Abstract
Background & Aims
Helicobacter pylori interact with epithelial cells resulting in activation of cellular signaling pathways leading to an inflammatory response. The pattern and timing of transcription factor activation in H pylori-infected gastric mucosa remain unclear. We investigated the roles of transcription factors in the gastric mucosa of H pylori-infected gerbils over the course of the infection.
Methods
Six-week-old male Mongolian gerbils were inoculated orally with H pylori TN2GF4 or isogenic cagE mutants and examined at 1, 3, 9, and 18 months. We examined the expression of 54 transcription factors using DNA/protein arrays and electrophoretic mobility shift assays. Phosphorylation status of mitogen-activated protein kinases and I κB were evaluated by immunoblot and immunohistochemistry.
Results
Ten transcription factors were up-regulated by H pylori infection. Six of these factors, including activator protein-1 (AP-1) and cAMP responsive element binding protein (CREB), reached maximal levels at 3 months and were strongly correlated with cellular inflammation and ulceration. Phosphorylation of extracellular signal-regulated kinase correlated with activation of AP-1 and CREB. Levels of nuclear factor-κB and interferon-stimulated responsive element (ISRE) peaked at 18 months and correlated with the presence of severe atrophy and with phosphorylation of Jun-N-terminal kinase (JNK), p38, and IκB.
Conclusions
The gastric mucosal transcription factors induced by H pylori infection differed according to the phase and outcome of infection; AP-1 and CREB levels were early responders related to inflammation and ulceration, whereas NF-κB and ISRE were late responders related to atrophy.
Helicobacter pylori causes chronic gastric inflammation in virtually all infected persons. The cellular inflammatory response initially consists of infiltration by neutrophils followed by T and B lymphocytes, plasma cells, and macrophages. Infiltration of the mucosa with inflammatory cells is associated with epithelial cell damage (reviewed in Suerbaum and Michetti1) and with a marked increase in the expression of proinflammatory cytokines, including interleukin (IL)-1β, IL-6, and IL-8,2,3 which are regulated at the level of transcription. The signal transduction pathways involve transcription factors that bind DNA at specific promoter or enhancer regions. Activated transcription factors regulate gene expression by modulating the frequency of transcription initiation by interacting with specific DNA-binding elements present in promoters.
The details and sequence of transcription factor activation in H pylori infection are not well understood (reviewed in Naumann and Crabtree4). Much of the available data comes from in vitro studies in which H pylori was cocultured with gastric cancer cells. In these experiments, transcription factor activation typically occurs within 1 hour, and the relationship between these in vitro observations and the phenomenon that occurs in H pylori-infected gastric mucosa remains largely unexplored. The Mongolian gerbil (Meriones unguiculatus) has proven to be a convenient animal model for studies of H pylori infection because the gross and histologic findings mimic the lesions induced by H pylori infection in the human gastric mucosa.5–9 In the present study, we investigated the roles of transcription factors in the gastric mucosa of H pylori-infected gerbils over the course of the infection in vivo.
Materials and Methods
Animals
Six-week-old male Mongolian gerbils (MGS/Sea; Harlan Sprague Dawley, Inc., Indianapolis, IN) were housed in an air-conditioned biohazard room designed for infectious animals with a 12-hour light/12-hour dark cycle. They were provided rodent diet and water ad libitum. The animal facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The experiment protocol was approved by the Animal Care Committee of Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX.
Bacterial Strains and Inoculation
H pylori strain TN2GF4 was isolated from a Japanese gastric ulcer patient and has been shown to colonize gerbils consistently for at least 1 year and to cause reproducible mucosal damage.6 This strain is cag pathogenicity island (PAI) positive, vacA s1 (production of the vacuolating cytotoxin) and has functional blood group antigen binding adhesin (BabA) and outer inflammatory protein (OipA). Long-term studies (eg, 18 months) used wild-type (WT) strain TN2GF4, whereas the cag PAI mutant (isogenic cagE mutant) was used for short-term studies (ie, 1 and 3 months). The isogenic cagE mutant was constructed previously10 and was chloramphenicol resistant.
H pylori were grown in brain heart infusion (BHI) broth supplemented with 15% fetal bovine serum (FBS) for 20–30 hours at 37°C under microaerobic conditions and saturated humidity, with shaking at 200 rpm. After fasting for 12 hours, each animal was orogastrically inoculated 3 times (days 0, 1, and 2) with 1.0 mL inoculum of H pylori (108 colony forming unit [CFU]/mL) or sterile BHI broth (as a control) using gastric intubation needles. No specific pretreatments were given before orogastric H pylori inoculation.
Time Course and Death
Infected gerbils were killed and necropsied 1, 3, 9, or 18 months after H pylori inoculation. Five or 6 H pylori-inoculated gerbils were used for the 1-, 3-, and 9-month time points, and 18 inoculated gerbils were used for the 18-month time point. Three to 5 age- and sex-matched uninfected gerbils were used as controls at each time point.
At necropsy, stomachs were opened along the greater curvature and were divided longitudinally into 2 parts. One half was fixed in 10% phosphate-buffered formalin for histologic examination. The other half was divided further into the pyloric gland mucosa (antrum) and the fundic gland mucosa (corpus) and stored at −80°C. The gastric mucosa was separated as much as possible from the underlying muscle by sharp dissection. In addition, one mm2 piece of gastric mucosa from the antrum was taken to culture H pylori. The culture of H pylori from the gastric mucosa of gerbils was performed as previously described.9,11 The recovered strains were tested for chloramphenicol resistance to confirm the stability of the mutation for the cagE mutant-infected cases. Polymerase chain reaction (PCR) analysis was also performed for cagE mutants to confirm the preservation of chloramphenicol cassettes inside the target genes as previously described.12
Histopathology
Tissues were fixed in 10% neutral-buffered formalin and sliced along the longitudinal axis into 4 to 7 slips of equal width, embedded in paraffin, and cut into 4-µm sections. The sections were stained with H&E for morphologic observation and with Genta stain to detect H pylori and mucin-containing cells. Because the inflammatory component of the mucosa was virtually identical to that found in human H pylori gastritis,7 the degree of inflammation and existence of intestinal metaplasia could be graded blindly by one pathologist (R.M.G) according to the Updated Sydney System.13 H pylori density was also evaluated by histology because only one mm2 piece of gastric mucosa from the antrum was taken to culture H pylori, and the number of H pylori by culture should not be more reliable than scoring the H pylori density by histology.
