Abstract
Epoxy quinol 1a was prepared on a multi-gram scale by Noyori transfer hydrogenative desymmetrization of the readily available meso epoxy diketone 4. Although the intrinsic enantioselectivity for the desymmetrization was modest (82:18 er at 4% conversion), a highly enantiopure product (99.6:0.4 er) could be obtained in one operation in 44% yield via kinetic resolution of the minor enantiomer with long reaction times (48 h), or in 73% yield by combination with an enzymatic resolution of a 93:7 er mixture.
1. Introduction
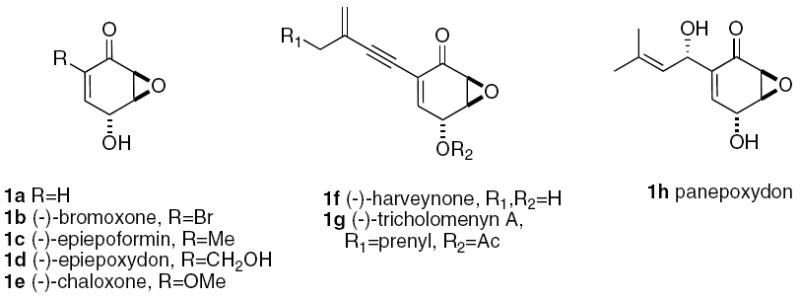
The trans-epoxy quinol moiety 1 is present in natural products 1b-h (Scheme 1),1 as well as being a useful synthon in complex molecule synthesis.2 Asymmetric approaches to these compounds have included whole cell oxidations of arenes to cis-arene diols,3 Baker’s yeast reduction of β-ketoesters,4 stoichiometric5 and catalytic6 asymmetric Diels-Alder reactions, asymmetric epoxidation,2a,7 use of a sulfoxide chiral auxiliary,8 enzymatic resolution of racemic9 or meso diols,10 from quinic acid,11 and by catalytic alkene isomerization of a meso bis-silyl ether.12
Scheme 1.
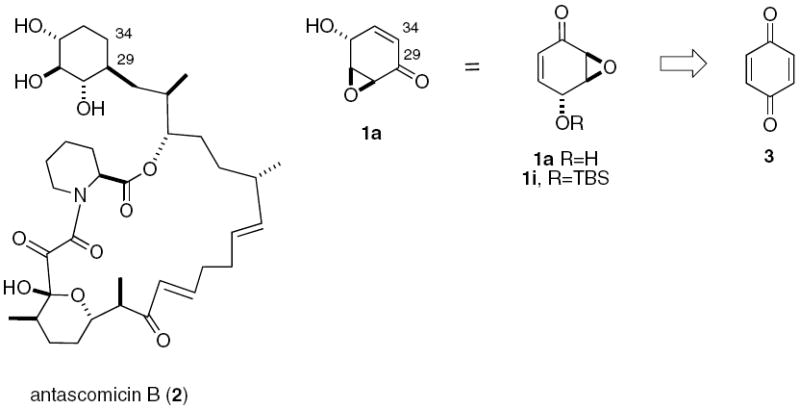
Over the course of our ongoing synthesis of antascomicin B 2,13,14,15 we identified parent epoxy quinol 1a as an early intermediate in the asymmetric synthesis of the C29-C34 cyclohexyl moiety (Scheme 2). We would thus need to be able to generate multi-gram quantities of 1a in an efficient manner. The existing approaches to epoxy quinols suffer from tedious and inefficient protection/deprotection and/or oxidation/reduction sequences, the use of expensive or hard to obtain starting materials, and/or lack of scalability. Thus, while racemic 1a has been prepared in 4 steps from benzoquinone,16 the reported asymmetric synthesis of the corresponding silyl ether 1i required eight steps from benzoquinone, a high enzyme loading for the desymmetrization step (lipase PS-D, 1 eq by weight), and a long reaction time (16 days).17,18
Scheme 2.
We sought a more efficient approach for preparing large quantities of 1a, which would be comparable to a short racemic synthesis.16,19 Asymmetric transfer hydrogenation offers a mild, readily scalable approach to enantiopure alcohols.20 The asymmetric reduction of meso-epoxy diketone 4 (available in two steps from benzoquinone)21 would shorten the synthesis of 1a to four steps (Scheme 3). The meso diketones have previously been desymmetrized via whole cell reductions,22 asymmetric hydride reductions,23 and asymmetric deprotonations.24 The asymmetric hydrogenation of meso diketones to keto alcohols has apparently not been explored.25
Scheme 3.

