Abstract
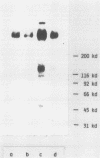
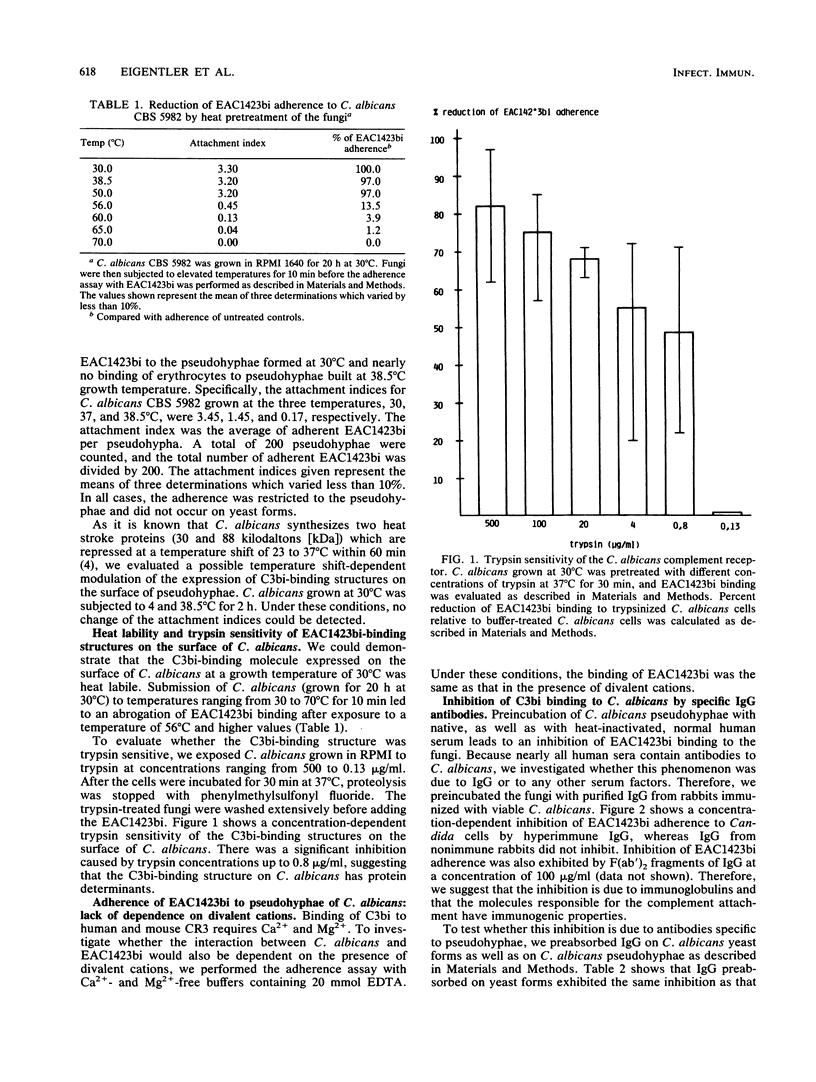
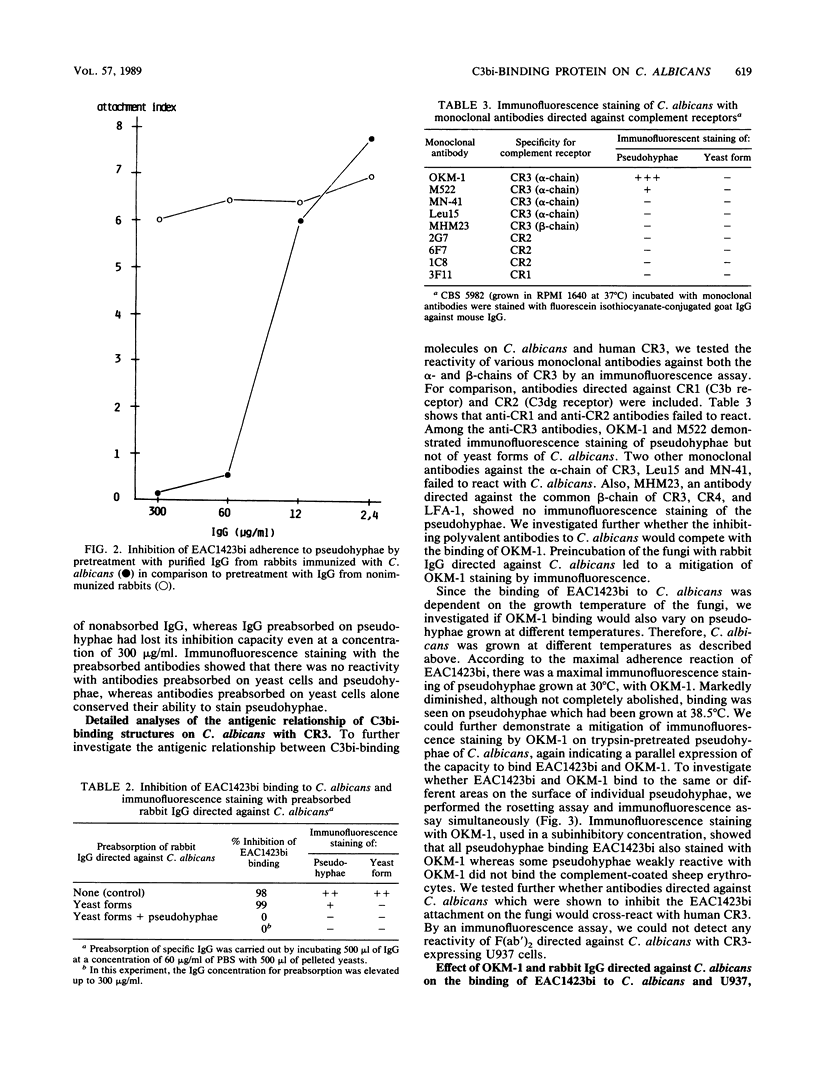
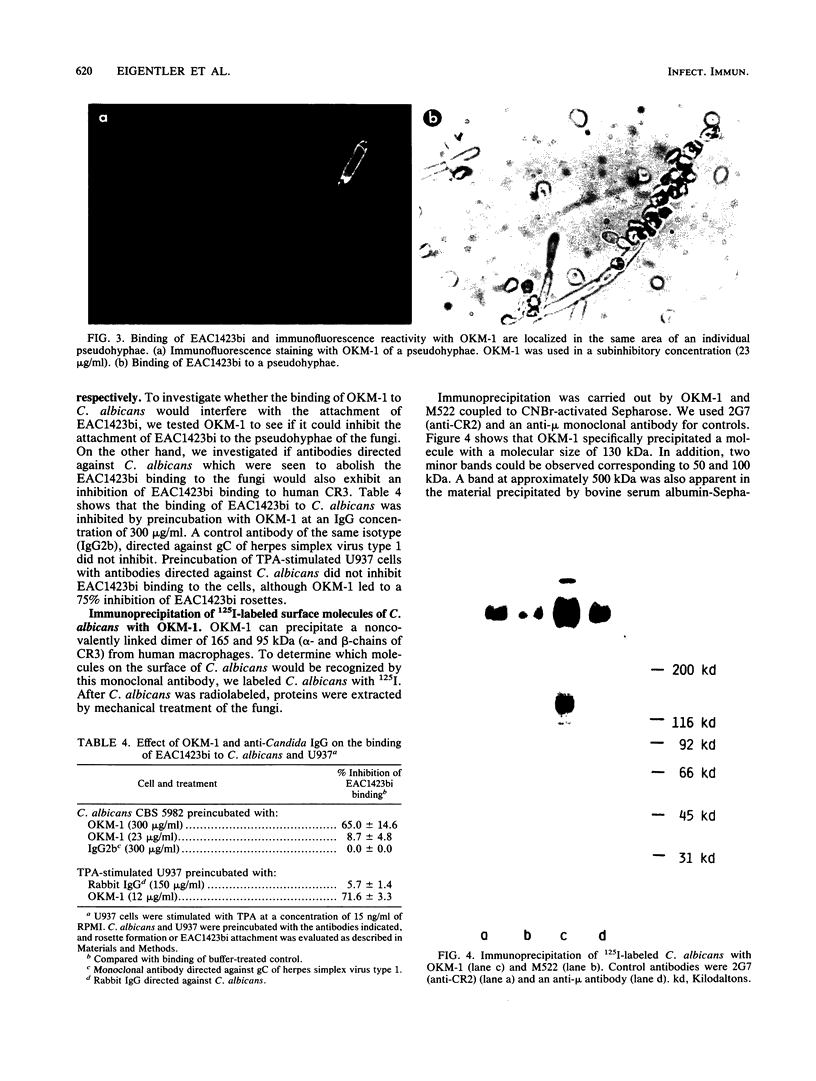
We investigated in detail the previously described capacity of pseudohyphae of Candida albicans to bind C3-coated particles. We show that the expression of the C3bi receptor of C. albicans was dependent upon the growth temperature of the fungi. C. albicans grown at 30 degrees C bound strongly to EAC1423bi, whereas those cells grown at 38.5 degrees C were completely devoid of this capacity. The molecule responsible for the attachment of EAC1423bi was heat labile and trypsin sensitive. Several, but not all, monoclonal antibodies to the alpha-chain of human complement receptor type 3 (CR3) stained C. albicans, and this reactivity was expressed in parallel with the capacity of C. albicans to bind EAC1423bi, i.e., both were dependent on the growth temperature of the fungi and were trypsin sensitive. In contrast to CR3, the binding of EAC1423bi to C. albicans did not require the presence of divalent cations. Rabbit immunoglobulin G antibodies directed against C. albicans inhibited the binding of EAC1423bi to C. albicans but not to human CR3. These inhibiting IgG antibodies recognized antigens expressed on the surface of pseudohyphae but not those of yeast cells. OKM-1, a monoclonal antibody to human CR3 inhibited the attachment of EAC1423bi to CR3 and also to C. albicans. OKM-1 precipitated a 130-kilodalton band from solubilized 125I-labeled C. albicans. We conclude that the complement receptors on C. albicans and human CR3 were antigenically related but not identical and that they differed in their functional characteristics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Melamed J., Tack B. F., Colten H. R. Characterization of the human complement (c3b) receptor with a fluid phase C3b dimer. J Immunol. 