Protein/DNA Array Analysis of Signal Transduction System Components
Nuclear extracts from gastric mucosal specimens were prepared using hypotonic/nonionic detergent lysis.14 After extraction, nuclear proteins were normalized by protein assay (Bio-Rad, Hercules, CA). Equal amounts (25 µg per sample) were used for the protein/DNA array (TranSignal Arrays, Panomics, Inc., Redwood City, CA). The protein/DNA array provides a profile of DNA binding activity for multiple transcription factors in a single array experiment and allows 54 transcription factors to be identified simultaneously. For quantitation, pooled nuclear extracts were prepared from a mixture of 3 gastric mucosal specimens obtained 9 months after inoculation of WT strains (standard sample). Six array membranes were set up simultaneously, with 1 membrane being used as a control. The density of dots for each transcription factor and the standard sample were scanned using Scion Image beta 4.02 software (Scion Corp. Frederick, MA). In the standard sample, the density of all dots from 54 transcription factors was summed (= density for all). The density of each transcription factor in the test samples was divided by the “density for all” and multiplied by 100.
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assay (EMSA) was performed using the same nuclear extracts used in the protein/DNA array. Equal amounts of nuclear proteins (10 µg per sample) were used to bind to duplex oligonucleotides corresponding to nuclear factor (NF)- κB, activator protein-1 (AP-1), cAMP responsive element binding protein (CREB), and interferon-stimulated responsive element (ISRE) binding sites. We used commercial consensus probes for these binding sites (Promega Corp, Madison, WI). EMSA was performed using standard methods as previously described.15 For semiquantitation, we used pooled nuclear extracts from the mixture of 3 WT H pylori-infected gastric mucosal specimens from the gastritis group at 18 months (standard sample). Density was measured by scanning, using the software Image J 1.36 software from the National Institutes of Health (http://rsbweb.nih.gov/ij/), and the density of samples was presented as a percentage of the standard sample. For competition assays, 5 pmol of unlabeled competitor was added at the time of probe addition. In the gel mobility supershift assays, commercial antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) against specific transcription factors were added to the binding reactions and incubated on ice prior to fractionation by 6% PAGE.
Immunoblot Analysis of Mitogen-Activated Protein Kinases and IκB
Immunoblot analysis was performed using standard methods. We used phospho-specific antibodies to detect phospho-p38, phospho-extracellular signal-regulated kinase (ERK), phospho-Jun-N-terminal kinase (JNK), phospho-inhibitor of IκB α, and control antibodies against total p38, ERK, and JNK as well as β-actin (1:1500 dilution) (antibodies for mitogen-activated protein kinase [MAPKs] were from Promega, and antibodies for phospho-IκBα and β-actin were from Cell Signaling Technology, Beverly, MA). These antibodies have been reported to cross-react with Mongolian gerbils.16–20
Cytoplasmic extract (10 µg per sample) obtained during the preparation of nuclear extracts was fractionated by SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane. Detection was performed using ECL reagents (Amersham Life Science, Arlington Heights, IL). For semiquantitation, pooled cytoplasmic extracts from the mixture of 3 WT H pylori-infected gastric mucosal specimens with gastritis at 18 months were used as the standard sample. Density was measured by scanning, using Image J 1.36 software (NIH), and the density of samples was calculated as a percentage of the standard sample.
Isolation of Primary Gastric Epithelial Cells From Gerbil Gastric Mucosa
Primary gastric epithelial cells were isolated enzy-matically from gerbil stomachs using previously established methods.21,22 Briefly, the surface mucosal layer (combined antrum and corpus) was removed with a razor blade, immediately minced, and then incubated in Ham’s F-12 culture medium containing collagenase type I (0.2 mg/mL) (Invitrogen) for 10 minutes. Cells from the final incubation were washed and cultured in Ham’s F-12 medium supplemented with 10% FBS and streptomycin (300 µg/mL) at 37°C in a humidified 5% CO2 atmosphere. Cultured cells (1 × 106 cells/mL) formed subcon-fluent monolayers within 24 hours of inoculation in a 24-well collagen-coated dish. Approximately 93% of cultured cells in the monolayers had periodic acid-Schiff-positive material in the cytoplasm, confirming that the population consisted of mucus-producing epithelial cells with minimal contamination by other cells. Epithelial cells could be maintained up to 1 week in culture.
In Vitro Experiments Using Primary Epithelial Cells From Gerbil Gastric Mucosa
H pylori TN2GF4 were suspended in cell culture medium, and the subconfluent epithelial cells (1 × 106 cells/mL) were cocultured with H pylori (multiplicity of infection [MOI] of 100) or kept uninfected in a 24-well collagen-coated dish for 3 hours. Cell viability was approximately 90% during the coculturing experiments (data not shown). Nuclear extracts were prepared using hypotonic/nonionic detergent lysis, and equal amounts (10 µg per sample) were used for protein/DNA arrays. Semiquantitation was performed as described previously.
In some experiments, we performed luciferase reporter gene assays using primary gastric epithelial cells isolated from gerbil stomachs as described above. Commonly used nonviral transfection methods (eg, Lipofectamine 2000 [Invitrogen]) did not succeed using primary gastric epithelial cells. Therefore, we used a novel nonviral transfection technology specially designed for primary cells and difficult to transfect cell lines, which allows trans-fected DNA to enter directly the nucleus of nondividing cells within a few hours (Nucleofector; Amaxa Biosystems, Allemagne, Germany). The PathDetect cis-reporting plasmids pNF- κBluc, pAP-1luc, and pCREluc, which contain the luciferase reporter gene driven by the TATA box plus multiple repeats of consensus NF- κB,AP-1, and CRE binding sequences, respectively, were purchased from Stratagene (La Jolla, CA). Luciferase assays were performed using the Dual-Luciferase reporter assay system according to the manufacturer’s instructions (Promega). Epithelial cells (1 × 106 cells/mL) were cultured in a 24-well collagen-coated dish for 24 hours before transfection, and, 6 hours after transfection, H pylori (MOI of 100) were added or kept uninfected. Nine hours after stimulation with H pylori, cells were harvested and lysed using passive lysis buffer (Promega), and the lysates were assayed for luciferase activity. Cell viability was approximately 85% to 90% during the coculturing experiments (data not shown). Normalized luciferase activity was calculated as firefly luciferase activity/Renilla luciferase activity. Luciferase activity is reported as fold increase of luciferase activity in treated cells relative to uninfected or mock-treated controls.
Immunohistochemistry
Immunohistochemistry was performed for p38, ERK, JNK, and IκBα. We used phospho-specific antibodies to detect phospho-p38, phospho-ERK, phospho-JNK, and phospho-IκBα (Promega and Cell Signaling Technology). Tissues were placed in a microwave oven at high power in 0.1 mol/L citrate buffer, pH 6.5, and treated 4 times for 5 minutes each. This was followed by a 2-hour incubation at room temperature with each antibody diluted 1:50. The immunoperoxidase reaction was developed using the biotin-streptavidin immunoperoxidase method (DAKO, Santa Barbara, CA). Areas of the surface/foveolar epithelium, glands, and submucosal lymphocytes were estimated, and the results were scored 0 to 3: 3, high level of staining (approximately 80%−100%); 2, medium level of staining (25%−80%); 1, low level of staining (1%–25%); and 0, virtually no staining (<1%).
