4 with comparable affinity (Fig. S2). Furthermore, 2-phenylethylamine did not similarly activate other olfactory TAARs with identified ligands (Fig. 2D), although it did activate TAAR1, which is not an olfactory receptor, and at 30-fold higher concentrations TAAR3, which detects many primary amines including benzylamine (Fig. S3). Mass spectrometry, fragmentation analysis, chromatographic retention time (see below), and functional activity all support 2-phenylethylamine being the major natural activator of TAAR4 present in bobcat urine.
Fig. 2.
2-phenylethylamine is a predator odor in bobcat urine. (A) Bobcat urine was fractionated by silica gel chromatography, and fractions were analyzed for the presence of TAAR4 activator with the reporter gene assay (triplicates ± SD). (B) An ion with the same mass and fragmentation pattern of 2-phenylethylamine was observed in a bobcat urine active fraction. (C) Commercial 2-phenylethylamine, but not benzylamine, activated TAAR4 (triplicates ± SD). (D) 2-phenylethylamine (10 μM) activated TAAR4 but did not similarly activate other olfactory TAARs with identified agonists.
Enriched 2-Phenylethylamine Production by Many Carnivores.
We next examined whether elevated 2-phenylethylamine levels were specific to bobcat urine or general to many carnivore urines. We used quantitative high performance liquid chromatography coupled with tandem mass spectrometry (LC/MS) to measure concentrations of 2-phenylethylamine in various specimens. Injection of pure 2-phenylethylamine and counting ions of appropriate mass (m/z = 122) over time yielded a single peak whose area was linearly correlated with concentration, enabling quantification (Fig. S4). Furthermore, LC/MS analysis of bobcat urine extracts revealed a single peak of ions with m/z = 122 that comigrated precisely with 2-phenylethylamine in spiked samples (Fig. 3A).
Fig. 3.
2-phenylethylamine is a component common to many carnivore odors. (A) LC/MS analysis of bobcat urine extracts, graphed as the number of ion counts with m/z = 122 over time, identified a single peak with identical retention time to 2-phenylethylamine. (B) 2-phenylethylamine (PEA) levels were quantified in multiple urine samples (#) from 38 species and 6 orders of mammals, as indicated. Samples were either purchased (p), provided by a zoo (z), or collected (c). (C) Average urinary 2-phenylethylamine levels were >50- to 500-fold higher in carnivores than in other mammalian orders.
Next, we quantified 2-phenylethylamine levels in urine extracts of 123 samples from 38 different mammalian species (Fig. 3B), including members of carnivore, rodent, artiodactyl, primate, lagomorph, and perissodactyl orders. Specimens were obtained from multiple collaborating zoos, commercial sources, or overnight collection in a metabolic cage. Zoo specimens were frozen immediately after collection to prevent bacterial growth. In cases where 2-phenylethylamine was not detected in specimen extracts, 20× concentrated extracts were also analyzed for enhanced sensitivity (detection limit <100 nM).
Urinary 2-phenylethylamine levels varied between species by several orders of magnitude. In 18/19 carnivore urines, 2-phenylethylamine levels were >2 μM, with highest levels observed in lion urine (340.1 μM). In contrast, urine from 0/19 noncarnivore species, representing five different mammalian orders, had 2-phenylethylamine levels >2 μM. 2-phenylethylamine was undetectable (<100 nM) in urine from 11/19 of these species. Average 2-phenylethylamine levels in samples from carnivore species examined (56.2 μM) were >50–500 fold higher (Fig. 3C) than average levels in samples from any other mammalian order (<100 nM to <1 μM). We did observe some variation in 2-phenylethylamine levels between specimens of the same carnivore species (Fig. S5). For example, levels in 11 bobcat specimens ranged from 5.3 μM to 72.6 μM, suggesting that its production might be further influenced by unknown physiological factors. However, levels of 2-phenylethylamine were higher in all 11 bobcat specimens than in any of 40 noncarnivore samples tested. Together, these data indicate that 2-phenylethylamine is a common metabolite whose production is elevated in many carnivores.
2-Phenylethylamine Activates Mouse Olfactory Sensory Neurons.
