Abstract
Superoxide dismutases (SOD) are considered to be antioxidant enzymes. This view came about because its substrate, superoxide, is a free radical; in the era of their discovery, 1960’s – 1970’s, the general mindset was that free radicals in biology must be damaging. Indeed SOD blunts the cascade of oxidations initiated by superoxide. However in the late 1970’s it was observed that cancer cells that have low activity of the mitochondrial form of SOD, MnSOD, grow faster than those with higher activities of MnSOD. These observations indicated that SOD, superoxide, and hydrogen peroxide affected the basic biology of cells and tissues, not just via damaging oxidation reactions. It is now realized that superoxide and hydrogen peroxide are essential for normal cellular and organism function. MnSOD appears to be a central player in the redox biology of cells and tissues.
Keywords: superoxide dismutase, mitochondria, coenzyme Q, hydrogen peroxide, superoxide, redox environment, hypoxia inducible factor, iron
Introduction
With the discovery of: 1) the superoxide dismutases by McCord and Fridovich in 1968–1969 [1]; 2) the concomitant discovery that superoxide can be produced by enzymes [2]; and 3) that free radicals can be substrates for an enzyme completely revolutionized the thinking on the role of free radicals and related oxidants in biology [3]. Because free radicals can bring about harmful oxidations, research initially concentrated on the chemistry of the individual reactive oxygen species and the damage they could inflict. In those early years much energy was invested in determining the kinetics and mechanisms of these detrimental oxidative reactions [4, 5, 6, 7, 8, 9].
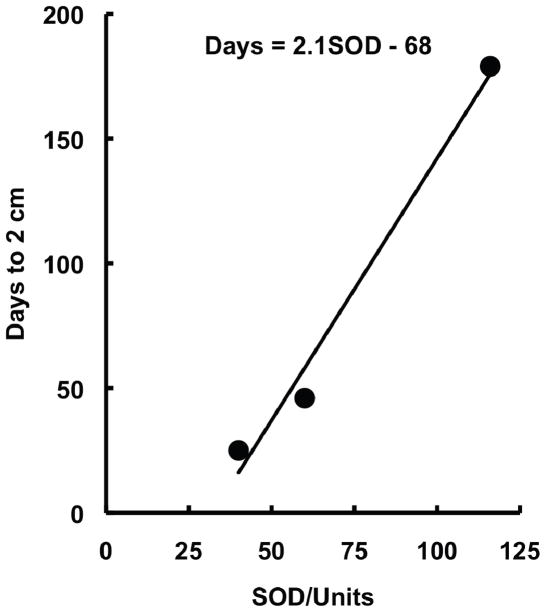
In the 1980’s the thinking in this new field was broadened with the introduction of a new, and then controversial, idea that at lower concentrations, superoxide or hydrogen peroxide could induce cell division [10, 11, 12]. This concept arose because it was observed that tumor cells have lower manganese superoxide dismutase (MnSOD) than corresponding normal cells, suggesting a role for superoxide in cell proliferation [10]. Most convincing of a role for SOD and superoxide was a study by Bize and Oberley on the Morris hepatomas [13]. In this tumor model, they found that the growth rate of the tumor was inversely proportional to the activity of MnSOD, Fig. (1). The idea that superoxide and hydrogen peroxide are important components of normal biology began to take firm hold in the 1990s with the development of molecular biology techniques that allowed controlled expression of proteins; these new tools revealed that increased expression of manganese superoxide dismutase suppresses the malignant phenotype of human melanoma cells [14]. At this time the idea that ROS could be signaling molecules gained broader acceptance and the field of redox biology began to grow exponentially [15, 16, 17, 18]. Redox biology is now viewed by many as a fundamental determinant of the basic biology of cells and tissues, disease and health [19, 20]. SOD serves not only as a protective enzyme, but also has a central role in determining the basic biology of cells and tissues [21].
Figure 1.
The rate of tumor growth is inversely related to SOD activity. Shown are total SOD activity in slow-, medium- and fast-growing Morris hepatomas. Data from [13].