Analysis of Cytokine mRNA Expression by Real-Time Quantitative Reverse Transcription-PCR
Total RNA was extracted from the gastric mucosa using an RNA extraction kit (Qiagen). After DNase treatment, 5 µg total RNA was subjected to reverse transcription (RT) using 200 U Moloney murine leukemia-virus reverse transcriptase (Life Technologies, Inc., Gaithersburg, MD) and 1 µmol/L of oligo(dT)16 primers. We used gerbil-specific IL-6 complementary DNA (cDNA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequences (GenBank accession number AB164706 and Yamaoka,10 respectively) and gerbil-specific keratinocyte-derived chemokine (KC) and interferon (IFN)-γ cDNA sequences (GenBank accession number: AJ877921 and L37782, respectively). Specific primers and TaqMan probes for KC, IL-6, IFN-γ, and GAPDH were described previously.23,24 Real-time PCR was performed as previously described.24 Cytokine messenger RNA (mRNA) levels were expressed as the ratio of cytokine mRNA to GAPDH mRNA (100,000 × cytokine mRNA [unit/µL]/ GAPDH mRNA [unit/µL]). Each assay was performed in triplicate.
In some experiments, we used RNA extracted from formalin-fixed, paraffin-embedded, tissue blocks. Serial 8-µm-thick sections of selected tissue blocks were placed onto glass slides, and the areas of interest (submucosal infiltrating cells) were microdissected visually after matching with an adjacent section stained with H&E. Microdissected samples were digested with proteinase K, and RNA was purified by phenol and chloroform extraction and reversed transcribed as published.25
Statistical Analysis
Statistical analysis was performed using the Mann-Whitney rank sum test and the paired t test depending on the data set. The data are presented as mean ± standard error (SE). A P value of less than .05 was accepted as statistically significant.
Results
Effect of H Pylori Infection on Macroscopic and Histopathologic Findings
Mongolian gerbils were inoculated orally with H pylori TN2GF4 or isogenic cagE mutants. H pylori infection was confirmed by culture and/or histology in all cases. All cagE mutants recovered from gerbils were resistant to chloramphenicol, confirming that the chloramphenicol cassette remained functional. The disruption of cagE gene was also confirmed based on the size of the amplicons by PCR. One month after inoculation, infiltration of neutrophils and monocytes into the gastric mucosa, mainly located in the submucosa, was observed in WT-infected gerbils. Infiltration into the stomach appeared to spread from the antrum to the corpus over time, with focal lymphocyte aggregates observed in the submucosa at the glandular border of the pyloric mucosa (antrum) and the fundic mucosa (corpus). Three months after inoculation, inflammation and H pylori density reached maximal levels (Figure 1). Inflammation and H pylori density were more severe in the antrum than in the corpus in each time point (P < .01). Macroscopically, pyloric channel ulcers were present in 1 of 5 gerbils (20%) at 9 months and in 8 of 18 gerbils (44%) at 18 months. Microscopically, intestinal metaplasia was present in 10 of 18 (56%) infected gerbils at 18 months, including 5 gerbils with gastric ulcers. The intestinal metaplasia occurred frequently in the transitional zone between pyloric and fundic mucosa, and Paneth cells were rarely found, indicating that the metaplasia was mostly of the incomplete type. Eighteen months after inoculation, gerbils were divided into 3 groups based on the gross and histologic outcomes. Eight gerbils had gastric ulcers (ulcer group), 5 gerbils had severe atrophic gastritis with intestinal metaplasia, but without ulcers (atrophy group), and 5 gerbils had gastritis without intestinal metaplasia or ulcers (gastritis group). Cellular infiltration in the antrum was more severe in the ulcer group than in the atrophy group (Figure 1). In contrast to a previous study using strain6 TN2GF4, no infected gerbils developed gastric cancer.
Figure 1.
Histologic scoring of cellular inflammation and H pylori density in the gastric mucosa of Mongolian gerbils infected with H pylori (time course [A] and according to the outcome at 18 months [B]). Neutrophils and monocyte infiltration scores and H pylori density scores are presented as mean ± SE. *P < .05 compared with 3 months (A).
Infiltration of neutrophils and monocytes was absent in control animals. cagE mutants did not induce inflammation during the period of observation of 3 months with the exception of a mild monocytic infiltration seen in the antrum 3 months after inoculation (score 1) in 2 of the 5 gerbils. Importantly, the H pylori density was not different between WT-infected gerbils and cagE mutant-infected gerbils (data not shown). The WT strain TN2GF4 induced an antral-dominant gastritis, whereas the cagE mutants induced little inflammation. These results are in agreement with previous studies using the same strain11 as well as with strain TN2, which shares the same origin20,27 as TN2GF4 and strain24 ATCC43504. These results differ from experiments using strain B128 as the parental strain.23 Thus, as in humans, the patterns of gastritis may differ depending on the H pylori strain infecting the individual.
H Pylori Infection Activates Multiple Transcription Factors in Mongolian Gerbils
Using the protein/DNA array, we assessed levels of activated transcription factors in the gastric mucosa. Three antral specimens and 1 corporal specimen were examined from animals killed after 1, 3, and 9 months (infected and uninfected) and from each of the 3 groups defined at 18 months (gastritis group, atrophy group, and ulcer group). Full quantitative analysis of all 54 transcription factors is available in Supplementary Table 1 and Supplementary Table 2 (see Supplementary Table 1 and Supplementary Table 2 online at www.gastrojournal.org).
Ten transcription factors were up-regulated by WT H pylori infection, both in the antrum and the corpus, at each time point (significantly up-regulated in the antrum and more than 2-fold up-regulated in the corpus) (Figure 2). Six factors including AP-1 and CREB reached maximal levels 3 months after infection, and 4 factors including NF-κB and ISRE reached maximal levels 18 months after infection. At 18 months after infection, the binding activity of AP-1 and CREB was significantly greater in the ulcer group than in the gastritis or atrophy groups in the antrum. In contrast, NFκB binding activity was significantly greater in the antrum of the atrophy group than in the ulcer group.
Figure 2.
The binding activity of 10 transcription factors was induced by H pylori infection both in the antrum and the corpus as determined by protein/DNA array analyses. Three antral specimens and 1 corporal specimen were examined from animals killed after 1, 3, and 9 months (infected and uninfected) and from each outcome group at 18 months (gastritis group, atrophy group, and ulcer group). The transcription factor binding activity of each sample was scanned and compared with that of standard nuclear extracts obtained from the mixture of 3 gastric mucosal specimens obtained 9 months after inoculation of WT strains. In the standard sample, the density of dots from all 54 transcription factors were summed (= density for all). The density of each transcription factor in the test sample then was divided by the “density for all” and multiplied by 100. We could perform statistical analyses only for the antral samples. Data are presented as mean ± SE. *P < .05 and **P < .01 compared with the uninfected control. †P < .05 and ††P < .01 compared with the ulcer group.
