Abstract
We showed previously that cruciferous vegetable constituent benzyl isothiocyanate (BITC) inhibits growth of cultured and xenografted human breast cancer cells, and suppresses mammary cancer development in a transgenic mouse model. We now demonstrate, for the first time, that BITC inhibits epithelial-to-mesenchymal transition (EMT) in human breast cancer cells. Exposure of estrogen-independent MDA-MB-231 and estrogen-responsive MCF-7 human breast cancer cell lines and a pancreatic cancer cell line (PL-45) to BITC resulted in up-regulation of epithelial markers (e.g., E-cadherin and/or occludin) with a concomitant decrease in protein levels of mesenchymal markers, including vimentin, fibronectin, snail, and/or c-met. The BITC-mediated induction of E-cadherin protein was accompanied by an increase in its transcription, whereas BITC-treated MDA-MB-231 cells exhibited suppression of vimentin, snail, and slug mRNA levels. Experimental EMT induced by exposure to transforming growth factor β1 (TGFβ) and tumor necrosis factor-α (TNFα) or Rb knockdown in a spontaneously immortalized non-tumorigenic human mammary epithelial cell line (MCF-10A) was also partially reversed by BITC treatment. The TGFβ/TNFα-induced migration of MCF-10A cells was inhibited in the presence of BITC, which was partially attenuated by RNA interference of E-cadherin. Inhibition of MDA-MB-231 xenograft growth in vivo in female athymic mice by BITC administration was associated with an increase in protein level of E-cadherin and suppression of vimentin and fibronectin protein expression. In conclusion, the present study reports a novel anticancer effect of BITC involving inhibition of EMT, a process triggered during progression of cancer to invasive state.
Keywords: Benzyl Isothiocyanate, E-Cadherin, Vimentin, EMT, Chemoprevention
Introduction
Breast cancer is a serious public health concern for women worldwide (1,2). Despite remarkable progress towards screening efforts and unremitting advancement of targeted therapies, breast cancer is still a leading cause of cancer related deaths in women (1–4). A number of mammary cancer risk factors have been identified, including family history, Li-Fraumeni syndrome, atypical hyperplasia of the breast, late age at first full-term pregnancy, early menarche and late menopause (5–7). Because many of the risk factors associated with mammary cancer development are not easily adjustable, other strategies for prevention of this dreadful disease are appealing to reduce disease-related cost, mortality, and morbidity for a large segment of female population. Prevention of breast cancer is feasible considering successful clinical applications of selective estrogen-receptor modulators, including tamoxifen and raloxifene (8–10). Unfortunately, this strategy is effective only against estrogen-receptor positive breast cancers and suffers from adverse side effects, including increased risk of uterine cancer, thromboembolism, and perimenopausal symptoms (8–10). Discovery of agents efficacious for prevention of breast cancer regardless of the estrogen-receptor status is desired. Dietary plants have drawn increasing attention in recent years for the discovery of cancer preventive and therapeutic agents (11–13).
Population-based case-control studies have documented an inverse association between intake of cruciferous vegetables and the risk breast cancer (14,15). For example, a case-control study involving >300 breast cancer cases and matched controls showed an inverse association between urinary levels of isothiocyanates (ITCs) as a biological measure of cruciferous vegetable intake and the risk of breast cancer (14). Broccoli consumption was also found to be inversely associated with the risk of mammary cancer in premenopausal women (15). Anticancer property of cruciferous vegetables is ascribed to organic ITCs, which are produced upon cutting or chewing of these vegetables (16). Benzyl isothiocyanate (BITC) is one such ITC compound that appears promising for prevention of breast cancer based on our own work. We showed previously that administration of BITC in the diet (3 μmol BITC/g diet) inhibited mammary hyperplasia and carcinoma incidence and/or burden in MMTV-neu transgenic mice in association with suppression of cell proliferation, apoptosis induction, and tumor infiltration of T cells (17). We also found that the growth of MDA-MB-231 human breast cancer cells implanted in female athymic mice was inhibited significantly by BITC administration (18).
Cellular studies have provided novel insights into the mechanisms underlying anticancer effects of BITC (19–23). For example, our own work has revealed that BITC treatment inhibits growth of cultured human breast cancer cells (MDA-MB-231 and MCF-7) by causing G2/M phase cell cycle arrest and p53-independent apoptotic cell death (19,22,23). Noticeably, a spontaneously immortalized non-tumorigenic human mammary epithelial cell line (MCF-10A) is significantly more resistant to growth inhibition and apoptosis induction by BITC compared with breast cancer cells (19,22). Molecular circuitry of BITC-induced apoptosis involves production of reactive oxygen species due to inhibition of complex III of the mitochondrial respiratory chain (22). The present study builds on these observations and demonstrates, for the first time, that BITC inhibits epithelial-mesenchymal transition (EMT), which is implicated in progression of cancers to invasive state (24–26).