The flow of electrons
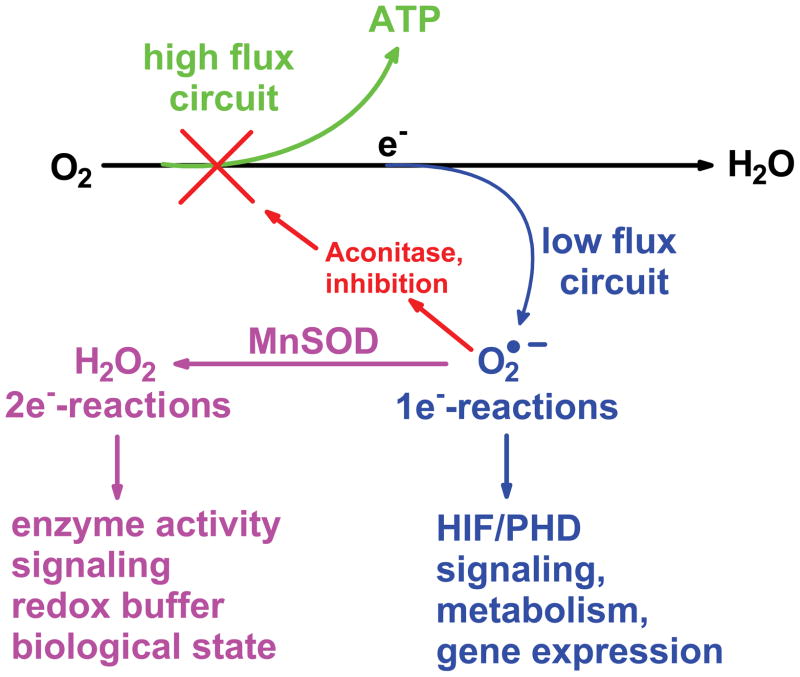
The major flow of electrons in normal cell metabolism is through the mitochondrial electron transport chain (ETC). There, oxygen is reduced to water producing energy that is harvested in ATP to build cellular structures and maintain them. A small percentage (≈ 1% or less [22, 23, 24]) is diverted via a one-electron reduction of dioxygen to produce the superoxide radical. These two pathways have been proposed as two distinct types of mitochondrial redox circuits [20]; a high flux circuit that results in energy production and a low flux circuit that results in regulation of metabolism and that initiates cell signaling, Fig. (2). The low flux circuit that produces O2•− and H2O2 appears to be controlled in part by superoxide dismutase (SOD) [21].
Figure 2. The mitochondrial electron transport chain has two principal circuits.
During the oxidation of carbon-based substrates, the vast majority of the electrons captured are shuttled to dioxygen to produce water, the high flux circuit; the energy is captured to produce ATP. In the low flux circuit a small fraction of the total electron flow moves to dioxygen in a one-electron reduction to produce superoxide. This superoxide can react with iron-sulfur centers such as aconitase to modulate the flux in the high flux circuit. Superoxide could also react with the iron-center of enzymes, such as the prolyl hydroxylases, inactivating the enzyme leading to accumulation of HIF-1α and subsequent gene expression. MnSOD will control the steady-state level of mitochondrially-produced superoxide as well as the flux of H2O2 [21]. This will have effects on two-electron signaling pathways, the redox buffer and the biological state of the cell.
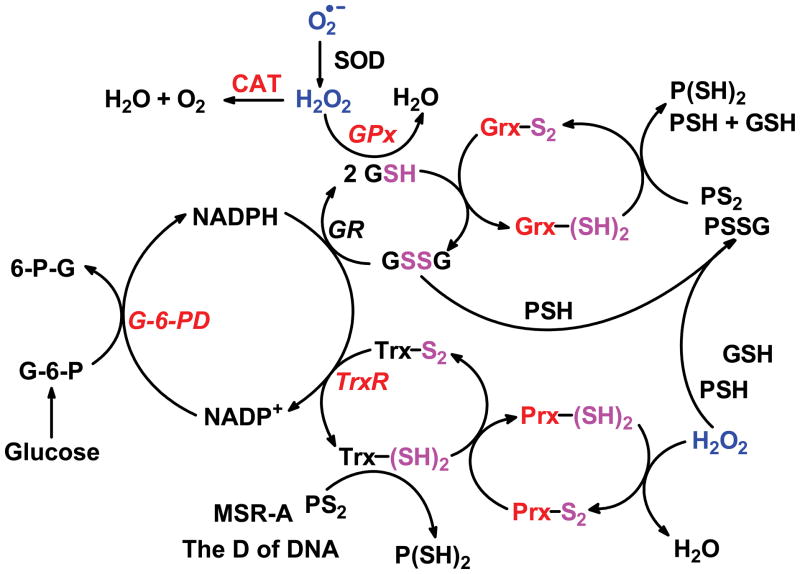
SOD and the peroxide-removing system assure that the steady-state levels of superoxide and H2O2 remain relatively low, Fig. (3). In addition to the electron transport chain, superoxide can be produced by the Nox-family of enzymes as well as xanthine oxidase [25]. H2O2 can be produced directly by several enzymes, for example oxidase enzymes in the peroxisomes [26, 27]. As signaling molecules, O2•− and H2O2, have quite different chemistry; superoxide can be either a one-electron oxidizing agent, mostly via HO2•, or a one-electron reducing agent; H2O2 is principally a two-electron oxidizing agent. These two different chemistries assure versatility as well as specificity in cellular signaling.
Figure 3. Overview of the thiol redox couples that set the redox status of cells and tissues.
SOD is central as it controls the steady-state level of superoxide as well as the flux of H2O2. The flux of H2O2 affects both nodes of the peroxide removal/redox system, i.e. both the GSH and Trx nodes.