The mammalian olfactory system encodes odor identity by using combinations of olfactory receptors (11). Population imaging of sensory neurons in tissue slices has provided a valuable strategy for understanding how the olfactory system recognizes pheromones, MHC peptides, and complex scent cues containing information about sex and individuality (12–15). Here, we used a confocal imaging strategy to record cytosolic calcium transients of single sensory neurons in real time. Viability of analyzed neurons was determined after odor exposures by KCl-induced depolarization.
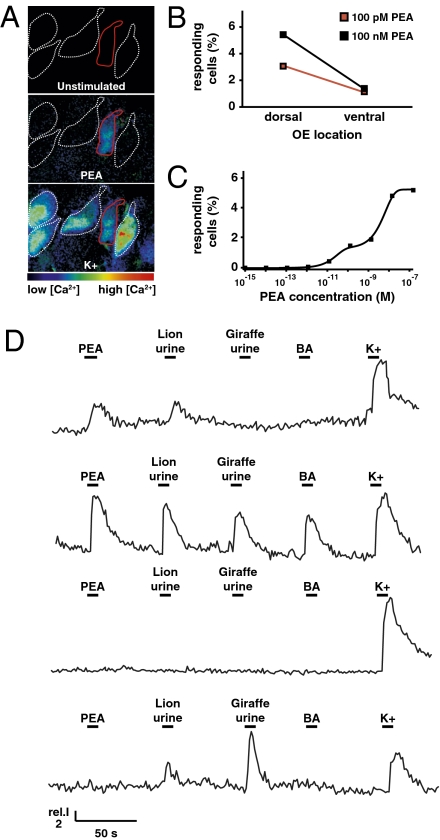
2-phenylethylamine activated a subset of KCl-responsive olfactory sensory neurons located in both the dorsal and ventral olfactory epithelium, although a higher percentage of responsive neurons were located dorsally (Fig. 4 A and B). The number of responding neurons in dorsal olfactory epithelium, which varied with test concentration, indicated that 2-phenylethylamine activated multiple olfactory receptors (Fig. 4C). The percentage of activated neurons was similar to what has been reported for other odors (11, 16). Because the dorsal region of the olfactory epithelium mediates behavioral responses to numerous aversive odors, including a carnivore urine (4), and is also the site of TAAR4 expression (Fig. S6), we focused on imaging dorsal olfactory epithelium in subsequent experiments.
Fig. 4.
2-phenylethylamine activates rodent olfactory sensory neurons. (A) Representative cytosolic calcium responses of individual olfactory sensory neurons in acute tissue slices. Fluo-4-loaded neurons (defined by contours indicated) were exposed to 2-phenylethylamine and elevated KCl (40 mM). Background-subtracted images of reporter dye intensity are coded in pseudocolors (rainbow spectrum). (B) Percentage of dorsal (n = 804) and ventral (n = 520) olfactory sensory neurons activated by 2-phenylethylamine at concentrations indicated. (C) Percentage of dorsal olfactory sensory neurons (n = 1,747) activated by 2-phenylethylamine at various concentrations. (D) Representative traces of integrated Fluo-4 fluorescence over time in individual dorsal olfactory sensory neurons exposed to test stimuli: 2-phenylethylamine (100 pM), lion urine (Fig. S5; specimen 5, 1:10,000), giraffe urine (Fig. S5; specimen 1, 1:10,000), benzylamine (100 pM), and KCl (40 mM).
In dorsal olfactory epithelium, we identified a small subset of carnivore odor-selective sensory neurons (21/1,268; 1.7%) that were activated by lion but not giraffe urine (diluted 10,000:1). Most, but not all, carnivore odor-selective neurons responded to 2-phenylethylamine (13/21 activated by 10,000:1 lion urine and 10/18 activated by 100:1 lion urine). These data indicate 2-phenylethylamine to be a major, but not exclusive, lion urine-enriched cue recognized by the main olfactory system. Furthermore, some 2-phenylethylamine-responsive neurons were effective at distinguishing lion urine and giraffe urine and did not respond to benzylamine (13/52, 25% of 2-phenylethylamine–responsive neurons; or 13/1,268, ≈1% of all dorsal KCl-responsive neurons), whereas others were activated by all four test stimuli (30/52; Fig. 4D). None of the carnivore odor-selective neurons that were activated by 2-phenylethylamine also responded to benzylamine (0/1,268). Together, these data indicate that 2-phenylethylamine is detected by the rodent olfactory system, activates multiple olfactory receptors, and is a major part of a lion odor blend recognized by rodents. Importantly, these data demonstrate that olfactory sensory neurons, like vomeronasal neurons shown previously (5, 7), have the capacity for interspecies cue recognition.