Using formic acid/trialkylamine26 as the stoichiometric reductant, we studied the effect of catalyst loading, the identity of the trialkylamine, acid:amine ratio and acid:ketone ratio on the enantioselectivity of reduction of epoxy diketone 4 using DMF as a cosolvent. The reaction was performed using using the Ru(p-cymene)[(S,S)-TsDPEN] catalyst from -10 °C to rt for approximately 16 h. The desired keto alcohol 5 was formed in 83:17 to 90:10 er (Scheme 3, Table 1). The catalyst loading, identity of the amine, acid:amine and acid:ketone ratio had relatively modest effects on the er.
Table 1.
Optimization of the reduction of diketone 4 on a 1.0 mmol scale.
| entry | catalyst (mol %) | acid:amine ratio | amine | acid:ketone ratio | er (5:ent-5) |
|---|---|---|---|---|---|
| 1 | 0.4 | 7:1 | NEt(iPr)2 | 5:1 | 83:17 |
| 2 | 0.8 | “ | “ | “ | 90:10 |
| 3 | 1.0 | “ | “ | “ | 90:10 |
| 4 | 1.6 | “ | “ | “ | 90:10 |
| 5 | 1.0 | “ | NEt3 | “ | 90:10 |
| 6 | 1.2 | “ | “ | “ | 90:10 |
| 7 | 2.0 | “ | “ | “ | 90:10 |
| 8 | 1.0 | “ | “ | 10:1 | 83:17 |
| 9 | 0.2 | 5:2 | NEt(iPr)2 | 3.2:1 | 86:14 |
| 10 | 0.4 | “ | “ | 3.2:1 | 86:14 |
| 11 | 1.0 | “ | “ | 3.2:1 | 86:14 |
| 12 | “ | “ | “ | 2.5:1 | 80:20 |
| 13 | “ | “ | NEt3 | 3.2:1 | 87:13 |
| 14 | “ | “ | “ | 2.5:1 | 85:15 |
In contrast to the racemic reduction of 4 with NBu4BH4, which gave ca. 6:1 dr,16 the Noyori reduction gave a single diastereomer. The absolute configuration of alcohol 4 was determined by chiral GC correlation to the compound prepared via the enzymatic desymmetrization route.
We then increased the reaction scale to 10 mmol (Table 2). A solvent survey indicated that acetonitrile was the optimal cosolvent for the reduction (entries 1-5). Changing the formic acid/ketone ratio from 3:1 to 1:1 resulted in a significant improvement in er (entries 3 and 6). However, a steady decrease in enantioselectivity was observed upon increasing the reaction scale beyond 10 mmol (entries 6-9). We were ultimately able to achieve a 93:7 er in the reduction on a 60 mmol scale in 85% yield. Chiral GC analysis at 4% conversion under the optimized conditions revealed a modest intrinsic enantioselectivity (82:18 er), which is, however, comparable to other dialkyl ketone transfer hydrogenations.25,27
Table 2.
Optimization of the scale-up of reduction of diketone 4.
| entry | solvent | acid:ketonea | scale (mmol) | conc (M) | er (5:ent-5) |
|---|---|---|---|---|---|
| 1 | None | 3:1 | 10 | “ | 80:20 |
| 2 | DMF | “ | “ | 0.5 | 80:20 |
| 3 | MeCN | “ | “ | “ | 82:18 |
| 4 | THF | “ | “ | “ | 70:30 |
| 5 | CH2Cl2 | “ | “ | “ | 70:30 |
| 6 | MeCN | 1:1 | “ | 0.15 | 90:10 |
| 7 | “ | “ | 30 | “ | 82:18 |
| 8 | “ | “ | 60 | “ | 79:21 |
| 9 | “ | “ | 120b | “ | 77:23 |
Molar ratio.
Reaction did not proceed to completion.
As expected, increasing the reaction time of the reduction resulted in material with a significantly improved er (99.6:0.4 at 48 h) as a consequence of the kinetic resolution of the minor enantiomer, although the isolated yield dropped to 44%. Alternatively, a 93:7 er mixture (12 h reaction time) could be enriched via enzymatic resolution with Amano lipase PS-IM to 99.5:0.5 er in an overall 73% yield.