Materials and Methods
Reagents
BITC (purity >98%) was purchased from LKT Laboratories. Reagents for cell culture including medium, fetal bovine serum, and antibiotics were purchased from Invitrogen-Life Technologies. Transforming growth factor-β1 (TGFβ) was from Calbiochem, and tumor necrosis factor-α (TNFα) was obtained from Sigma-Aldrich. Antibodies against E-cadherin and β-catenin were purchased from BD Transduction Laboratories; anti-snail antibody was from Abgent; antibodies against vimentin and actin were from Sigma-Aldrich; anti-fibronectin and anti-c-met antibodies were from Santa Cruz Biotechnology; anti-Rb antibody was from Cell Signaling; anti-occludin antibody was from Invitrogen-Life Technologies; and anti-p53 antibody was from Calbiochem. The vimentin antibody used for immunofluorescence microscopy was from Santa Cruz Biotechnology. Alexa Fluor 488-conjugated goat anti-mouse antibody and Alexa Fluor 568-conjugated donkey anti-goat antibodies were from Invitrogen-Life Technologies. E-cadherin promoter reporter construct was purchased from Addgene. E-cadherin-targeted small interfering RNA (siRNA) was from Ambion; Rb-targeted siRNA was from Thermo Fisher Scientific-Dharmacon, and a control non-specific siRNA was purchased from Qiagen.
Cell lines
The MDA-MB-231, MCF-7, MCF-10A, and PL-45 cell lines were obtained from the American Type Culture Collection (ATCC), and cultured according to the supplier’s instructions. Authentication of MDA-MB-231, MCF-7, and MCF-10A cell lines was done by Research Animal Diagnostic Laboratory (University of Missouri, Columbia, MO). The cells were last tested in February 2011. The MDA-MB-231, MCF-7, and MCF-10A cells were found to be of human origin and no mammalian inter-species contamination was detected. Moreover, the genetic profile for each cell line was consistent with the corresponding genetic profile in the ATCC database. The PL-45 cell line was not authenticated.
Immunoblotting
Stock solution of BITC was prepared in dimethyl sulfoxide (DMSO) and an equal volume of DMSO (final concentration <0.05%) was added to the controls. Control and BITC-treated cells and tumor tissues from control and BITC-treated mice were processed for immunoblotting as described by us previously (18,19,27). Lysate proteins were resolved by sodium-dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane. After blocking with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween-20, the membrane was incubated with the desired primary antibody for 1 hour at room temperature or overnight at 4°C. Immunoreactive bands were visualized by enhanced Chemiluminescence method. Densitometric quantitation was done using UN-SCAN-IT software version 5.1 (Silk Scientific Corporation, Orem, Utah, USA).
Immunocytochemical analysis for E-cadherin and vimentin
Cells (2.5×104 cells/mL) were cultured on coverslips, allowed to attach, and then exposed to DMSO (control), 2.5 μmol/L BITC, and/or a combination of 10 ng/mL each of TGFβ and TNFα for specified time period. The cells were fixed with 2% paraformaldehyde for 1 hour, permeabilized with phosphate-buffered saline (PBS) containing 0.05% Triton X-100 for 10 minutes, and blocked with 0.5% bovine serum albumin in PBS for 1 hour at room temperature. For single-antibody staining (E-cadherin and vimentin), the cells were incubated overnight with anti-E-cadherin or anti-vimentin antibody at 4°C, washed with PBS, and incubated with Alexa Fluor 568-conjugated secondary antibody (Molecular Probes) for 1 hour at room temperature. For E-cadherin/actin double staining, the cells were incubated with E-cadherin antibody overnight at 4°C, followed by extensive washing with PBS and incubation with Alexa Fluor 568-conjugated secondary antibody (Molecular Probes) for 1 hour at room temperature. A monoclonal anti-actin antibody was then added followed by 2 hours of incubation at room temperature. Cells were then washed three times with PBS and stained with Alexa Fluor 488-conjugated secondary antibody (Molecular Probes) for 1 hour at room temperature. Cell nuclei were stained with DAPI (10 ng/mL; 5 minutes at room temperature). Cells were washed twice with PBS, mounted, and examined under a Leica DC300F microscope.
Reverse transcription-PCR (RT-PCR)
Total RNA was prepared from DMSO-treated control and BITC-treated MDA-MB-231 or MCF-7 cells using RNeasy kit (Invitrogen) and reverse transcribed with reverse transcriptase and oligo(dT)20 to synthesize complementary cDNA. RT-PCR reaction was carried out using high fidelity Taq polymerase (Invitrogen-Life Technologies), gene-specific primers, and 2 μL of cDNA. The E-cadherin primers were: forward- 5′-TGGGTTATTCCTCCCATCAG-3′ and reverse- 5′-TTTGTCAGGGAGCTCAGGAT-3′. The amplification conditions were: 94°C for 5 minutes, 25 (MCF-7 cells) or 55 (MDA-MB-231 cells) cycles 94°C for 60 s, 60°C for 60 s, 68°C for 60 s, and 68°C for 10 minutes. The primers used for vimentin were: forward- 5′-CTCTTCCAAACTTTTCCTCCC -3′ and reverse- 5′-AGTTTCGTTGATAACCTGTCC-3′ the primers used for Snail were: forward- 5′-CGAAAGGCCTTCAACTGCAAAT-3′ and reverse- 5′-ACTGGTACTTCTTGACATCTG -3′ and the primers used for Slug were: forward- 5′-CGCCTCCAAAAAGCCAAAC-3′ and reverse-5′-CGGTAGTCCACACAGTGATG -3′. The amplifications conditions used were: 94°C for 5 minutes, 30 cycles 94°C for 30 s, 58°C for 50 s, 68°C for 50 s, and 68°C for 7 minutes. The house keeping gene β-actin or GAPDH was used as a control. The β-actin was amplified using the primers: forward- 5′-CAAAGACCTGTACGCCAACAC-3′ and reverse- 5′-ATACTCCTGCTTGCTGATCC-3′ and the following amplification cycles: 95°C for 3 minutes, 18 cycles 95°C for 60 s, 56°C for 60 s, 68°C for 60 s, and 68°C for 10 minutes. The GAPDH was amplified using the primers: forward-5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and reverse-5′-TCCTTGGAGGCCATGTGGGCCAT-3′, and the following amplification cycles: 94°C for 2 minutes, 25 cycles 94°C for 30 s, 55°C for 30 s, 68°C for 30 s, and 68°C for 5 minutes. PCR products were resolved by 1–2% agarose gel pre-stained with ethidium bromide and visualized under an UV illuminator.