Comparison of the transcription factor binding activity with neutrophil and monocyte infiltration score among WT Hpylori-infected animals (n = 18 for antrum and n = 6 for corpus) showed that AP-1 and CREB binding activities strongly correlated with cellular inflammation in the antrum (r = 0.63–0.73, P < .01). AP-2 and Smad3/4 were also correlated with cellular inflammation in the antrum (r = 0.56–0.61, P < .01). Activities of the remaining transcription factors were independent of the degree of cellular infiltration (data not shown).
The induction of most transcription factor activity including AP-1 and CREB binding activities by cagE mutants was similar to uninfected controls (see Supplementary Table 1 and Supplementary Table 2 online at www.gastrojournal.org). In the antrum, 5 transcription factors, including NF-κB and ISRE, showed significantly greater binding activity when infected with cagE mutants than in controls. In the corpus at 3 months after infection with cagE mutants, NF-κB and ISRE binding activities were more than 2-fold greater than uninfected controls (Figure 2). However, the ability of cagE mutants to induce the binding activities of NF-κB and ISRE was significantly less than that of WT H pylori. In contrast, antral levels of upstream stimulatory factor (USF)-1 binding activity were similar in cagE mutants and WT H pylori at 3 months.
H Pylori Infection Activates NF-κB, ISRE, AP-1, and CREB in Mongolian Gerbils
We performed EMSA and supershift assays using probes containing consensus binding site sequences for NF-κB, AP-1, ISRE, and CREB to confirm the results of the protein/DNA arrays. Two major NF-κB binding complexes (C1 and C2) were weakly detected in uninfected gastric mucosa (Figure 3) and increased in a time-dependent manner in mucosa infected with WT H pylori, reaching maximal levels at 18 months. These results were consistent with those obtained using the protein/DNA array. Two additional binding complexes (C3 and C4) first appeared at 3 months and also were maximal at 18 months. The 4 inducible complexes were sequence specific as demonstrated by competition with unlabeled WT but not mutant oligonucleotides (Figure 3). Supershift assays demonstrated that p65 was a component of the C1 complex. We were unable to identify components of the other complexes. In previous in vitro studies using human gastric cancer cell lines, we showed that both p50 and p65 were components of NF-κB binding complexes.15,22,26 However, because the accuracy of commercially available antibodies against p50 in Mongolian gerbils is unknown, we cannot conclude that p50 was not present.
Figure 3.
EMSA of NF-κB, ISRE, AP-1, and CREB binding complexes. Four to 5 antral and corporal specimens were used from animals killed at 1,3, and 9 months (infected and uninfected). We also examined samples from each of the 3 outcome groups at 18 months (gastritis, atrophy, and ulcer groups). Left column: Nuclear extracts were prepared from uninfected control mucosa at 18 months; infected mucosa from 1, 3, 9, and 18 months. Middle column: semiquantitative EMSA analyses. The densities of nuclear extracts from a mixture of 3 WT H pylori-infected gastric mucosal specimens from the gastritis outcome group at 18 months (standard sample) are presented as the percentage of the standard samples after standardization with free probe. Data are presented as mean ± SE. Right column: lanes 1 to 3: competition analysis. Nuclear extracts from infected samples (18 months) were used to bind probe in the absence (−) or presence of 100-fold excess of unlabeled competitors (WT or mutated [Mut]). Lanes 4 and later: supershift interference assay. EMSA was performed using nuclear extracts of infected samples (18 months) and commercial antibodies against specific transcriptional factors.
Two ISRE binding complexes (C1 and C2) were weakly detected in uninfected gastric mucosa and increased in a time-dependent manner in mucosa infected with WT H pylori, reaching maximal levels at 18 months (Figure 3), again confirming the results obtained from protein/DNA arrays. The inducible complexes were sequence specific as demonstrated by competition with unlabeled WT but not with mutant oligonucleotides. In contrast, strong signals observed in both uninfected and infected gerbils were judged to be nonspecific because there was no competition with unlabeled WT oligonucleotides. Supershift assays demonstrated that interferon regulatory factor (IRF)-1, IRF-3, and IRF-7 were components of the H pylori-inducible ISRE complex. Of note, the density of the C1 and C2 complexes was not decreased by these antibodies, suggesting that several IRF families might be present in the complexes.
One clear AP-1 binding complex was weakly detected in the antral mucosa of uninfected gerbils but was not seen clearly in the corporal mucosa (Figure 3). The complex increased in the mucosa of gerbils infected with WT H pylori and reached maximal levels at 3 months. This pattern was similar to the histologic changes (Figure 1). The density of the AP-1 complex was significantly correlated with neutrophil and monocyte infiltration scores in the antrum (r = 0.61 and 0.63, respectively; P < .01 for each), similar to data obtained using the protein/DNA arrays. The inducible complex was sequence specific, and supershift assays demonstrated that c-Jun and c-Fos were components of the AP-1 complex.
At least 3 CREB binding complexes (C1, C2, and C3) were weakly detectable in uninfected gastric mucosa and increased in mucosa of infected WT H pylori peaking at 18 months (Figure 3). Complex C2 was variable at each time point and among different experiments, although the conditions and buffers were identical (eg, very faint in Figures 4 and 5). The 3 complexes were sequence specific as demonstrated by competition with unlabeled WT but not mutant oligonucleotides; however, competition with the complex C2 was less efficient than the other 2 complexes (Figure 3). Therefore, complex C2 was not included in the semiquantitative analyses. Consistent with results of the protein/DNA array, the density of CREB complexes significantly correlated with neutrophil and monocyte infiltration scores in the antrum (r = 0.58 and 0.61, respectively; P < .01 for each). Use of anti-CREB-1, anti-ATF-2, and anti-c-Jun antibodies in EMSAs resulted in supershifted bands, whereas use of anti-CREB-2 and anti-c-Fos antibodies resulted in weak supershifted bands, suggesting that members of the CREB/ATF family are major components of the H pylori-inducible CRE complexes in gerbils.
Figure 4.
Expression of ERK, p38, JNK, and IκB. Four to 5 antral and corporal specimens were analyzed from animals killed at 1, 3, and 9 months (infected and uninfected) and at 18 months (infected only). Semiquantitative analyses were performed by comparing the phospho-MAPK signal after standardizing with total MAPK or the phospho-IκB signal after standardizing with β-actin and calculated as a percentage of the standardized signals from antral mucosal samples infected with WT H pylori for 18 months (gastritis outcome group). Data are presented as mean ± SE. *P < .05 and **P < .01 compared with uninfected controls.