One-electron reactions: radicals and antioxidants
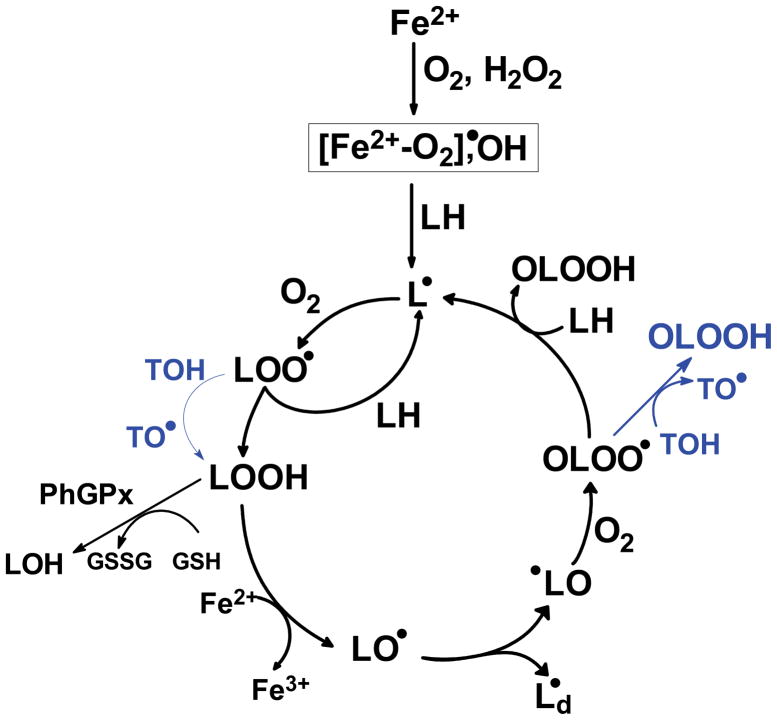
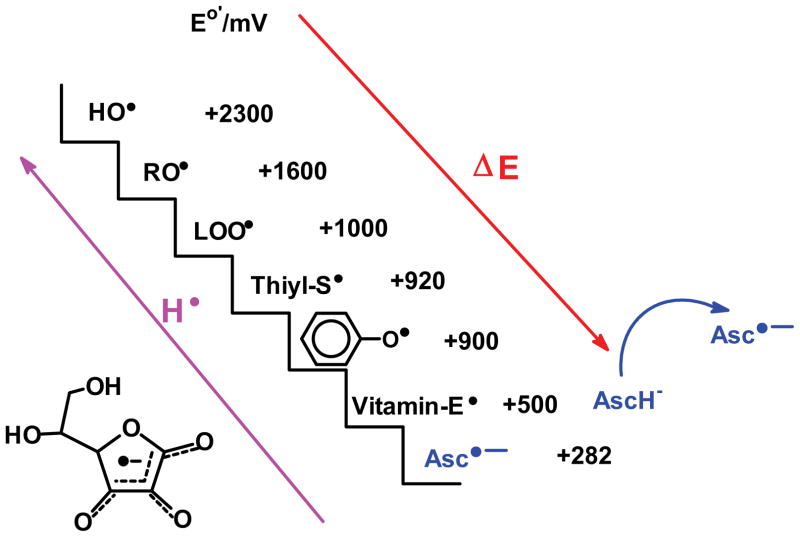
Most chemical reactions in living systems that involve organic molecules are two-electron reactions. In general one-electron reactions are avoided because they can result in damaging free radical processes. The damaging free radicals can be removed by one-electron reactions with small molecule antioxidants [28]. For example, in free radical-mediated lipid peroxidation, one-electron oxidants generate a carbon-centered radical on the lipid chain, Fig. (4). This radical reacts rapidly with oxygen forming reactive peroxyl radicals. Donor antioxidants such as tocopherol can convert lipid peroxyl radicals (LOO•) to a less reactive lipid hydroperoxide (LOOH). The tocopheroxyl radical can be recycled in a one-electron reaction by ascorbate. The ascorbate radical is quite unreactive and thus far less damaging than the original peroxyl radical, Fig. (5).
Figure 4. Shown is an overview of lipid peroxidation and points where tocopherol can act as a donor antioxidant.
In this example iron-derived oxidants are shown as initiators. In addition Fe2+ is shown as a facilitator of chain-branching reactions. Although lipid-derived alkoxyl radicals (LO•) are quite oxidizing and could initiate new chains, i.e. LO• + LH → LOH + L•, rearrangement to an epoxide is the dominant reaction [29, 30]. Shown also is an example of how two-electron processes remove LOOH, here phospholipid hydroperoxide glutathione peroxidase (GPx4) using GSH as a source of reducing equivalents.
Figure 5. Ascorbate is the terminal, water-soluble, small-molecule antioxidant [48].
Among its many functions as a reducing agent, ascorbate can serve as a one-electron reductant to recycle tocopherol. In this way it exports free radicals that are in a lipid structures (here LOO• and TO•) to the aqueous phase where enzymes and other reducing agents can recycle Asc•− back to AscH−. By reducing TO• (vitamin-E•) back to TOH further oxidation to tocopherol quinone is prevented; tocopherol quinone will form irreversible products and thus vitamin E lost. In addition it prevents TO• from initiating further oxidations [49].