Rodents Avoid a 2-Phenylethylamine Odor Source.
We next examined behavioral responses of rodents to 2-phenylethylamine. Rats avoid predator urines in an open field paradigm (17), so we asked whether 2-phenylethylamine elicits a similar reaction. Behaviors of rats in a square-shaped arena were recorded and analyzed after placement of test stimuli in a pseudorandom corner (Fig. 5A). Animals did not display spatial preference for any corner after exposure to water, whereas animals actively avoided corners containing lion and coyote urine. A significant avoidance response was also observed to corners containing 2-phenylethylamine (Fig. 5 B and C) in a dose-dependent manner (Fig. 5D), but not benzylamine, a highly related amine with similar physical properties. The percentage of time rats were located in the odor quadrant during a 10-min exposure to various test stimuli was measured to be 26.7 ± 6.8% for water, 5.2 ± 1.4% for lion urine, 4.6 ± 1.1% for coyote urine, 29 ± 7.2% for benzylamine, and 8.0 ± 2.0% for 2-phenylethylamine (Fig. 5C, 12 animals ± SEM). Thus, 2-phenylethylamine, in the absence of other predator odor cues, was sufficient to evoke rat avoidance behavior.
Fig. 5.
2-phenylethylamine elicits an innate avoidance response in rodents. (A) A cartoon depiction of the experimental arena and ligand structures are shown. Movements of rats in response to test stimuli were recorded automatically by using infrared detectors. (B) 3D surface plots depict the percentage of time 12 rats were in regions of a square arena after exposure to test stimuli (1 mL of water or lion urine, 5 μL of PEA or BA) in the corner indicated (circle). Similar responses were observed when PEA and BA were diluted in 1 mL of water. Color scaling from red to blue indicates increased time spent in a particular region. (C) Mean percentage of time rats were located in the quadrant containing test stimuli was measured (12 animals, ± SEM, **P < 0.01). (D) Mean percentage of time rats occupied the quadrant containing 10% lion urine and 2-phenylethylamine (0, 0.4, 4, and 40 μmol) diluted in water or giraffe urine (1 mL), (12 animals, ± SEM, **P < 0.01). (E) Corticosterone levels in rat plasma determined by radioimmunoassay after exposure to odors indicated (1 mL of water, 2% TMT, 10% PEA, or 10% BA, 30 min, 8–20 animals, ± SEM, *P < 0.05). (F) Responses of wild-type or TrpC2−/− mice to odors indicated (aerosolized from 10 μL) were measured as a change in percentage occupancy of an odor compartment during a 3-min stimulus presentation (n = 5–7, ± SEM, *P < 0.05).
Avoidance to 2-phenylethylamine in rats was associated with acute changes in circulating levels of the stress hormone corticosterone. Using a competitive radioactive binding assay, plasma levels of corticosterone were measured (Fig. 5E) after exposure to water (103 ± 16 ng/mL, n = 16), TMT (238 ± 21 ng/mL, n = 16), 2-phenylethylamine (194 ± 18 ng/mL, n = 20), and benzylamine (130 ± 32 ng/mL, n = 8). Increases in plasma corticosterone levels after exposure to TMT or 2-phenylethylamine, but not benzylamine, were statistically significant compared with exposures involving water. Thus, 2-phenylethylamine activates olfactory circuits that provide input to the hypothalamic-pituitary-adrenal axis that orchestrates systemic stress responses.