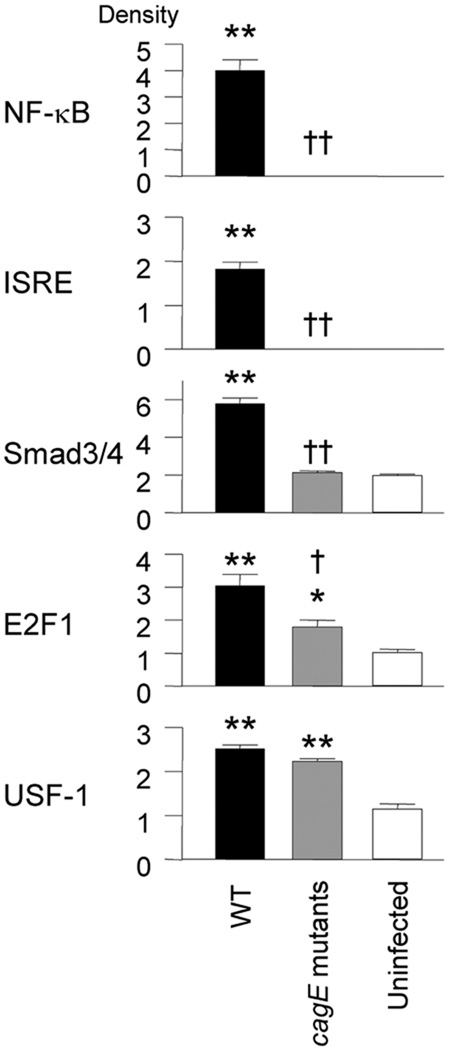
Figure 5.
Activation of 5 transcription factors by H pylori infection of primary gastric epithelial cells using protein/DNA array. The subconfluent primary epithelial cells (1 × 106 cells/mL) were cocultured with H pylori (MOI of 100) or kept uninfected in 24-well collagen-coated dishes for 3 hours. Nuclear extracts were prepared using hypotonic/nonionic detergent lysis and equal amounts (10 µg per sample) were used. The vertical axis shows the density of each transcription factor adjusted by the density of all dots from 54 transcription factors that were summed in the standard sample. The standard sample was prepared from pooled nuclear extracts from 3 gastric mucosal specimens from animals killed 9 months after inoculation of WT strains. Data are presented as mean ± SE. *P < .05 and **P < .01 compared with uninfected controls. †P < .05 and ††P < .01 compared with WT H pylori infected samples.
EMSA confirmed the results of protein/DNA array data, showing that AP-1 and CREB binding activities were greater in the ulcer group than in the gastritis or atrophy groups and that NF-κB and ISRE binding activities were greater in the atrophy group in the antrum (see Supplementary Figure 1 online at www.gastrojournal. org). EMSA also confirmed that infection with the cagE mutant resulted in more NF-κB and ISRE binding activity than uninfected controls both in the antrum and the corpus, although the degree of activation was less than that with the WT strain (see Supplementary Figure 2 online at www.gastrojournal.org).
Immunoblot Analysis of MAPKs and IκB Pathways in Gastric Mucosa of Gerbils
The MAPKs and IκB pathways are involved in signaling upstream of many transcription factors including NF-κB, ISRE, AP-1, and CREB. Therefore, we performed immunoblots for MAPKs and IκB using phospho-specific antibodies to detect phospho-ERK1/2, p38, JNK, and IκB, as well as control antibodies to detect unphosphorylated forms of ERK, p38, and JNK.
Phosphorylation in both pathways was greater in infected tissues than in uninfected controls (Figure 4). ERK phosphorylation reached maximal levels at 3 months, decreased at 9 months, and increased again at 18 months, similar to the pattern of cellular inflammation and activation of AP-1 and CREB binding. ERK phosphorylation almost disappeared at 9 months. ERK phosphorylation levels correlated with cellular inflammation scores and AP-1 and CREB binding levels measured by both protein/ DNA array and EMSA (data not shown). p38 Phosphorylation reached maximal levels at 3 months and plateaued until 18 months. Phosphorylation of JNK and IκB was induced in a time-dependent manner through 18 months, a pattern similar to the activation of NF-κB and ISRE binding. These data agree with results obtained from human gastric cells infected with H pylori, showing that JNK and IκB are involved in upstream NF-κB signaling and that p38 and IκB are upstream of ISRE 4,15,22,26
Immunoblots were used to examine mucosal samples from the 3 groups at 18 months. Levels of phosphorylated ERK were highest in the ulcer group, followed by the gastritis and the atrophy groups (see Supplementary Figure 3A online at www.gastrojournal.org). Levels of phosphorylated IκB were higher in the atrophy group than in the ulcer group (P = .07). In contrast, levels of phosphorylated p38 and JNK were independent of the different groups.
Infection with cagE mutants induced phosphorylation of p38, JNK, and IκB, although the levels were lower than with WT strains (see Supplementary Figure 3B online at www.gastrojournal.org). This pattern was similar to the one observed upon NF-κB and/or ISRE activation, confirming the relationship between p38/JNK/IκB and NF-κB and/or ISRE activation. In contrast, cagE mutants did not induce ERK phosphorylation, which is consistent with their inability to activate AP-1 and CREB.
H Pylori Infection Activates NF-κB and ISRE but not AP-1 and CREB in Primary Epithelial Cells Isolated From Gerbil Gastric Mucosa
One disadvantage of the above approach was that the mucosal specimens contained many different cells including epithelial cells, stromal cells, and inflammatory cells infiltrating the mucosa, such that the origin of cells containing activated transcription factors could not be specifically determined. We attempted to establish primary epithelial cell cultures from the gastric mucosa, but we were unable to isolate pure epithelial cells from H pylori-infected gastric mucosa without contamination from nonepithelial cells (unpublished data). Therefore, we isolated epithelial cells from 7-week-old male uninfected gerbils (combined antrum and corpus) and performed the protein/DNA array. Five factors (NF-κB, ISRE, USF-1, E2F1, and Smad3/4) showed increased binding activity following WT H pylori infection of epithelial cells (Figure 5). Full quantitative analysis of all 54 transcription factors is available in Supplementary Table 3 (see Supplementary Table 3 online at www. gastrojournal.org). Importantly, AP-1 binding activity was not detected in either Hpylori-infected or uninfected epithelial cells, and CREB binding activity was similar in uninfected and infected epithelial cells. Infection with cagE mutants did not induce NF-κB, ISRE, or Smad3/4 binding activity in epithelial cells but did induce USF-1 binding activity similar to WT H pylori. Infection with cagE mutants also induced E2F1, although the levels were lower than in WT H pylori infection (Figure 5). Finally, infection with cagE mutants did not induce NF-κB or ISRE binding activities, which differed from results of whole gastric mucosa but agreed with previous reports using human gastric epithelial cell lines.15 With the caveat that experiments in primary gerbil gastric mucosal epithelial cells may differ from stable gastric epithelial cell lines in many ways, NF-κB and ISRE binding activities appear to be activated in epithelial and submucosal cells. Activation of NF-κB and ISRE binding activities in epithelial cells appears to be cag PAI dependent, whereas activation in submucosal cells is cag PAI independent.