1981 Oct;127(4):1348–1354. [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowa N., Howard D. H. Heat shock and heat stroke proteins observed during germination of the blastoconidia of Candida albicans. Infect Immun. 1984 May;44(2):537–539. doi: 10.1128/iai.44.2.537-539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich M. P., Landen B. Complement bridges between cells analysis of a possible cell-cell interaction mechanism. J Exp Med. 1977 Dec 1;146(6):1484–1499. doi: 10.1084/jem.146.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich M. P., Schulz T. F., Eigentler A., Huemer H., Schwäble W. Structural and functional relationships among receptors and regulators of the complement system. Mol Immunol. 1988 Nov;25(11):1043–1051. doi: 10.1016/0161-5890(88)90136-8. [DOI] [PubMed] [Google Scholar]
- Eddy A., Newman S. L., Cosio F., LeBien T., Michael A. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984 Jun;31(3):371–389. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Crevon M. C., Barel M., Vazquez A., Krikorian L., Charriaut C., Galanaud P. Enhancement of human B cell proliferation by an antibody to the C3d receptor, the gp 140 molecule. Eur J Immunol. 1985 Jan;15(1):73–76. doi: 10.1002/eji.1830150114. [DOI] [PubMed] [Google Scholar]
- Frade R., Myones B. L., Barel M., Krikorian L., Charriaut C., Ross G. D. gp140, a C3b-binding membrane component of lymphocytes, is the B cell C3dg/C3d receptor (CR2) and is distinct from the neutrophil C3dg receptor (CR4). Eur J Immunol. 1985 Dec;15(12):1192–1197. doi: 10.1002/eji.1830151210. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Friedman H. M., Cohen G. H., Eisenberg R. J., Hammer C. H., Frank M. M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986 Sep 1;137(5):1636–1641. [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Nussenzweig V. Endogenous association of decay-accelerating factor (DAF) with C4b and C3b on cell membranes. J Immunol. 