This sequence of reactions has two major consequences:
The dangerous one-electron oxidant is exported from the vulnerable lipid phase into the aqueous phase of cells where enzyme systems can detoxify and “recycle” ascorbate.
The remaining oxidant in the lipid phase (LOOH) is now available to the 2-electron antioxidant system for use or removal.
One-electron reactions, redox active metals and electron flux
One-electron reactions are common when redox active metal ions such as copper, manganese, or iron are involved. These metals are essential parts of the active sites of many enzymes, including the superoxide dismutases. Superoxide can be an oxidant, a reductant, or both, depending on the coordination environment of the metal. The production of superoxide by the mitochondrial electron transport chain is the gateway to the low-flux signaling circuits of cells and tissues, Fig. (2); it provides the connection to the high flux, energy-harvesting circuits of ATP production. Superoxide reacts with the metal-containing active site of SODs alternately as a reductant and then as an oxidant, yielding oxygen and H2O2. The steady-state level of O2•− is inversely related to [SOD] [21]. Thus, SOD controls the flux of electrons entering the one-electron signaling and regulatory pathways, Fig. (2). Hydrogen peroxide is a product of the SOD-catalyzed dismutation of O2•−. This H2O2 is a key regulator of the redox states of the various redox couples of two-electron signaling circuits. Thus, in the mitochondria, superoxide is the entry point for both the one-electron and two-electron signaling circuits and MnSOD is at the node of the bifurcation point for these pathways.
One-electron reactions, redox active metals and gene expression
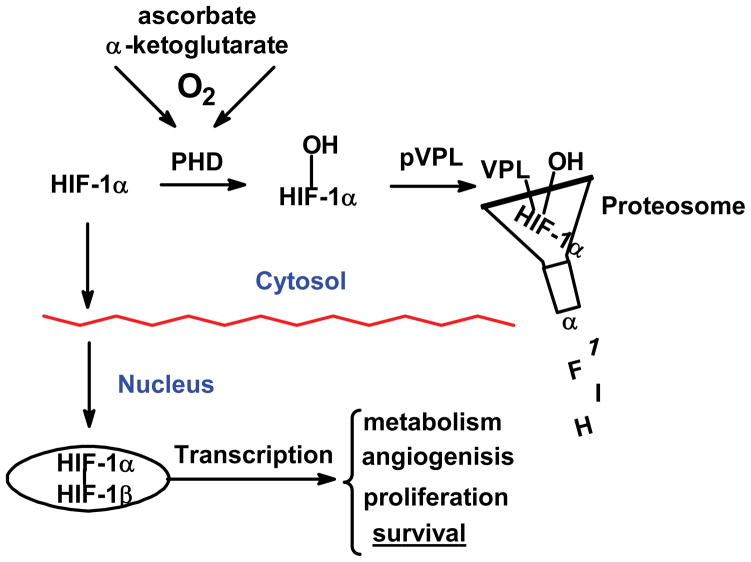
Superoxide can influence gene expression by one-electron reactions. It has recently been shown that superoxide, via manganese-containing superoxide dismutase (MnSOD), can regulate the levels of the transcription factor hypoxia inducible factor-1α (HIF-1α) [29, 30]. The HIF-system is responsible for sensing the local levels of oxygen and regulates literally hundreds of genes ranging from basic metabolism, angiogenesis, to cell proliferation [31]. There are many routes to the modulation of the levels of HIF-1α; the primary route centers on the hydroxylation of HIF-1α by prolyl hydroxylase (PHD), Fig. (6). When oxygen is present, PHD can hydroxylate HIF-1α marking it for degradation [31]. However, when the levels of dioxygen are low, HIF-1α accumulates and moves to the nucleus where it assembles into the transcription factor HIF-1.
Figure 6. Prolyl hydroxylases serve as sensors of cell/tissue dioxygen controlling HIF-1α accumulation and subsequent gene expression.
PHD is an iron-containing enzyme; the iron must be present as Fe2+. This ferrous iron activates dioxygen resulting in the oxidation of proline residues, leading to degradation. A lack of oxygen or disturbance of this iron will lead to accumulation of HIF-1α and subsequent gene expression. This simplified scheme does not show all the routes to regulation of HIF system.