E-Cadherin luciferase assay
MDA-MB-231 cells (2×104 cells/well) were plated in 12-well plates and allowed to attach by overnight incubation at 37°C. The cells were then co-transfected with 8 μg of pGL2Basic-EcadK1-Luc plasmid with human E-cadherin promoter sequence from −108 to +125 and 0.8 μg of pRL-CMV plasmid using Fugene6. Twenty-four hours after transfection, the cells were treated with DMSO or BITC for 8 or 16 hours. Luciferase activity was determined using dual-luciferase reporter assay kit (Promega).
Migration assay
Effect of BITC treatment on MCF-10A cell migration was determined using Transwell Boyden chamber (Corning) containing a polycarbonate filter (8 μm). MCF-10A cell suspension (1×105 cells/mL) in complete medium was mixed with DMSO (control) or BITC in the absence or presence of TGFβ and TNFα (10 ng/mL each), and the suspension was added to the upper compartment of the chamber. Lower compartment of the chamber contained 0.6 mL of complete medium containing the same concentrations of DMSO, BITC and/or TGFβ/TNFα. Following incubation at 37°C for 24 hours, non-migrant cells from the upper face of the membrane were removed using a cotton swab. The membrane was washed with PBS and the migrated cells on the bottom face of the membrane were fixed with 90% ethanol followed by staining with H&E.
RNA interference
MCF-10A cells (1×105 cells/well) were seeded in six-well plates one day before transfection. The cells were washed thrice with serum/growth factor-free OptiMEM (Invitrogen-Life Technologies) and then transfected with 150 nmol/L E-cadherin-targeted siRNA or Rb-targeted siRNA or control siRNA using OligofectAMINE. Forty-eight hours after transfection, the cells were treated with DMSO, BITC and/or TGFβ and TNFα (10 ng/mL of each) for 24 hours. The cells were then collected and processed for immunoblotting and migration assay.
Immunohistochemistry for E-cadherin and vimentin in MDA-MB-231 xenografts
We have shown previously that BITC administration significantly retards growth of MDA-MB-231 cells implanted in female athymic mice (18). We used tumor tissues from the same study to determine the effect of BITC administration on expression of E-cadherin and vimentin by immunohistochemistry. Representative tumor sections from control and BITC-treated mice were processed for immunohistochemical analysis of E-cadherin and vimentin essentially as described by us previously for other proteins (17,18,23).
Results
BITC up-regulated E-cadherin protein expression in cancer cells
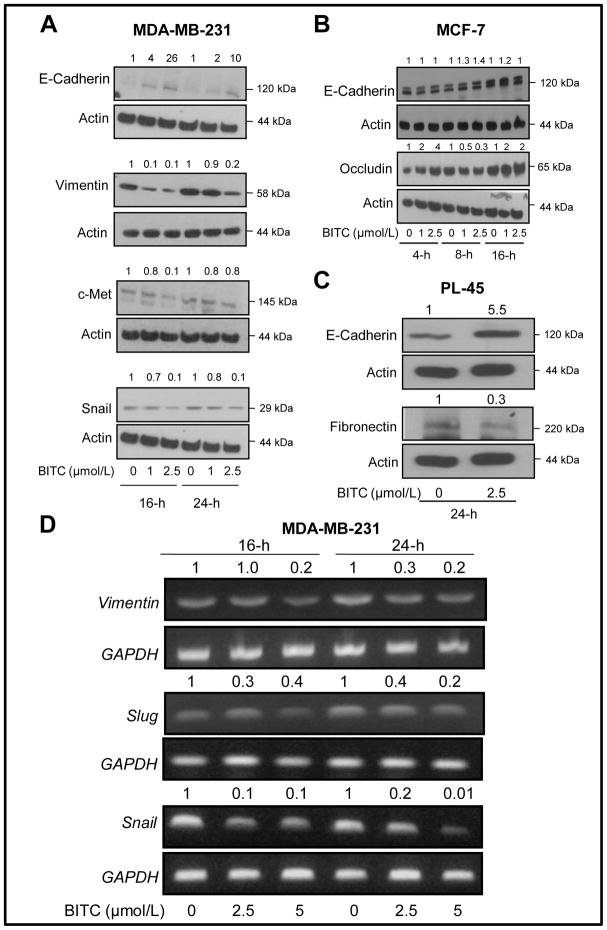
Loss of expression of epithelial adherens junction proteins (e.g., E-cadherin and occludin) with a concomitant gain of mesenchymal marker expression (e.g., vimentin, fibronectin) is a biochemical hallmark of EMT (24–26,28–30). Loss of E-cadherin expression is associated with increased invasion in breast cancer cells (25,26). Expression of E-cadherin is very low in the MDA-MB-231 cell line, which is a highly invasive “basal B” type and estrogen-independent fibroblastic human breast cancer cell line with stellate morphology (31). Initially, we used this cell line to test whether BITC treatment affected EMT. As can be seen in Fig. 1A, exposure of MDA-MB-231 cells to pharmacologic concentrations of BITC (1 and 2.5 μmol/L) for 16 and 24 hours resulted in up-regulation of E-cadherin expression. The BITC-mediated induction of E-cadherin expression in this cell line was accompanied by a marked decrease in mesenchymal marker vimentin especially at the 2.5 μmol/L concentration (Fig. 1A). The c-met along with E-cadherin constitutes a marker signature associated with angiogenic and lymphangiogenic factors in breast ductal carcinoma in situ (32). The c-met expression inversely correlates with E-cadherin expression (32). We observed a decrease in the level of c-met protein in MDA-MB-231 