1986 May 1;136(9):3390–3395. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Erdei A., Schulz T., Dierich M. P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985 Sep 19;317(6034):264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Sim R. B. Isolation of complement-fragment-iC3b-binding proteins by affinity chromatography. The identification of p150,95 as an iC3b-binding protein. Biochem J. 1985 Oct 1;231(1):233–236. doi: 10.1042/bj2310233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., McNaughton M. E., Cooper N. R. Binding of monoclonal antibody to the Epstein Barr virus (EBV)/CR2 receptor induces activation and differentiation of human B lymphocytes. J Immunol. 1985 Nov;135(5):3068–3073. [PubMed] [Google Scholar]
- Petzer A. L., Schulz T. F., Stauder R., Eigentler A., Myones B. L., Dierich M. P. Structural and functional analysis of CR2/EBV receptor by means of monoclonal antibodies and limited tryptic digestion. Immunology. 1988 Jan;63(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T. F., Helmberg A., Hosp M., Petzer A., Föger B., Dierich M. P. Enzyme-linked immunoassay to monitor the purification of, and to screen for monoclonal antibodies against, C3b receptor. Complement. 1985;2(4):219–229. doi: 10.1159/000467865. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Scharfenberger H., Lambris J. D., Rieber P., Riethmüller G., Dierich M. P. Antigenic relationship between the alpha-chain of C3, a leucocyte-surface antigen involved in the activation of phagocytic cells, and a 50,000 MW B-cell antigen. Immunology. 1985 Apr;54(4):791–800. [PMC free article] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Springer T. A. The LFA-1, Mac-1 glycoprotein family and its deficiency in an inherited disease. Fed Proc. 1985 Jul;44(10):2660–2663. [PubMed] [Google Scholar]
- Weis J. J., Tedder T. F., Fearon D. T. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]