To test generality across rodent species, we assessed behavioral responses of mice to 2-phenylethylamine. Valence responses to odors were measured by using a modified version of a two-choice compartment assay that was established for mouse aversion behavior (4). Male mice were exposed to aerosolized stimuli delivered to a test compartment in an otherwise odor-free arena. Time spent in the odor compartment was measured before and during odor delivery, and the odor-evoked change in occupancy recorded (Fig. 5F). Female urine, a powerful attractant for male mice, increased test compartment occupancy (+102 ± 34.2%, n = 6), whereas water alone had no effect (−4.0 ± 9.2%, n = 6). In contrast, TMT (−58.9 ± 11.2%, n = 7) and 2-phenylethylamine (−51.3 ± 10.0%, n = 7) decreased test compartment occupancy. Mice lacking TrpC2 displayed similar innate avoidance responses to 2-phenylethylamine (−42.0 ± 14.0%, n = 5), suggesting that signaling through the vomeronasal organ is not required. These data indicate that 2-phenylethylamine is aversive to mice, as well as rats, and that response patterns are conserved in at least two rodent species.
2-Phenylethylamine Is Required for Aversion Responses to Lion Urine.
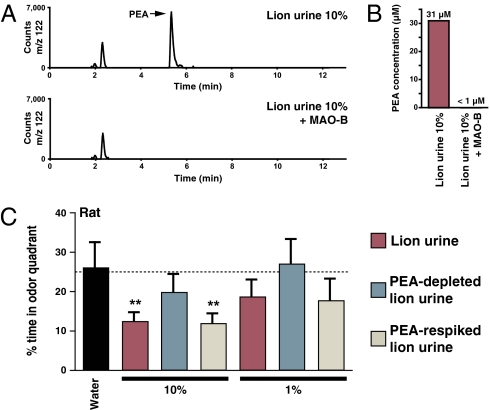
We next asked whether 2-phenylethylamine was required for lion urine-evoked avoidance responses in the rat. To address this possibility, we developed a method of depleting 2-phenylethylamine from lion urine. Lion urine (Specimen 6, Fig. S5; 309 μM 2-phenylethylamine) was diluted 10-fold and treated with monoamine oxidase B (MAO-B), an enzyme that oxidizes certain aromatic amines with preferred substrate preference for 2-phenylethylamine and dopamine (18). After addition of MAO-B to 10% lion urine, 2-phenylethylamine was undetectable by quantitative HPLC, with a detection limit of 1 μM (Fig. 6 A and B). Because MAO-B could potentially oxidize other biogenic amines present in lion urine, we created a third test specimen “PEA-respiked lion urine,” in which 2-phenylethylamine was reintroduced to original, physiological levels after MAO-B inhibition.
Fig. 6.
2-phenylethylamine is a key aversive component of a predator odor blend. (A and B) Quantitative LC/MS analysis of lion urine (10%) before and after addition of MAO-B was used to measure PEA concentration. (C) Mean percentage of time rats were located in odor quadrants containing water, 1%, or 10% lion urine, PEA-depleted lion urine, or PEA-respiked lion urine (11 animals, ±SEM, **P < 0.01).
Rat avoidance responses were measured to dilutions of (i) lion urine, (ii) “PEA-depleted lion urine” (lion urine treated with MAO-B), and (iii) PEA-respiked lion urine (Fig. 6C). Rats showed significant avoidance behavior to 10% lion urine, but not to 10% PEA-depleted lion urine. Furthermore, full aversion was restored to 10% PEA-respiked lion urine, indicating that 2-phenylethylamine is the relevant MAO-B substrate required for the full avoidance response to lion urine. Other potential MAO-B substrates, if present, are not important for avoidance behavior because rat responses are identical to 10% lion urine and 10% PEA-respiked lion urine despite different levels of such substrates. Furthermore, based on this analysis, 2-phenylethylamine evokes avoidance behavior at physiological concentrations in the context of other lion-derived odor cues. Together, our data provide evidence that 2-phenylethylamine is a key component of a carnivore odor blend detected and avoided by rodents.