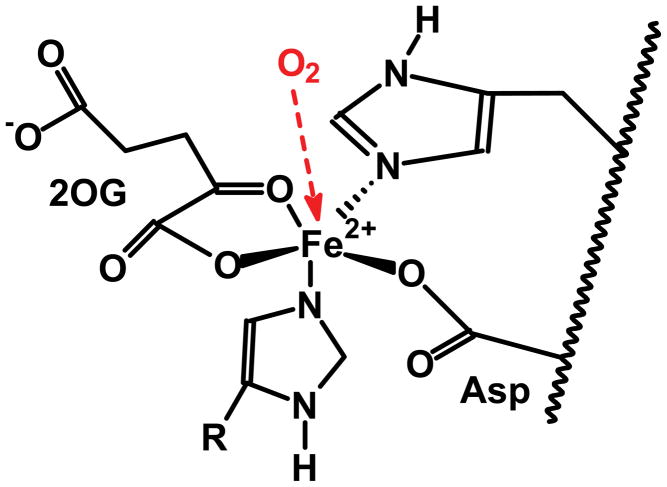
PHD is an iron-containing enzyme; the sensing of oxygen as well as the hydroxylation reaction are all centered on iron. This iron has a very loose coordination environment, with two histidines and a carboxylate group (Asp or Glu) from the PHD protein as ligands, Fig. (7). The ligands for the remaining three coordination sites, α-keto-glutarate and dioxygen, are transient. During the catalytic reaction, molecular oxygen is fully reduced; two electrons come from α-keto-glutarate, which leaves as succinate and CO2, the remaining two electrons come from a proline. The coordination environment of the Fe2+ in PHD would be amenable to reaction with O2•− (oxidation via HO2•). In addition the ferryl (i.e. Fe(IV)) intermediate that is formed during the catalytic cycle [32] would be especially vulnerable to a reducing agent such as superoxide. In both of these possible reactions of superoxide, the Fe2+ of the resting state enzyme and the ferryl intermediate generated in the catalytic cycle would be converted to Fe3+; the result would be the inactivation of PHD and subsequent accumulation of HIF-1α. Thus, for example, decreasing the activity of MnSOD in mitochondria could inactivate PHD, leading to increased HIF and subsequent gene expression [29, 30].
Figure 7. The coordination environment of typical prolyl hydroxylases.
Only three of the six coordination sites are from the protein. The remaining three sites are open so substrates, 2-oxoglutarate (2OG) and dioxygen, can “come and go”. This results in the iron being susceptible to oxidants, e.g. superoxide. (Protonation of superoxide yields HO2•, a strong oxidant.) Protons from ionizable amino acids near the active site can facilitate this change in reactive character of superoxide. This type of structural feature and mechanism converts superoxide, a weak reductant, to HO2• an oxidant in SOD [50, 51].
This example shows how one-electron reactions via superoxide with the iron of PHD-type enzymes initiates signaling cascades that result in gene expression, Fig. (2).
One-electron reactions, redox active metals and metabolism
The mitochondrial enzyme aconitase is a key enzyme in the Krebs cycle and thus is a fundamental link in the production of ATP. The active site of aconitase contains an iron sulfur cluster. Superoxide reacts with this cluster rapidly, k ≈ 107 M−1 s−1 [33, 34]. In this reaction superoxide actually oxidizes the Fe4S4 cluster of aconitase. There are amino acid residues near the active site that provide hydrogen bonding for the substrate, e.g. isocitrate, during the reaction [35]. These residues could also serve to protonate superoxide, facilitating the oxidation of the cluster. This oxidative reaction of superoxide inactivates the enzyme thereby slowing the basic energy metabolism of the cell. This inactivation provides a feedback mechanism from the low flux signaling circuit to the high flux circuit of energy metabolism, Fig. (2). Superoxide can be viewed as a brake for aconitase and ATP production. This is a good example of O2•− being an efficient effecter-molecule in controlling metabolic switches. The inhibition of aconitase has additional ramification. Its inactivation would lower the production of α-ketoglutarate, which could lead to accumulation of HIF-1α because α-ketoglutarate is an essential cofactor of PHD.
The one-electron, low-flux circuits originating in mitochondria provide feedback to the high flux circuit of ATP production. In addition these low flux circuits connect to metabolism and gene expression. The H2O2 produced from superoxide is an entry point to two-electron signaling processes.
Two-electron signaling
The low flux, two-electron circuitry has NADPH and thiols as fundamental redox couples. The conversion of hydrogen peroxide to water requires two reducing equivalents. The glutathione peroxidase family of enzymes (GPx) uses glutathione as this source of reducing equivalents.
The peroxiredoxin (Prx) family of enzymes also removes H2O2 with thioredoxin as the principal source of reducing equivalents.
Each system gathers electrons from NADPH to return GSSG and Trx(SS) back to their corresponding reduced forms, GSH and Trx(SH)2, Fig. (3).
Changes in the flux of H2O2 will present differing demands on these reducing systems and thereby change the redox state of these redox couples; the flux of H2O2 would contribute to the redox state of the GSSG,2H+/2GSH couple, the principal component of the redox buffer of cells and tissues [19, 20]. Changes in the redox state of these couples will have consequences on signaling cascades and thereby on the biological state.