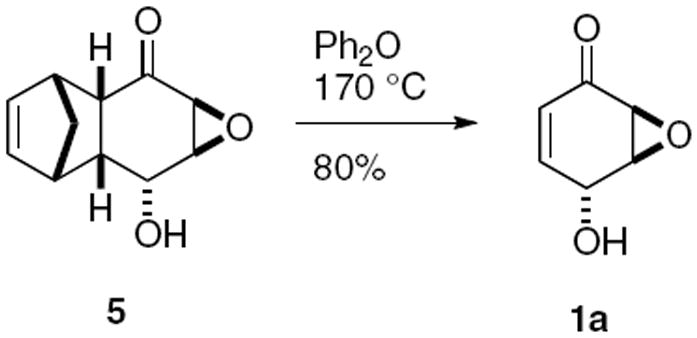
To complete the preparation of epoxyquinol 1a, we employed the Taylor variation19 of the Lubineau procedure16 for the retro-Diels-Alder reaction of 5 to 1a. Heating 10 g of keto alcohol 5 at 210 °C in diphenyl ether afforded the desired epoxy quinol 1a in 80% yield.
3. Conclusion
In conclusion, we have prepared epoxy quinol 1a in 4 steps from benzoquinone in a concise manner with a synthetically useful yield and high enantiopurity.
4. Experimental
Method A (93:7 er)
At first, Ru(p-cymene)[(S,S)-TsDPEN] (0.25 g, 0.39 mmol, 0.65 mol %) and epoxy diketone 4 (11.4 g, 60 mmol) were added to a solution of formic acid (3.85 mL), triethylamine (14.3 mL) and acetonitrile (500 mL) at -10 °C. The reaction mixture was allowed to slowly warm to rt and stirred for ca. 16 h. The mixture was concentrated in vacuo and then stirred for ca. 16 h in 30/70 ethyl acetate/hexanes (300 mL) with activated charcoal (10 g). The mixture was filtered through a plug of Celite with 30/70 ethyl acetate/hexanes and concentrated in vacuo. At this point, the residue could be purified by flash chromatography (20/80 ethyl acetate/hexanes) to give keto alcohol 5 as colorless crystals (9.8 g, 85%, er 93:7). Spectroscopic data matched those previously reported.16
Method B (99.6:0.4 er)
As in Method A, but the reaction mixture was allowed to stir for 48 h at rt. Flash chromatography (20/80 ethyl acetate/hexanes) gave keto alcohol 5 as colorless crystals 5.1 g, 44%, er 99.6:0.4).
Method C (99.5:0.5 er)
Prior to chromatography, the residue from Method A was purified by enzymatic resolution: vinyl acetate (1.34 mL, 1.26 g, 14.6 mmol), Lipase PS-IM (1.4 g), and keto alcohol 7 (10 g, 52 mmol, 93:7 er) were added to a solution of THF (100 mL) and NEt3 (10 mL) and stirred at rt for 4 d. The reaction mixture was filtered through a plug of Celite and concentrated in vacuo. The crude material was purified by flash chromatography on silica gel with 20/80 ethyl acetate/hexanes to give epoxy keto alcohol 5 as colorless crystals (8.5 g, 73% from 4, 99.5:0.5 er). The alcohol could be further crystallized to enantiopurity from diethyl ether.
The experimental procedure for the retro-Diels-Alder is as follows. A solution of keto epoxide 5 (10 g, 52 mmol) in diphenyl ether (200 mL) was heated under nitrogen at 210 °C for 2 h. Upon cooling to rt, the mixture was loaded directly on a silica gel column. Hexanes were used to elute the diphenyl ether, followed by 20/80 EtOAc/hexanes to give epoxy quinol 1a as white crystals (5.2 g, 42 mmol, 80%); mp 84-86 °C; [α]D19 = +3.8 (c 1.65, CH2Cl2). Spectroscopic data matched those previously reported.16
Scheme 4.

Scheme 5.
Acknowledgments
NSF (CHE0616154, CHE0911638), NIH (RR15569) and the Arkansas Biosciences Institute; David T. Williams and Andrew S. Gibson, University of Arkansas, for supporting experiments.