cells after treatment with 2.5 μmol/L BITC for 16 hours, which was somewhat reversible at the 24 hour time point (Fig. 1A). In addition to regulating canonical Wnt signaling pathway, β-catenin also serves as a component of adherens junctions and links E-cadherin to the cytoskeleton (33). Expression of β-catenin was not affected by BITC treatment (results not shown). We also determined the effect of BITC treatment on protein level of snail, a transcription factor that functions as a repressor of E-cadherin gene in epithelial tumor cells (34). Expression of snail was also reduced in MDA-MB-231 cells after treatment with 2.5 μmol/L BITC at both 16 and 24 hour time points (Fig. 1A).
Fig. 1.
Immunoblotting for expression of epithelial and mesenchymal marker proteins using lysates from (A) MDA-MB-231, (B), MCF-7, and (C) PL-45 cells after treatment with the indicated concentrations of BITC or DMSO (control) for specified time periods. Number on top of the immunoreactive band represents fold change in protein level relative to corresponding DMSO-treated control. D, expression of vimentin, slug and snail mRNA in MDA-MB-231 cells after 16- or 24-hour treatment with DMSO (control) or the indicated concentrations of BITC. GAPDH was used as a loading control. Each experiment was repeated at least twice and representative data from one such experiment are shown.
Next, we questioned if the BITC-mediated induction of E-cadherin protein was unique to the “basal” type breast cancer cells. We addressed this question using MCF-7 and PL-45 cells. The MCF-7 cell line is a well-accepted representative of estrogen-receptor positive “luminal” type breast cancer (31). Unlike MDA-MB-231 cells, the MCF-7 cell line exhibits epithelial phenotype with high expression of E-cadherin and occludin (31). Expression of mesenchymal marker vimentin is not detectable in MCF-7 cells (31). In contrast to the MDA-MB-231 cell line, expression of E-cadherin and occludin proteins was only modestly increased by BITC treatment in the MCF-7 cell line (Fig. 1B). Exposure of a highly invasive pancreatic cancer cell line (PL-45) to BITC (2.5 μmol/L for 24 hours) resulted in a robust induction of E-cadherin protein (5.5-fold increase over DMSO-treated control), which was accompanied by a 70% decrease in fibronectin protein level (Fig. 1C). We were unable to detect vimentin protein expression in this cell line.
Fig. 1D shows effect of BITC treatment on mRNA levels of vimentin, snail, and slug in MDA-MB-231 cells. The levels of vimentin, snail, and slug mRNA were decreased after treatment of MDA-MB-231 cells with BITC, especially at the 2.5 μmol/L concentration compared with DMSO-treated control at both 16 and 24 hour time points (Fig. 1D).
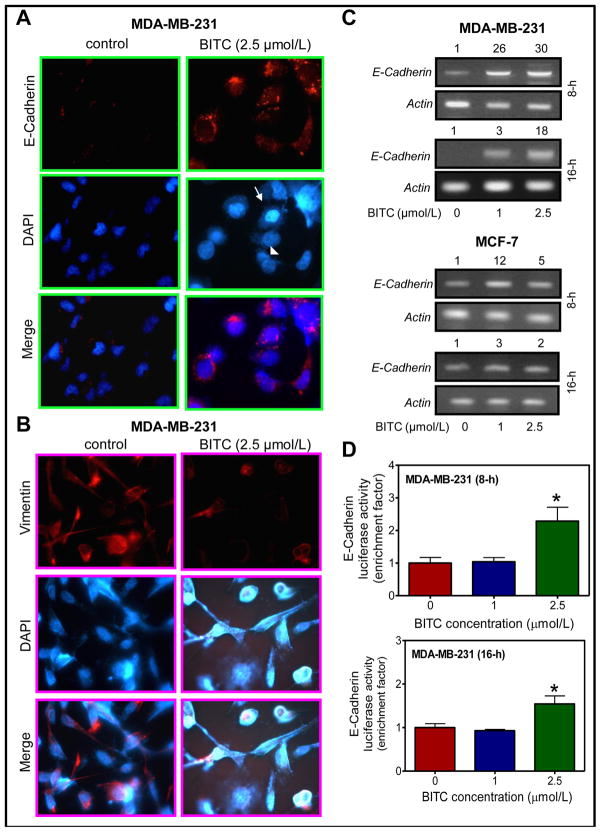
We performed immunocytochemistry to confirm results of the immunoblotting regarding effect of BITC treatment on protein levels of E-cadherin and vimentin using MDA-MB-231 cells. Constitutive expression of E-cadherin (red fluorescence) was very weak in DMSO-treated control MDA-MB-231 cells (Fig. 2A). Immunocytochemical staining for E-cadherin was clearly visible around DAPI stained nuclei in MDA-MB-231 cells following 24 hour exposure to 2.5 μmol/L BITC (Fig. 2A). Noticeably, the BITC-mediated induction of E-cadherin protein expression was evident in both apoptotic (marked by an arrow in Fig. 2A) and non-apoptotic cells (marked by an arrowhead in Fig. 2A). Constitutive expression of vimentin protein, characterized by filamentous microtentacle like protrusions, was clearly visible in DMSO-treated control MDA-MB-231 cells (Fig. 2B). Expression of vimentin protein was markedly suppressed after 24 hour treatment of MDA-MB-231 cells with 2.5 μmol/L BITC in association (Fig. 2B). The BITC treatment also resulted in disruption of the vimentin network (Fig. 2B).