In agreement with the protein/DNA array results, luciferase reporter gene assays showed that AP-1 and CREB binding activities were not up-regulated by WT H pylori infection (1.1- ± 0.1-fold and 1.0- ± 0.1-fold, respectively). In contrast, NF-κB binding activity was significantly up-regulated by WT H pylori infection (fold increase of luciferase activity in treated cells over uninfected, or mock-treated controls = 3.4- ± 0.5-fold), but not by infection with cagE mutants (1.1- ± 0.1-fold).
Immunohistochemistry Detection of Phosphorylation Forms of MAPKs and IκB
As described above, the phosphorylation status of ERK, p38, JNK, and IκB could be determined by immunoblot. Therefore, immunohistochemistry was used to examine the source of these factors in the gastric mucosa (data for 1 and 3 months after infection are presented in Figure 6). In control uninfected gerbils and in gerbils infected with cagE mutants, phospho-ERK-positive cells were generally absent in the antrum and were only weakly present in the surface epithelium of the corpus. In WT H pylori-infected gerbils, cells containing phospho-ERK were markedly observed in the surface epithelium as well as in submucosal cells. Because immunoblot analyses showed that phospho-ERK had largely disappeared at 9 months after infection, we also performed immunohistochemistry for phospho-ERK at 9 months. At 9 months after infection, cells containing phospho-ERK were weakly observed in the surface epithelium (score, 1.2 ± 0.2 in the antrum and 1.7 ± 0.3 in the corpus, P < .01 and P < .05 compared with 3 months in the antrum and the corpus, respectively) and were not observed in the gland and submucosal cells. These results are in agreement with immunoblot analyses showing that the phospho-ERK levels were markedly decreased at 9 months.
Figure 6.
Immunohistochemical staining for phosphorylated MAPKs (ERK and p38) and IκB. Bar, 50 µm. Representative sections from gerbils infected with WT strains, cagE mutants, or uninfected controls for 3 months. Areas of the surface/foveolar epithelium, glands, and submucosal lymphocytes were estimated, and the results were scored 0 to 3 where 3 = high level of staining (approximately 80%–100%), 2 = medium level of staining (25%–80%), 1 = low level of staining (1%–25%), and 0 = virtually no staining (<1%). Data are presented as mean ± SE. *P< .05 and **P < .01 compared with uninfected controls. †P < .05 and ††P < .01 compared with WT H pylori infected samples.
More phospho-IκB-positive cells were observed in epithelial and submucosal cells of gerbils infected with WT strains than in uninfected gerbils. Importantly, the number of phospho-IκB-positive submuocsal cells was increased in both WT- and cagE mutant-infected gerbils, whereas the number of phospho-IκB-positive epithelial cells was similar between cagE mutant-infected gerbils and uninfected controls (Figure 6). These data suggested that activation of IκB→NF-κB pathways in epithelial cells is cag PAI dependent, whereas activation in submucosal cells is cag PAI independent.
Phospho-JNK staining was homogenous throughout the gastric mucosa of both H pylori-infected and uninfected gerbils (data not shown). Several nonspecific JNK bands were seen in the immunoblots, possibly because of the fact that the antibodies used to detect phospho-JNK may not work for immunohistochemistry with gerbils. Staining patterns were not different between the sources of antibodies (Promega or Cell Signaling Technology), except for straining for phospho-p38 (data not shown), indicating that the antibodies used to detect phospho-p38 may not be reliable for immunohistochemistry with gerbils. We therefore performed immunoblot using antibody to detect phospho-p38 (Cell Signaling Technology) in randomly selected 30 samples and confirmed the accuracy of the immunoblot for phospho-p38 described above because the results were identical irrespective of the antibodies we used (data not shown).
Expression of IFN-γ, IL-6, and KC in Gastric Mucosa of Mongolian Gerbils
It is well-known that proinflammatory cytokines, especially the cysteine-X-cysteine CXC chemokines (eg, IL-8), IL-6 and IFN-γ are involved in gastric mucosal injury. The IFN-γ promoter primarily is regulated by AP-1, whereas the IL-6 promoter is regulated by NF-κB, AP-1, and CREB. The promoter of KC, a CXC chemokine, is regulated by NF-κB and CREB. We extracted RNA from frozen gerbil gastric mucosal tissues and performed real-time RT-PCR. Consistent with previous studies,22,23 IFN-γ, IL-6, and KC mRNA levels were greater in WT H pylori-infected gerbils than in uninfected or cagE mutant-infected gerbils (see Supplementary Figure 4 online at www.gastrojournal.org). IFN-γ mRNA levels were decreased at 9 months, which differs from previous reports that IFN-γ mRNA levels remained high at 9 months.9,28 The reason was unclear; however, the IFN-γ mRNA levels correlated well with cellular infiltration, ERK phosphorylation, and AP-1 binding activity. These results are consistent with the fact that the IFN-γ promoter is regulated primarily by AP-1. IL-6 and KC mRNA levels were maximal at 3 months. At 18 months, mRNA levels of all 3 cytokines were significantly higher in the ulcer group than in the other groups.
We also extracted mRNA from formalin-fixed paraffin-embedded specimens from the 18-month time point. The erosive regions with infiltrating cells, but without epithelial cells, were easily microdissected by sharp dissection, and RT-PCR was performed. For comparison, mRNA was extracted from paraffin-embedded samples of whole mucosa from the same gerbil, and the same amount of RNA was used as template. Cytokine mRNA was undetectable in all uninfected controls. In infected samples, all 3 mRNA levels were significantly higher in whole mucosal and submucosal infiltrating samples than in uninfected controls (P < .01 for each) (Figure 7). IFN-γ mRNA levels were similar in the whole mucosa and in regions with infiltrating cells, consistent with the infiltrating cells being the primary origin of IFN-γ mRNA. IL-6 and KC mRNA levels were lower in regions with infiltrating cells, but the signals were still strong.
Figure 7.
Cytokine mRNA levels in Mongolian gerbil gastric mucosa using formalin-fixed paraffin-embedded specimens from samples 18 months postinfection. Cytokine mRNA levels (100,000 × cytokines mRNA/GAPDH mRNA) are presented as mean ± SE.