References
- 1.Bromoxone: Higa T, Okuda RK, Severns RM, Scheuer PJ, He C-H, Changu X, Clardy J. Tetrahedron. 1987;43:1063–1070.. Epiepoxydon and epiepoformin: Nagasawa H, Suzuki A, Tamura S. Agric Biol Chem. 1978;42:1303–1304.. Chaloxone: Fex T, Wickberg B. Acta Chem Scand. 1981;B35:97–98.. Harveynone: Nagata T, Ando Y, Hirota A. Biosci Biotechnol Biochem. 1992;56:810–811. doi: 10.1271/bbb.56.810.. Tricholemnyn A: Garlaschelli L, Magistrali E, Vidaria G, Zuffard O. Tetrahedron Lett. 1995;36:5433–5436.. Panepoxydon: Kis Z, Closse A, Sigg HP, Hruban L, Snatzke G. Helv Chim Acta. 1970;53:1577–1597..
- 2.See, for example: Lei X, Johnson RP, Porco JA. J Angew Chem Int Ed. 2003;42:3913–3917. doi: 10.1002/anie.200351862.; Porco J, JA, Su S, Lei X, Bardhan S, Rychnovsky SD. Angew Chem Int Ed. 2006;45:5790–5792. doi: 10.1002/anie.200602854.. Mehta G, Ramesh SS. Tetrahedron Lett. 2004;45:1985–1987.. Mauvais A, Winterfeldt E. Tetrahedron. 1993;49:5817–5822.; Riviere P, Mauvais A, Winterfeldt E. Tetrahedron: Asymmetry. 1994;5:1831–1846.. Konno H, Ogasawara K. Synthesis. 1999:1135–1140..
- 3.Pinkerton DM, Banwell MG, Willis AC. Org Lett. 2009;11:4290–4293. doi: 10.1021/ol9016657.; Labora Maitia, Pandolfi Enrique M, Schapiro Valeria . For a recent review, see: Hudlicky T, Reed JW. Synlett. 2009:685–703..
- 4.Tachiharaa T, Kitahara T. Tetrahedron. 2003;59:1773–1780. [Google Scholar]
- 5.Jin MY, Hwang G-S, Chae HI, Jung SH, Ryu DH. Bull Korean Chem Soc. 2010;31:727–730. [Google Scholar]
- 6.Okamura H, Shimizu H, Yamashita N, Iwagawaa T, Nakatani M. Tetrahedron. 2003;59:10159–10164. [Google Scholar]
- 7.Li J, Park S, Miller RL, Lee D. Org Lett. 2009;11:571–574. doi: 10.1021/ol802675j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreno MC, Merino E, Ribagorda M, Somoza A, Urbano A. Chem Eur J. 2007;13:1064–1077. doi: 10.1002/chem.200601330. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CR, Miller MW. J Org Chem. 1995:6674–6675. [Google Scholar]; Block O, Klein G, Altenbach H-J, Brauer DJ. J Org Chem. 2000;65:716–721. doi: 10.1021/jo991324c. [DOI] [PubMed] [Google Scholar]; Mehta G, Ramesh SS. Tetrahedron Lett. 2004;45:1985–1987. [Google Scholar]
- 10.Takano S, Higashi Y, Kamikubo T, Moriya M, Ogasawara K. Synthesis. 1993:948–950. [Google Scholar]
- 11.Barros MT, Maycock CD, Ventura MR. Chem Eur J. 2000;6:3991–3996. doi: 10.1002/1521-3765(20001103)6:21<3991::aid-chem3991>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Kamikubo T, Hiroya K, Ogasawara K. Tetrahedron Lett. 1996;37:499–502. [Google Scholar]
- 13.Isolation: Fehr T, Sanglier JJ, Schuler W, Gschwind L, Ponelle M, Schilling W, Wioland C. J Antibiot. 1996;49:230–233. doi: 10.7164/antibiotics.49.230..
- 14.Total synthesis: Brittain DEA, Griffiths-Jones CM, Linder MR, Smith MD, McCusker C, Barlow JS, Akiyama R, Yasuda K, Ley SV. Angew Chem Int Ed. 2005;44:2732–7. doi: 10.1002/anie.200500174..