Fig. 2.
A, immunocytochemical analysis for (A) E-cadherin and (B) vimentin protein expression in MDA-MB-231 cells after 24-hour treatment with DMSO (control) or 2.5 μmol/L BITC. C, E-cadherin mRNA expression in MDA-MB-231 and MCF-7 cells after 8- or 16-hour treatment with DMSO (control) or BITC (1 or 2.5 μmol/L). D, E-cadherin luciferase activity in MDA-MB-231 cells treated for 8 or 16 hours with DMSO (control) or BITC (1 or 2.5 μmol/L). Columns, mean (n=3); bars, SD. *Significantly different (P<0.05) compared with DMSO-treated control by Student’s t-test. Each experiment was repeated at least twice. Representative data from one such experiment are shown.
BITC treatment increased E-cadherin transcription in breast cancer cells
The BITC treatment caused an increase in the levels of E-cadherin mRNA in MDA-MB-231 and MCF-7 cells at both 8 and 16 hour time points (Fig. 2C). Consistent with the immunoblotting results (Fig. 1), the BITC-mediated increase in E-cadherin mRNA expression was relatively more pronounced in the MDA-MB-231 cell line in comparison with MCF-7 cells (Fig. 2C). These results indicate cell line-specific differences in BITC-mediated induction of E-cadherin mRNA at least in breast cancer cells.
Luciferase activity in MDA-MB-231 cells transiently transfected with an E-cadherin promoter reporter construct (E-cadherin promoter sequence from −108 to +125) was also significantly increased upon treatment with 2.5 μmol/L BITC in comparison with DMSO-treated control at both 8 and 16 hour time points (Fig. 2D). These results indicated that BITC treatment increased transcription of E-cadherin in both MDA-MB-231 and MCF-7 cells.
Reversal of TGF-β/TNF-α-induced EMT by BITC in MCF-10A cells
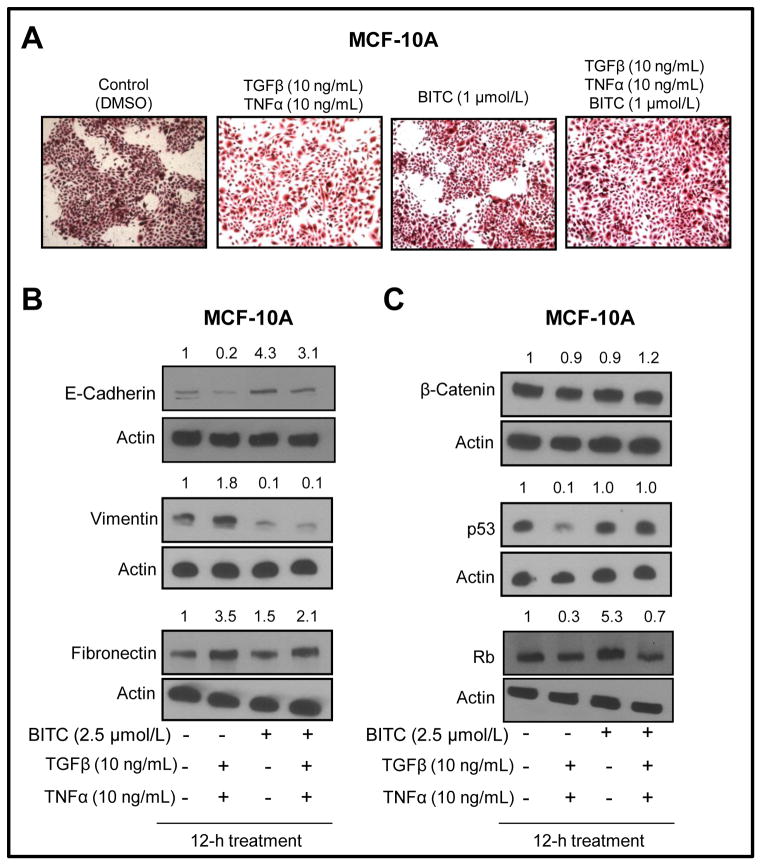
We used an experimental system involving TGFβ/TNFα (10 ng/mL of each) and MCF-10A cells to confirm BITC-mediated inhibition of EMT. The MCF-10A cell line, which was isolated from fibrocystic breast disease and spontaneously immortalized, is non-tumorigenic and widely used as a representative normal mammary epithelial cell line. The DMSO-treated control MCF-10A cells exhibited round and well-packed cobblestone appearance, a morphological feature of epithelial cells (Fig. 3A). Morphology of the MCF-10A cells was altered by a 24-hour exposure to known EMT inducers TGFβ/TNFα with a large fraction of cells exhibiting mesenchymal phenotype characterized by spindle shaped morphology with cell scattering and loss of cell-cell contact (Fig. 3A). The BITC treatment alone did not have an appreciable effect on MCF-10A morphology. The TGFβ/TNFα-induced EMT was partially reversible in the presence of BITC with restoration of cell-cell contact (Fig. 3A).