Discussion
Numerous studies have examined the effect of H pylori cocultivation with gastric cancer cells on the expression of transcription factors, especially NF-κB and AP-1 (reviewed in Naumann and Crabtree4). The applicability and transferability of the findings from these acute coculture experiments to in vivo conditions remain unclear. Natural H pylori infections tend to be stable or slowly progressing chronic infections with an inflammatory response involving many different cell types including inflammatory cells that infiltrate into the mucosa. We used the Mongolian gerbil model to examine the in vivo response. The gerbil model provides an opportunity to examine responses to H pylori that display different specific virulence factors both over time and with different patterns of mucosal damage including gastric mucosal inflammation, ulcers, and intestinal metaplasia. A few prior studies have investigated the roles of transcription factors (AP-1 and NF-κB) in gerbils with brain damage.29–32 This study focused on effects of H pylori infection on transcription factors in the gastric mucosa of gerbils. We also used DNA/protein arrays for 54 different transcription factors, based on the premise that consensus transcription factor binding sites are conserved among species. Although it is possible that failure to identify transcription factor up-regulation may have occurred related to differences across species, the possibility of false-positive results is low considering that our results of the arrays for the 4 transcription factors selected were confirmed by EMSA and supershift assays.
The pattern of transcription factor binding activity induced by H pylori infection in the gastric mucosal differed according to the phase and outcome of the infection. In the early phase of infection, activation of AP-1 and CREB was correlated with gastric cellular infiltration and activation of AP-1 and CREB was strongly dependent on the presence of the cag PAI as reflected by a reduced inflammatory response in the gastric mucosa of gerbils infected with cagE mutants. In addition, in the chronic phase of infection, activation of AP-1 and CREB was associated with gastric ulcerations. Although we could not determine the in vivo source of AP-1 and CREB directly, coculture experiments using primary gerbil gastric epithelial cells, as well as the identification of MAPKs as upstream inducers of AP-1 and CREB, suggested that cells infiltrating the submucosa are the main origin of activated AP-1 and CREB. Because direct adherence of H pylori to the infiltrating cells should be a rare occurrence, other factors induced by H pylori-infected epithelial cells likely are required to activate AP-1 and CREB in submucosal cells, which then induce proinflammatory factors such as IFN-γ (whose promoter mainly is regulated by AP-1). Although we could not directly measure the pathways, signaling pathways in human cells are well described and, along with our in vivo data, suggest that the ERK→AP-1 and CREB pathways are involved in H pylori-induced gastric cell infiltration and possibly in the development of gastric ulcers.
The binding activities of NF-κB and ISRE, as well as AP-1 and CREB, were induced in the chronic phase of infection. NF- κB and ISRE binding activities reached maximal levels 18 months after infection and correlated with the presence of gastric atrophy and intestinal metaplasia but not with severe cellular inflammation or development of gastric ulcers. H pylori also induced the phosphorylation of p38, JNK, and I κB; however, these phosphorylations also did not correlate with the severe cellular inflammation or the development of gastric ulcers. Combing these data with the well-known signaling pathways in human suggests that the p38→ISRE, JNK→NF-κB, and I κB →NF-κB/ISRE pathways appear to be related to the development of gastric atrophy. It has long been suspected that NF-κB signaling plays a pivotal role in malignancies associated with chronic inflammation.33–35 Several reports suggest that the main effects of NF-κB signaling on tumor development are exerted at the promotion and progression stages by preventing apoptosis of premalignant cells (reviewed in Li et al33 and Karin and Greten34). Although no cancers developed during the 18-month observation period of our study, activated NF-κB in gastric atrophy and intestinal metaplasia is consistent with a role for NF-κB in precursor stages of gastric malignancy.
Immunohistochemistry showed that phosphorylation of IκB in epithelial cells appears to be cag PAI dependent, whereas activation in submucosal cells is cag PAI independent. These data are in agreement with previous in vitro studies using gastric epithelial gastric cell lines15 and monocytic cell lines.36 Shibata et al also performed immunohistochemistry and reported that IκB phosphorylation levels were similar between cagE mutant-infected gerbils and uninfected gerbils20; however, the source of immunostaining cells was unclear in their study.
We used a DNA/protein array to examine the status of a number of transcription factors. We found that E2F1 binding activity was up-regulated by H pylori infection and that it increased in a time-dependent manner, becoming maximum at 18 months. The E2F transcription factor induces coordinated transactivation of genes such as dihydrofolate reductase, DNA polymerase α, thymidine kinase, and proliferating cell nuclear antigen (PCNA), which regulate cell proliferation, particularly at the G1 to S phase transition of the cell cycle.37 E2F has also been reported to be expressed in the nuclei of gastric epithelial cells within the gastric pit of human gastric mucosa, and its expression was significantly up-regulated in H pylori-infected gastric mucosa.38 In gerbils, similar levels of E2F1 were observed in the ulcer and atrophy groups. Further experiments are needed to determine the role, if any, of E2F family members in gastric injury.
We also found that USF-1 binding activity was increased by H pylori infection. USF transcription factors belong to the family of “basic helix-loop-helix” proteins and represent ubiquitously expressed mammalian nuclear proteins.39 Interestingly, infection with cagE mutants or WT H pylori resulted in similar levels of USF-1 activation in both the gastric mucosa and in primary epithelial cells. This finding agrees with recent in vitro studies showing that USF-1 activation of the cyclooxygenase-2 promoter during H pylori infection of AGS human gastric epithelial cells was independent of cag PAI status.40 Of interest, among the 54 transcription factors we examined, only USF-1 binding activity was independent of cag PAI status. It will be of interest to investigate other factors regulated by USF-1 to determine the effects of H pylori infection independent of cag PAI status.
Supplementary Material
Acknowledgments
Supported in part by grants from the National Institutes of Health DK62813 (to Y.Y.) and a Public Health Service grant (DK56338), which funds the Texas Gulf Coast Digestive Diseases Center.