- 15.McIntosh MC, Qi W. Tetrahedron. 2008;64:7021–7025. doi: 10.1016/j.tet.2008.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubineau A, Billault I. Carbohydr Res. 1999;320:49–60. [Google Scholar]
- 17.Takano S, Higashi Y, Kamikubo T, Moriya M, Ogasawara K. Synthesis. 1993:948–950. [Google Scholar]
- 18.(a) Konno and Ogasawara later reported that the enzymatic desymmetrization of the meso-diol intermediate on a 500 mg scale required only 3 h using lipase LIP.2d However, one weight equivalent of the enzyme is required, and it costs $40-45/g from Toyobo depending upon the quantity purchased. (b) In our hands, 40 h were required using Amano lipase PS-D or PS-IM. (c) Brimble et al. reported the desymmetrization of the same meso-diol on a 35 mg scale under microwave irradiation with Novozyme 435 in 12 h: Bachu P, Gibson JS, Sperry J, Brimble MA. Tetrahedron: Asymmetry. 2007;18:1618–1624..
- 19.Genski T, Taylor RJK. Tetrahedron Lett. 2002;43:3573–3576. [Google Scholar]
- 20.For recent reviews, see: Ohkuma T, Noyori R. In: Handbook of Homogeneous Hydrogenation. de Vries JG, Elsevier CJ, editors. Vol. 3. WILEY-VCH; Weinheim: 2007. pp. 1105–1163.; Blacker AJ. ibid. :1215–1244. and references cited therein.
- 21.Alder K, Flock FH, Beumling H. Chem Ber. 1960;93:1896–1899. [Google Scholar]; O’Brien DF, Gates JW. J J Org Chem. 1965;30:2593–2601. [Google Scholar]
- 22.(a) Brooks DW, Grothaus PG, Irwin WL. J Org Chem. 1982;47:2820–2821. [Google Scholar]; Brooks DW, Mazdiyasni H, Grothaus PG. J Org Chem. 1987;52:3223–3232. [Google Scholar]; Marchand AP, Xing D, Wang Y, Bott SG. Tetrahedron: Asymmetry. 1995;6:2709–2714. [Google Scholar]; Fuhshuku K-i, Funa N, Akeboshi T, Ohta H, Hiroyuki Hosomi, Ohba S, Sugai T. J Org Chem. 2000;65:129–135. doi: 10.1021/jo991192n. [DOI] [PubMed] [Google Scholar]; Wei Z-L, Li Z-Y, Lin G-Q. Synthesis. 2000:1673–1676. [Google Scholar]; Wei Z-L, Li Z-Y, Lin G-Q. Tetrahedron: Asymmetry. 2001;12:229–233. [Google Scholar]; Fuhshuku K-i, Tomita M, Sugai T. Adv Synth Catal. 2003;345:766–774. [Google Scholar]; Synthesis. 2006:2643–2645. [Google Scholar]; Shimoda K, Kubota N, Hamadà H, Hamada H. Tetrahedron Lett. 2006;47:1541–1544. [Google Scholar]; (b) Iwamoto M, Kawada H, Tanaka T, Nakada M. Tetrahedron Lett. 2003;44:7239–7243. [Google Scholar]; Watanabe H, Iwamoto M, Nakada M. J Org Chem. 2005;70:4652–4658. doi: 10.1021/jo050349a. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu M, Yamada S, Fujita Y, Kobayashi F. Tetrahedron: Asymmetry. 2000;11:3883–3886.; Ohtsuka Y, Koyasu K, Ikeno T, Yamada T. Org Lett. 2001;3:2543–2546. doi: 10.1021/ol016204h.; Nising CF, Ohnemueller UK, Bräse S, Yeung Y-Y, Chein R-J, Corey EJ. J Am Chem Soc. 2007;129:10346–7. doi: 10.1021/ja0742434.. See also ref 11b.
- 24.Butler B, Schultz T, Simpkins NS. J Chem Soc Chem Commun. 2006:3634–3636. doi: 10.1039/b606864b. [DOI] [PubMed] [Google Scholar]
- 25.For Noyori reduction of an achiral diketone, see: Shi B, Merten S, Wong DKY, Chu JCK, Liu LL, Lam SK, Jaeger A, Wong W-T, Chiu P, Metz P. Adv Synth Catal. 2009;351:3128–3132..
- 26.Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R. J Am Chem Soc. 1996;118:2521–2522. [Google Scholar]; Hamada T, Torii T, Izawa K, Noyori R, Ikariya T. Org Lett. 2002;4:4373–4376. doi: 10.1021/ol020213o. [DOI] [PubMed] [Google Scholar]
- 27.For a review, see: Noyori R, Ohkuma T. Angew Chem Int Ed. 2001;40:40–73..