Fig. 3.
A, microscopic images of MCF-10A cells after 24-hour treatment with DMSO (control), TGFβ and TNFα (10 ng/mL of each), 2.5 μmol/L BITC, and combined TGFβ/TNFα and BITC (×100 magnification). Immunoblotting for (B) E-cadherin, vimentin, and fibronectin, and (C) β-catenin, p53, and Rb using lysates from MCF-10A cells treated for 12 hours with DMSO (control), TGFβ and TNFα (10 ng/mL of each), 2.5 μmol/L BITC, and combined TGFβ/TNFα and BITC. Number on top of the immunoreactive band represents fold change in protein level relative to DMSO-treated control (first lane from left). Each experiment was repeated at least twice and representative data from one such experiment are shown.
Next, immunoblotting was done using lysates from TGFβ/TNFα and/or BITC-treated cells to confirm reversal of EMT by BITC. As shown in Figure 3B, exposure of MCF-10A cells to TGFβ/TNFα resulted in down-regulation of E-cadherin protein (80% decrease compared with DMSO-treated control) and induction of vimentin (1.8-fold induction compared with DMSO-treated control) and fibronectin (3.5-fold induction compared with DMSO-treated control) proteins. The TGFβ/TNFα-mediated suppression of E-cadherin and induction of fibronectin protein expression was partially reversed in the presence of BITC. The level of β-catenin was not altered by TGFβ/TNFα and/or BITC treatments. On the other hand, the TGFβ/TNFα-treated MCF-10A cells exhibited a decrease in protein levels of p53 and Rb tumor suppressors (Fig. 3C), which are implicated in EMT (35,36). The TGFβ/TNFα-mediated suppression of p53 and Rb protein expression was also fully (p53) or partially reversible (Rb) in the presence of BITC (Fig. 3C).
Next, we proceeded to determine the role of Rb in BITC-mediated inhibition of EMT using MCF-10A cells. Rb depletion results in deregulation of E-cadherin and EMT induction (35). Knockdown of Rb protein level by transient transfection of MCF-10A cells with an Rb-targeted siRNA (Supplemental Fig. S1A) was accompanied by suppression of E-cadherin (60% decrease) and occludin protein expression (90% decrease) with a concomitant upregulation of fibronectin protein expression (1.5-fold increase) (Supplemental Fig. S1B). These effects mediated by Rb protein knockdown were partially reversible in the presence of BITC (Supplemental Fig. S1B). Another phenotype associated with Rb protein knockdown was increased migratory potential of MCF-10A cells (Supplemental Fig. S1C). The MCF-10A cell migration was markedly reduced in the presence of BITC regardless of Rb expression status (Supplemental Fig. S1C). Together, these results indicated that BITC cooperated with Rb to inhibit EMT in our model.
BITC treatment inhibited TGFβ/TNFα induced cell migration
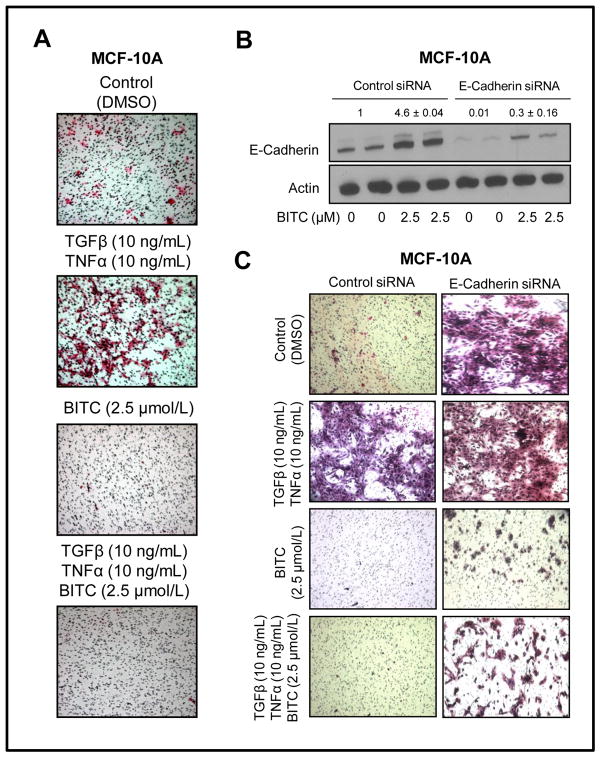
We designed experiments to determine anti-migratory effect of BITC in TGFβ/TNFα stimulated MCF-10A cells. As shown in Fig. 4A, migratory potential of MCF-10A cell was increased markedly in the presence of TGFβ/TNFα. Basal as well as TGFβ/TNFα-inducible migration of MCF-10A cells was markedly abolished in the presence of 2.5 μmol/L BITC (Fig. 4A). These results led us to conclude that BITC-mediated inhibition of experimental EMT translates into suppression of cell migration. We utilized RNA interference to further test role of E-cadherin in BITC-mediated inhibition of EMT and cell migration. The BITC treatment (2.5 μmol/L for 24 hours) increased level of E-cadherin protein by about 4.6-fold over DMSO-treated control in MCF-10A cells transiently transfected with a control siRNA (Fig. 4B). Protein level of E-cadherin was reduced by over 98% in MCF-10A cells transfected with an E-cadherin-specific siRNA (Fig. 4B). Similar to un-transfected cells (Fig. 4A) migratory potential of control siRNA-transfected MCF-10A cells was increased markedly in the presence of 10 ng/mL each of TGFβ and TNFα (Fig. 4C). Quantitation of migrated cells was not possible in this experiment due to cell clumping. Knockdown of E-cadherin protein also caused a marked increase in migration capability of MCF-10A cells even in the absence of TGFβ and TNFα (Fig. 4C). Migration ability of E-cadherin-depleted MCF-10A cells was only slightly increased in the presence of TGFβ and TNFα in comparison with control siRNA transfected cells (Fig. 4C). Nevertheless, BITC treatment exhibited significant inhibitory effect against migration in E-cadherin siRNA-transfected MCF-10A cells regardless of the TGFβ and TNFα stimulation.