Discussion
Understanding the molecular basis of predator odor recognition by the rodent olfactory system will provide tools to study neural circuitry associated with innate behavior. Here, we purify a predator odor from bobcat urine and identify it to be a biogenic amine, 2-phenylethylamine. Based on data presented, 2-phenylethylamine (i) is a component general to many carnivore odors, (ii) activates a rodent olfactory receptor in heterologous cells and multiple populations of olfactory sensory neurons in tissue slices, (iii) elicits innate avoidance behavior in rats and mice, and (iv) is a required component of a lion odor blend that evokes aversion responses. Together, these data indicate that 2-phenylethylamine is a predator odor-derived kairomone detected and avoided by prey species.
Based on our quantitative analysis of 2-phenylethylamine–evoked aversion (Fig. 5D), we consider it likely that behavioral responses to carnivore urine involve cooperative recognition of multiple cues, one of which is 2-phenylethylamine. This notion is consistent with neuronal imaging results indicating 2-phenylethylamine to be a major, but not exclusive, component of predator urine recognized by the olfactory system. In analogy, some aggression-promoting mouse pheromones elicit innate responses when presented in the context of an odor blend (19). It is important to note that 2-phenylethylamine is key and contributes to the aversive quality of a carnivore odor, because lion urine depleted of this chemical does not elicit a significant avoidance response.
The increased production of 2-phenylethylamine might reflect metabolic or dietary differences in the carnivore order. 2-phenylethylamine is a metabolite of phenylalanine, an essential amino acid found in dietary protein (20). One attractive model to explain our data is that elevated levels of dietary protein in meat-eating species directly lead to enhanced 2-phenylethylamine levels in urine. However, manipulation of protein levels in the diet of mouse and rat had no effect on lower levels of 2-phenylethylamine production in these species. This result does not exclude that manipulation of protein levels in carnivore species could affect 2-phenylethylamine production. Alternatively, enhanced 2-phenylethylamine production in carnivores could be explained by order-particular differences in phenylalanine use and metabolism rather than on levels consumed in diet. Last, it is also possible that 2-phenylethylamine is released by some carnivores as an odor in scent marks involved in social behavior.
Olfactory receptors that activate hard-wired neural circuits underlying 2-phenylethylamine avoidance are unknown. TAAR4 is an excellent candidate to function as a kairomone receptor, although based on population imaging, other olfactory receptors contribute to 2-phenylethylamine recognition. A role for vomeronasal receptors is unlikely because TrpC2 knockout mice still avoid 2-phenylethylamine. Consistent with this observation, avoidance responses to one carnivore urine are ablated in mice lacking function in dorsal olfactory epithelium (4), indicating that this carnivore urine response is distinct from some other predator odor responses (5, 7) in requiring main olfactory rather than vomeronasal signaling. Rats actively avoided 2-phenylethylamine but not benzylamine, suggesting that the innate avoidance we observed was due to activation of an olfactory receptor that can effectively distinguish these highly related amines. Based on calcium imaging data (Fig. 4), ≈1% of dorsal olfactory sensory neurons are activated by 2-phenylethylamine but not benzylamine. Humans, who lack a TAAR4 ortholog (21), perceive undiluted 2-phenylethylamine as a mildly unpleasant odor. Rats and mice might detect 2-phenylethylamine with higher sensitivity and selectivity than humans and, perhaps, display distinct behavioral responses to this chemical. Results presented here provide a basis for future experiments to probe aversion responses in genetically altered mice lacking TAAR4, or other key genes expressed in peripheral olfactory circuits or central limbic regions of the brain.
It is interesting to note that several TAAR ligands are highly aversive odors. Trimethylamine activates TAAR5, and although behavioral responses of mice to this cue are uncharacterized, it is a repugnant odor to humans associated with bacterial contamination, bad breath, and illness (22). Isoamylamine activates TAAR3 and, although speculated to be a mouse pheromone that influences reproductive physiology (23), was also shown to be an aversive odor to mice (4). Furthermore, we show here that TAAR4 detects a predator odor-enriched cue that repels rodents.