Two-electron reactions: redox buffer and enzyme activity
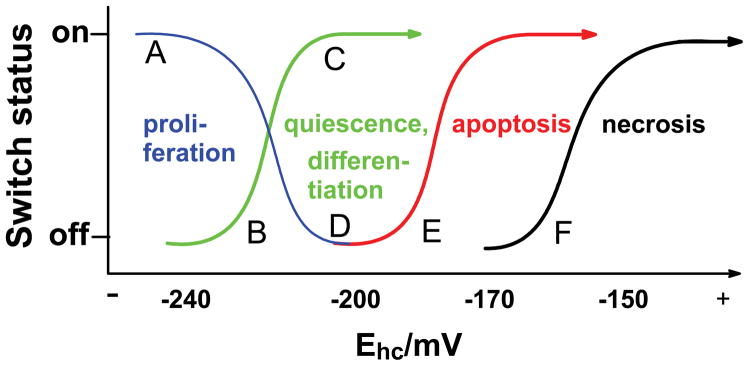
Cells and tissues must maintain a reducing environment to survive. The reducing environment is maintained by a redox buffer that consists of an array of linked redox couples, Fig. (3). The redox environment of a cell is a reflection of the redox state of these couples [19]. Glutathione is considered to be the major component of this redox buffer. H2O2 produced by the mitochondrial low flux circuit, via superoxide and MnSOD, can change the state of the redox buffer by drawing on the reducing equivalents of the buffer [36]. The flux of H2O2 can change the status of the GSSG/2GSH couple to a more oxidized state as H2O2 is reduced to water by GPx. The metabolic flux of H2O2 and glutathione disulfide reductases can keep the GSSG/2GSH buffer within its normal range for the appropriate biological state. However, if the flux of H2O2 is increased, then GSSG may accumulate to higher levels; this can alter the redox state of the GSSG/2GSH couple and potentially change the biological state of the cell, Fig. (8).
Figure 8. Reduction potential-driven nano-switches move cells through different biological stages [19, 52].
The intracellular redox environment changes throughout its life cycle. For example, cancer cells have disruptions in the low-flux and high flux circuits that result in their not progressing to the quiescent or differentiated state. Rather they retain a more reduced intracellular environment and remain the proliferative state.
(A) During proliferation the half-cell reduction potential (Ehc) for the GSSG,2H+/2GSH couple has the most negative value. The switches for proliferation are fully on.
(B) When Ehc (GSH) becomes more positive, the differentiation switches can be turned on or the cells are quiescence while proliferation decreases.
(C) The more positive Ehc (GSH) becomes the more differentiation switches are turned on until they reach a maximum where nearly all cells are differentiating.
(D) While cells undergo differentiation, proliferation switches are turned down and finally turned off. Cells that are not terminally differentiated could undergo proliferation with an appropriate signal and associated redox environment. We would expect that these partially differentiated cells might shift to a more negative reduction potential, i.e., to the left. Quiescent cells are considered to be in this state; however, this state is reversible [38, 39]; quiescent cells can return to a more reduced environment and re-enter the cell cycle; terminally differentiated cells cannot.
(E) If Ehc (GSH) becomes too positive, then death signals are activated and apoptosis is initiated. This mechanism provides for the orderly removal of cells that have lost their ability to control their redox environment and therefore, are not functioning normally. It should also coincide with signaling pathways to purposely dispose of unneeded cells.
(F) Very high values of Ehc (GSH), i.e. more positive values, perhaps resulting from severe oxidative stress, leave only necrosis as a path to cell death.
In general, cancer cells are considered to be under oxidative stress. However, there is good evidence that the redox state of the intracellular GSSG/2GSH couple remains relatively reduced (EGSSG/2GSH = ≈−240 mV); this reduced status correlates with proliferation [19]. The intracellular redox state of the GSSG/2GSH couple is more positive in cells that are not proliferating. Changes in the redox state of the intracellular redox buffer are associated with the cell cycle; as initially proposed by Goswami et al., there is a redox cycle within the cell cycle and MnSOD appears to be fundamental element in this redox cycle [37, 38, 39].
Although oxidative stress is often thought of as producing damage, a better view is that oxidative stress disrupts cellular redox circuits [20]. This disruption would occur when there is an imbalance of oxidant production and removal. MnSOD in the mitochondria would be a key player in establishing the appropriate balance in the redox circuitry of mitochondria.