Fig. 4.
A, migration of MCF-10A cells after 24-hour treatment with DMSO (control) or TGFβ and TNFα (10 ng/mL of each) in the absence or presence of 2.5 μmol/L BITC (×100 magnifications). B, Western blot analysis for E-cadherin in MCF-10A cells transiently transfected with a control siRNA or an E-cadherin-targeted siRNA and treated for 24 hours with DMSO (control) or 2.5 μmol/L BITC. C, migration of MCF-10A cells transiently transfected with a control siRNA or an E-cadherin-targeted siRNA and treated for 24 hours with DMSO (control) or TGFβ and TNFα (10 ng/mL of each) in the absence or presence of 2.5 μmol/L BITC (×100 magnifications). Experiments were repeated twice with comparable results.
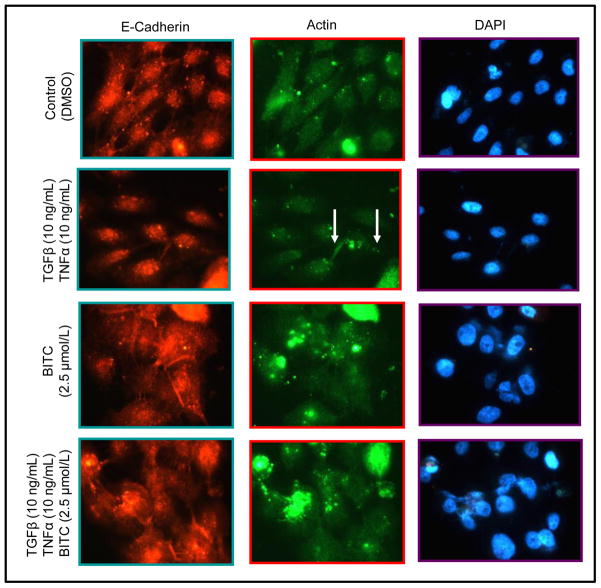
Formation of adherens junctions is important for maintenance of the epithelial phenotype (24–26). Expression of E-cadherin protein was decreased in TGFβ and TNFα-treated MCF-10A cells leading to loss of cell-cell contact and appearance of lamellipodia in some cells (identified by arrows). Moreover, when the cells were co-treated with 2.5 μmol/L BITC and 10 ng/mL each of TGFβ and TNFα, a reversion to epithelial phenotype was discernible as evidenced by an increase in E-cadherin staining and emergence of cell-cell contact (Fig. 5).
Fig. 5.
Immunocytochemical analysis for E-cadherin and actin in MCF-10A cells treated for 24 hours with DMSO (control) or 10 ng/mL each of TGFβ and TNFα in the absence or presence of 2.5 μmol/L BITC (×100 magnifications). Experiment was repeated twice and representative data from one such experiment are shown.