Two distinct models, that are not mutually exclusive, could explain how rodents detect and avoid divergent predator odors. One model would involve a myriad of distinct predator odor constituents, each of which is produced with high species and tissue selectivity, and each of which activates distinct olfactory circuits that trigger innate defensive behavior. Species-specific predator odors might be particularly relevant in predator–prey relationships with a long evolutionary history. A second model would involve detection of signals commonly produced by many predators, such as 2-phenylethylamine, that provide animals with the ability to avoid novel and dangerous species not previously encountered, an evolutionary benefit.
Predator–prey relationships provide a powerful paradigm to understand the neuronal basis of instinctive behavior. Avoidance of 2-phenylethylamine illustrates how a single volatile chemical detected in the environment can drive an elaborate behavioral response in mammals through activation of the olfactory system.
Materials and Methods
Details of materials and methods used are given in SI Materials and Methods. Methods described include specimen collection, TAAR functional assays, preparation, fractionation, and MS of urine extracts, quantitative LC/MS analysis, confocal calcium imaging, modulation of 2-phenylethylamine levels in predator odor, corticosterone measurements, and behavioral assays.
Supplementary Material
Acknowledgments
We thank Jon Clardy and Dong-chan Oh for assistance with quantitative LC/MS, Steven Gygi and Wilhelm Haas for assistance with MS, Richard Axel for generously providing TrpC2 knockout mice, Gholamreza Rahmanzadeh for assistance with corticosterone measurements, and the Franklin Park Zoo, Stone Zoo, Great Plains Zoo, and Capron Park Zoo for providing urine specimens. This work was supported by National Institute on Deafness and Other Communicative Disorders Grant R01DC010155 (to S.D.L.) and Deutsche Forschungsgemeinschaft Grant SP724/2-1 (to M.S.). M.S. is a Lichtenberg-Professor of the Volkswagen Foundation. D.M.F. is supported by a Boehringer Ingelheim Fonds PhD Fellowship. Financial support for S.R.D. was provided by a Career Award in the Medical Sciences from the Burroughs Wellcome Fund and an NIH Director's Office New Innovator Award (DP2-OD-007109).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103317108/-/DCSupplemental.
References
- 1.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Burger BV. Mammalian semiochemicals. Top Curr Chem. 2005;240:231–278. [Google Scholar]
- 3.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Kobayakawa K, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 5.Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berton F, Vogel E, Belzung C. Modulation of mice anxiety in response to cat odor as a consequence of predators diet. Physiol Behav. 1998;65:247–254. doi: 10.1016/s0031-9384(98)00126-7. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shaul Y, Katz LC, Mooney R, Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci USA. 2010;107:5172–5177. doi: 10.1073/pnas.0915147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leypold BG, et al. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 10.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 11.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 12.He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–538. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leinders-Zufall T, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- 14.Leinders-Zufall T, et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- 15.Spehr M, et al. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Chaim Y, Cheng MM, Yau KW. Unitary response of mouse olfactory receptor neurons. Proc Natl Acad Sci USA. 2011;108:822–827. doi: 10.1073/pnas.1017983108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol. 2006;32:2617–2627. doi: 10.1007/s10886-006-9186-9. [DOI] [PubMed] [Google Scholar]
- 18.Grimsby J, et al. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet. 1997;17:206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 19.Novotny M, Harvey S, Jemiolo B, Alberts J. Synthetic pheromones that promote inter-male aggression in mice. Proc Natl Acad Sci USA. 1985;82:2059–2061. doi: 10.1073/pnas.82.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman S. A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc Natl Acad Sci USA. 1999;96:3160–3164. doi: 10.1073/pnas.96.6.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindemann L, et al. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell SC, Smith RL. Trimethylaminuria: The fish malodor syndrome. Drug Metab Dispos. 2001;29:517–521. [PubMed] [Google Scholar]
- 23.Nishimura K, Utsumi K, Yuhara M, Fujitani Y, Iritani A. Identification of puberty-accelerating pheromones in male mouse urine. J Exp Zool. 1989;251:300–305. doi: 10.1002/jez.1402510306. [DOI] [PubMed] [Google Scholar]
- 24.Krautwurst D, Yau KW, Reed RR. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell. 1998;95:917–926. doi: 10.1016/s0092-8674(00)81716-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.