MnSOD, the bifurcation point between 1-e− and 2-e− signaling
Superoxide dismutase has traditionally been considered as an antioxidant enzyme. However, as presented above, it is clear that MnSOD also is central in setting the basic biology of cells. It is clear that the steady-state level superoxide is inversely proportional to the activity of MnSOD [21]. However, SOD can also influence the flux of O2•− and consequently the flux of H2O2 in some settings. SOD will not affect the flux of O2•− or H2O2 from typical sources in which flavins are the conduit for electrons to dioxygen, such as xanthine oxidase. However, SOD will be able to alter the flux of O2•− or H2O2 from some quinone/semiquinone/hydroquinone triads, providing the thermodynamics and kinetics are appropriate [40]. The coenzyme Q triad appears to have the appropriate thermodynamics and kinetics for SOD to “pull” the reaction of coenzyme Q semiquinone with dioxygen to form superoxide [21],
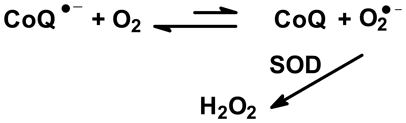 |
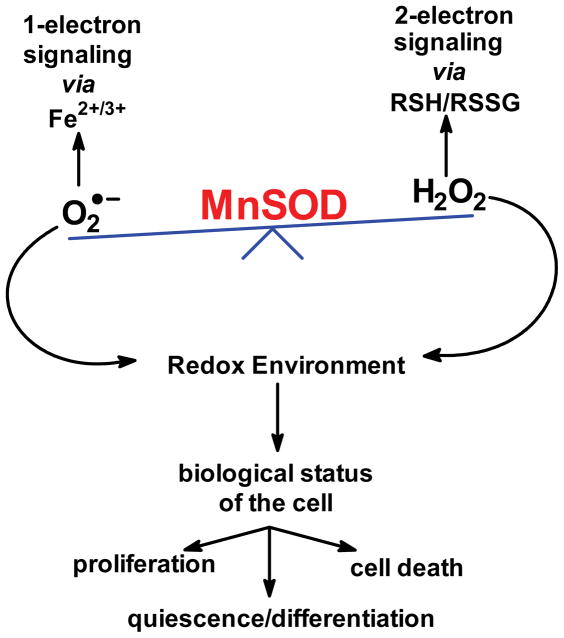
An increase in the levels of H2O2 with increasing activity of SOD in cells has been observed [21, 41, 42, 43, 44 ]. These reactions allow SOD to affect both the 1-electron and 2-electron signaling pathways, i.e. MnSOD is at the bifurcation point between these two separate signaling pathways Fig. (9). These possibilities explain in part how changing the activities and ratios of SOD and GPx in tumor cells can affect cell doubling time and tumorigenicity [41, 45, 46]. This source of H2O2 also appears to have a major role in metastases, reviewed in [47]. Thus, it is clear that MnSOD is a central redox enzyme, i.e. it plays a major role in establishing the biology of cells and tissues.
Figure 9. MnSOD in the mitochondrial matrix is at the bifurcation point between 1-electron and 2-electron signaling.
The different signaling cascades provide a feedback mechanism for the high-flux metabolic circuit as well as low flux circuits that lead to gene expression, such as with the HIF pathway as presented in Fig. (2).
Acknowledgments
Supported by grant R01GM073929 from the NIGMS/NIH. The content is solely the responsibility of the author and does not represent views of the NIGMS or the NIH. The University of Iowa ESR Facility provided invaluable support.
References
- 1.McCord JM, Fridovich I. Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 2.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 3.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 4.Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of hydroperoxyl/superoxide radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1041–1100. [Google Scholar]
- 5.Gordon S, Sullivan JC, Ross AB. Rate constants for reactions of radiation-produced transients in aqueous solution of actinides. J Phys Chem Ref Data. 1988;15:1637–1755. [Google Scholar]
- 6.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J Phys Chem Ref Data. 1988;17:513–886. [Google Scholar]
- 7.Neta P, Huie RE, Ross AB. Rate constants for reactions of inorganic radicals in aqueous solutions. J Phys Chem Ref Data. 1988;17:1027–1284. [Google Scholar]
- 8.Hoffman MZ, Bolletta F, Moggi L, Hug GL. Rate constants for the quenching of excited states of metal complexes in fluid solution. J Phys Chem Ref Data. 1989;18:219–543. [Google Scholar]
- 9.Wardman P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J Phys Chem Ref Data. 1989;18:1637–1755. [Google Scholar]
- 10.Oberley LW, Buettner GR. The role of superoxide dismutase in cancer: A review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 11.Oberley LW, Oberley TD, Buettner GR. Cell differentiation, aging and cancer: The possible roles of superoxide and superoxide dismutase. Med Hypotheses. 1980;6:249–268. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- 12.Oberley LW, Oberley TD, Buettner GR. Cell division in normal and transformed cells: The possible role of superoxide dismutase and hydrogen peroxide. Med Hypotheses. 1981;7:21–42. doi: 10.1016/0306-9877(81)90018-9. [DOI] [PubMed] [Google Scholar]
- 13.Bize IB, Oberley LW, Morris HP. Superoxide dismutase and superoxide radical in Morris hepatomas. Cancer Res. 1980;40:3686–3693. [PubMed] [Google Scholar]
- 14.Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS, Trent JM. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci U S A. 1993;90:3113–3117. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiavone JR, Hassan HM. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem. 1988;263(9):4269–4273. [PubMed] [Google Scholar]
- 16.Malter JS, Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991;266(5):3167–3171. [PubMed] [Google Scholar]