Abbreviations used in this paper
- AP-1
activator protein-1
- CREB
cAMP responsive element binding protein
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- IFN
interferon
- IL
interleukin
- IRF
interferon regulatory factor
- ISRE
interferon-stimulated responsive element
- JNK
Jun-N-terminal kinase
- MAPK
mitogen-activated protein kinase
- PAI
pathogenicity island
- USF
upstream stimulatory factor
- WT
wild-type
Appendix
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at dx.doi:10.1053/j.gastro.2007.01.009.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene-positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naumann M, Crabtree JE. Helicobacter pylori-induced epithelial cell signaling in gastric carcinogenesis. Trends Microbiol. 2004;12:29–36. doi: 10.1016/j.tim.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31 Suppl 9:24–28. [PubMed] [Google Scholar]
- 6.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 7.Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta RM, Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O’Brien DP, Romero-Gallo J, Peek RM., Jr Activation of fS-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaoka Y, Yamauchi K, Ota H, Sugiyama A, Ishizone S, Graham DY, Maruta F, Murakami M, Katsuyama T. Natural history of gastric mucosal cytokines expression in Helicobacter pylori gastritis in Mongolian gerbils. Infect Immun. 2005;73:2205–2212. doi: 10.1128/IAI.73.4.2205-2212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaoka Y, Kwon DH, Graham DY. A Mr 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A. 2000;97:7533–7538. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai T, Fukui H, Franceschi F, Penland R, Sepulveda AR, Fujimori T, Terano A, Genta RM, Graham DY, Yamaoka Y. Cyclooxygenase expression during Helicobacter pylori Infection in Mongolian gerbils. Dig Dis Sci. 2003;48:2139–2146. doi: 10.1023/b:ddas.0000004517.83166.26. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka Y, Kita M, Kodama T, Imamura S, Ohno T, Sawai N, Ishimaru A, Imanishi J, Graham DY. Helicobacter pylori infection in mice: role of outer membrane proteins in colonization and inflammation. Gastroenterology. 2002;123:1992–2004. doi: 10.1053/gast.2002.37074. [DOI] [PubMed] [Google Scholar]
- 13.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Brasier AR, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo RP. A promoter recruitment mechanism for tumor necrosis factor-α-induced interleukin-8 transcription in type II pulmonary epithelial cells Dependence on nuclear abundance of RelA, NF- κB1, and c-Rel transcription factors. J Biol Chem. 1998;273:3551–3561. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 15.Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126:1030–1043. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Walton KM, DiRocco R, Bartlett BA, Koury E, Marcy VR, Jarvis B, Schaefer EM, Bhat RV. Activation of p38 MAPK in microglia after ischemia. J Neurochem. 1998;70:1764–1767. doi: 10.1046/j.1471-4159.1998.70041764.x. [DOI] [PubMed] [Google Scholar]
- 17.Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawano T, Fukunaga K, Takeuchi Y, Morioka M, Yano S, Hamada J, Ushio Y, Miyamoto E. Neuroprotective effect of sodium or-thovanadate on delayed neuronal death after transient forebrain ischemia in gerbil hippocampus. J Cereb Blood Flow Metab. 2001;21:1268–1280. doi: 10.1097/00004647-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Koyama M, Spicer SS, Schulte BA. Distribution of IκB proteins in gastric mucosa and other organs of mouse and gerbil. J Histo-chem Cytochem. 2000;48:191–200. doi: 10.1177/002215540004800204. [DOI] [PubMed] [Google Scholar]
- 20.Shibata W, Hirata Y, Maeda S, Ogura K, Ohmae T, Yanai A, Mitsuno Y, Yamaji Y, Okamoto M, Yoshida H, Kawabe T, Omata M. CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J Pathol. 2006;210:306–314. doi: 10.1002/path.2040. [DOI] [PubMed] [Google Scholar]
- 21.Tanahashi T, Kita M, Kodama T, Yamaoka Y, Sawai N, Ohno T, Mitsufuji S, Wei YP, Kashima K, Imanishi J. Cytokine expression and production by purified Helicobacter pylori urease in human gastric epithelial cells. Infect Immun. 2000;68:664–671. doi: 10.1128/iai.68.2.664-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H, Wu JY, Kudo T, Ohno T, Graham DY, Yamaoka Y. Regulation of interleukin-6 promoter activation in gastric epithelial cells infected with Helicobacter pylori. Mol Biol Cell. 2005;16:4954–4966. doi: 10.1091/mbc.E05-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229–1242. doi: 10.1053/j.gastro.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 24.Saito H, Yamaoka Y, Ishizone S, Maruta F, Sugiyama A, Graham DY, Yamauchi K, Ota H, Miyagawa S. Roles of virD4 and cagG genes in the cag pathogenicity island of Helicobacter pylori using a Mongolian gerbil model. Gut. 2005;54:584–590. doi: 10.1136/gut.2004.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419–429. doi: 10.1016/S0002-9440(10)63985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo T, Lu H, Wu JY, Graham DY, Casola A, Yamaoka Y. Regulation of RANTES promoter activation in gastric epithelial cells infected with Helicobacter pylori. Infect Immun. 2005;73:7602–7612. doi: 10.1128/IAI.73.11.7602-7612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197–207. doi: 10.1002/path.1498. [DOI] [PubMed] [Google Scholar]
- 29.Kuramoto N, Azuma Y, Inoue K, Ogita K, Mitani A, Zhang L, Yanase H, Masuda S, Kataoka K, Yoneda Y. Correlation between potentiation of AP1 DNA binding and expression of c-Fos in association with phosphorylation of CREB at serine133 in thalamus of gerbils with ischemia. Brain Res. 1998;806:152–164. doi: 10.1016/s0006-8993(98)00693-3. [DOI] [PubMed] [Google Scholar]
- 30.Domanska-Janik K, Bong P, Bronisz-Kowalczyk A, Zajac H, Zablocka B. AP1 transcriptional factor activation and its relation to apoptosis of hippocampal CA1 pyramidal neurons after transient ischemia in gerbils. J Neurosci Res. 1999;57:840–846. doi: 10.1002/(sici)1097-4547(19990915)57:6<840::aid-jnr9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Kapinya K, Penzel R, Sommer C, Kiessling M. Temporary changes of the AP-1 transcription factor binding activity in the gerbil hippocampus after transient global ischemia, and ischemic tolerance induction. Brain Res. 2000;872:282–293. doi: 10.1016/s0006-8993(00)02503-8. [DOI] [PubMed] [Google Scholar]
- 32.Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjanheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor κ-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004;91:755–765. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF- κB is the lynchpin. Trends Immunol. 2005;26:318–325. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 35.Pikarsky E, Ben-Neriah Y. NF-κB inhibition: a double-edged sword in cancer? Eur J Cancer. 2006;42:779–784. doi: 10.1016/j.ejca.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, Shiratori Y, Omata M. Distinct mechanism of Helicobacter pylori-mediatedNF-kB activation between gastric cancer cells and monocytic cells. J Biol Chem. 2001;276:44856–44864. doi: 10.1074/jbc.M105381200. [DOI] [PubMed] [Google Scholar]
- 37.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 38.Isomoto H, Furusu H, Shin M, Ohnita K, Miyazaki M, Omagari K, Mizuta Y, Murase K, Inoue K, Murata I, Koji T, Kohno S. Enhanced expression of transcription factor E2F in Helicobacter pylori-infected gastric mucosa. Helicobacter. 2002;7:152–162. doi: 10.1046/j.1523-5378.2002.00075.x. [DOI] [PubMed] [Google Scholar]
- 39.Sirito M, Lin Q, Maity T, Sawadogo M. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res. 1994;22:427–433. doi: 10.1093/nar/22.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jüttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, Wunder C, Weber M, Fischer SM, Schmidt WE, Wiedenmann B, Meyer TF, Naumann M, Höcker M. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol. 2003;5:821–834. doi: 10.1046/j.1462-5822.2003.00324.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.