- 17.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Buettner GR, Oberley LW. Oxygen free radicals mediate the induction of manganese superoxide dismutase gene expression by TNF-α in human oral carcinoma SCC-25 cells. Free Radic Biol Med. 2000;28:1197–1205. doi: 10.1016/s0891-5849(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 19.Schafer FQ, Buettner GR. Redox state of the cell as viewed though the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 20.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chemical–Biological Interactions. 2006;163:38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Buettner GR, Ng CF, Wang W, Rodgers VGJ, Schafer FQ. A new paradigm: Manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006;41:1338–1350. doi: 10.1016/j.freeradbiomed.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 26.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 27.Bonekamp NA, Völkl A, Fahimi HD, Schrader M. Reactive oxygen species and peroxisomes: struggling for balance. Biofactors. 2009;35:346–355. doi: 10.1002/biof.48. [DOI] [PubMed] [Google Scholar]
- 28.Buettner GR. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Kirk JS, Venkataraman S, Domann FE, Zhang HJ, Schafer FQ, Flanagan SW, Weydert CJ, Spitz DR, Buettner GR, Oberley LW. Manganese superoxide dismutase suppresses hypoxic induction of hypoxia inducible factor-1α and vascular endothelial growth factor. Oncogene. 2005;24:8154–8166. doi: 10.1038/sj.onc.1208986. [DOI] [PubMed] [Google Scholar]
- 30.Kaewpila S, Venkataraman S, Buettner GR, Oberley LW. Manganese superoxide dismutase modulates Hypoxia Inducible Factor-1α induction via superoxide. Cancer Res. 2008;68(8):2781–2788. doi: 10.1158/0008-5472.CAN-07-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 32.Purpero V, Moran GR. The diverse and pervasive chemistries of the alpha-keto acid dependent enzymes. J Biol Inorg Chem. 2007;12:587–601. doi: 10.1007/s00775-007-0231-0. [DOI] [PubMed] [Google Scholar]
- 33.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 34.Gardner PR, Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- 35.Lauble H, Kennedy MC, Beinert H, Stout CD. Crystal structures of aconitase with trans-aconitate and nitrocitrate bound. J Mol Biol. 1994;237:437–451. doi: 10.1006/jmbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- 36.Ng CF, Schafer FQ, Buettner GR, Rodgers VGJ. The rate of cellular hydrogen peroxide removal shows dependency on GSH: Mathematical insight into in vivo H2O2 and GPx concentrations. Free Rad Res. 2007;41:1201–1211. doi: 10.1080/10715760701625075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26(8):1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 38.Sarsour EH, Venkataraman S, Kalen AL, Oberley LW, Goswami PC. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell. 2008;7(3):405–417. doi: 10.1111/j.1474-9726.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11(12):2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y, Buettner GR. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic Biol Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkataraman S, Jiang X, Weydert CJ, Zhang Y, Zhang HJ, Goswami PC, Ritchie JM, Oberley LW, Buettner GR. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene. 2005;24:77–89. doi: 10.1038/sj.onc.1208145. [DOI] [PubMed] [Google Scholar]
- 42.Venkataraman S, Wagner BA, Jiang X, Wang HP, Schafer FQ, Ritchie JM, Burns CP, Oberley LW, Buettner GR. Overexpression of manganese superoxide dismutase promotes the survival of prostate cancer cells exposed to hyperthermia. Free Rad Res. 2004;38:1119–1132. doi: 10.1080/10715760400010470. [DOI] [PubMed] [Google Scholar]
- 43.Zhang HJ, Zhao W, Venkataraman S, Robbins ME, Buettner GR, Kregel KC, Oberley LW. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002;277(23):20919–20926. doi: 10.1074/jbc.M109801200. [DOI] [PubMed] [Google Scholar]
- 44.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J, Van De Water L, Mian BM, Melendez JA. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007;67:10260–10267. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 45.Ridnour LA, Oberley TD, Oberley LW. Tumor suppressive effects of MnSOD overexpression may involve imbalance in peroxide generation versus peroxide removal. Antioxid Redox Signal. 2004;6(3):501–512. doi: 10.1089/152308604773934260. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60(14):3927–3939. [PubMed] [Google Scholar]
- 47.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anti-Cancer Agents in Medicinal Chemistry. 2011;11(2):191–201. doi: 10.2174/187152011795255911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buettner GR, Jurkiewicz BA. The ascorbate free radical as a marker of oxidative stress: An EPR study. Free Rad Biol Med. 1993;14:49–55. doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- 49.Bowery VW, Stocker R. Tocopherol-mediated peroxidation. The prooxidants effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc. 1993;115:6029–6043. [Google Scholar]
- 50.Cudd A, Fridovich I. Electrostatic interactions in the reaction mechanism of bovine erythrocyte superoxide dismutase. J Biol Chem. 1982;257:11443–11447. [PubMed] [Google Scholar]
- 51.Benovic J, Tillman T, Cudd A, Fridovich I. Electrostatic facilitation of the reaction catalyzed by the manganese-containing and the iron-containing superoxide dismutases. Arch Biochem Biophys. 1983;221:329–332. doi: 10.1016/0003-9861(83)90151-0. [DOI] [PubMed] [Google Scholar]
- 52.Schafer FQ, Buettner GR. Redox state and redox environment in biology. In: Forman HJ, Torres M, Fukuto J, editors. Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles. Chapter 1. Kluwer Academic Publishers; Dordrecht, Netherlands: 2003. pp. 1–14. [Google Scholar]