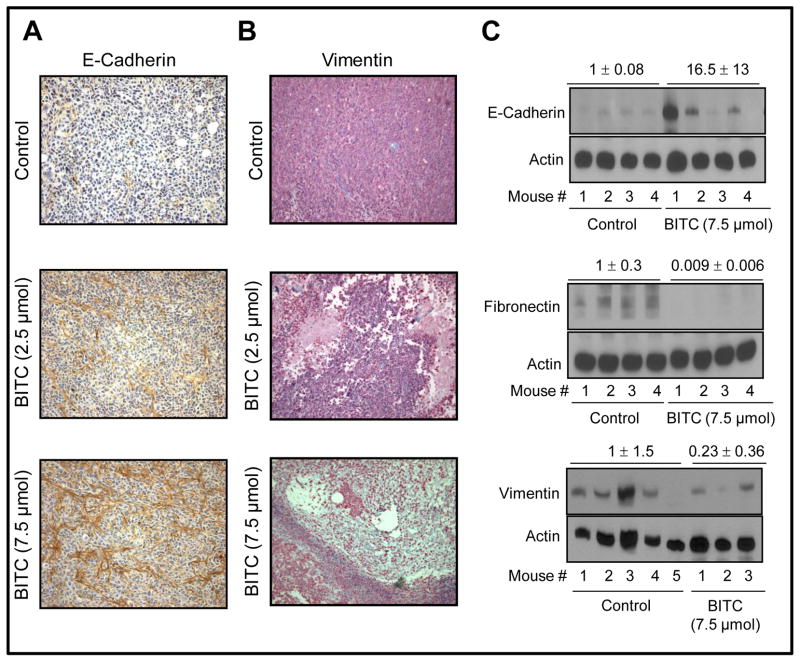
In vivo effect of BITC administration on EMT markers in MDA-MB-231 xenografts
We have shown previously that growth of MDA-MB-231 xenografts in female athymic mice is inhibited significantly by i.p. BITC administration (2.5 and 7.5 μmol BITC/mouse, five times per week) (18). For example, 50 d after tumor cell injection the average tumor volume in control mice (1581 ± 240 mm3) was about 2.5- to 3-fold higher compared with BITC-treated mice (18). We used tumor tissues from the same experiment to determine the effect of BITC administration on expression of EMT markers. E-cadherin protein expression was very low in the tumors from control mice (Fig. 6A). The BITC administration dose-dependently caused up-regulation of E-cadherin protein expression in MDA-MB-231 xenografts (Fig. 6A). Expression of vimentin protein was markedly decreased in MDA-MB-231 tumors from BITC-treated mice compared with those of control mice (Fig. 6B). Results of immunohistochemical analyses were confirmed by Western blotting using tumor supernatants from control and 7.5 μmol BITC-treated mice (Fig. 6C). Tumors from the BITC-treated mice exhibited increased expression of E-cadherin protein compared with control. In agreement with cellular data, MDA-MB-231 tumors from BITC-treated mice revealed a marked decrease in protein levels of fibronectin and vimentin in comparison with those of control mice (Fig. 6C). Together, these results provided in vivo evidence for BITC-mediated suppression of EMT.
Fig. 6.
Immunohistochemistry for (A) E-cadherin and (B) vimentin in MDA-MB-231 xenograft from a representative control mouse and a mouse of 2.5 or 7.5 μmol BITC-treated groups (magnification ×400). C, immunoblotting for E-cadherin, fibronectin, and vimentin using MDA-MB-231 tumor supernatants from mice of control group (n= 4–5) and 7.5 μmol BITC treatment group (n= 3–4). Densitometric quantitation normalized to control group is shown on top of the immunoreactive bands.
Discussion
The EMT is essential for normal physiological processes such as embryonic development, tissue remodeling, and wound healing (37). During EMT, epithelial phenotype characterized by tight cell-cell junctions and polarity changes to a mesenchymal phenotype typified by disruption of the cell-cell contact with conversion to fibroblastic morphology and increased motility (37). Moreover, the EMT assumes a central place in pathogenesis of aggressive cancers (33). For example, in biopsies of human breast carcinoma detection of overexpressed EMT indicators are associated with tumor aggressiveness, disease recurrence, unfavorable clinicopathologic variables, and shorter survival (25,29). Recent studies have identified many diet-derived natural products as inhibitors of EMT, including garlic-derived chemicals (38,39), soy constituent genistein (40,41), and green tea polyphenols (42). The present study demonstrates that cruciferous vegetable constituent BITC inhibits EMT in cancer cells and this effect is not a cell line-specific phenomenon. The BITC-mediated inhibition of EMT is discernible in breast cancer cells as well as in a pancreatic cancer cell line. More importantly, BITC administration elicits E-cadherin induction and suppression of mesenchymal markers in vivo in MDA-MB-231 xenografts.
Adherens junctions are important for maintenance of epithelial integrity (33,37,43). The E-cadherin is regarded as a tumor suppressor because of its role in maintenance of epithelial phenotype (44–46). The E-cadherin is frequently down-regulated during cancer progression and correlates with poor prognosis (45). In mesenchymal cells, E-box elements located within 108 bp of the 5′-region of the E-cadherin promoter are responsible for its transcriptional repression by Snail and other transcriptional factors (34,47). Because BITC treatment suppresses expression of snail and slug mRNA and increases transcription of E-cadherin, it is reasonable to conclude that BITC-mediated induction of E-cadherin involves E-box elements. Further studies are needed to test this hypothesis.
Vimentin, a mesenchymal filament protein, increase motility of cells undergoing mesenchymal conversion in coordination with detyrosinated microtubules to provide support to microtentacle extension of detached tumor cells (48). The BITC treatment clearly decreases vimentin protein level in MDA-MB-231 cells as well as during experimental EMT in MCF-10A cells. Rb, a classical tumor suppressor, in association with the transcription factor activator protein-2α (AP-2α) binds to E-cadherin promoter sequence, and regulates expression of E-cadherin in epithelial cells. During EMT, Rb is degraded and it depletion results in deregulation of E-cadherin and induction of an EMT-like phenotype with increased cell motility (35). We found that the TGFβ and TNFα-treated MCF-10A cells display morphological alterations from epithelial to mesenchymal phenotype in association with down-regulation of Rb, p53, and E-cadherin concomitant with gain of vimentin and fibronectin protein levels. Whereas BITC-mediated reversal of morphological and molecular changes in TGFβ/TNFα stimulated MCF-10A cells prompt us to speculate that E-cadherin up-regulation in BITC-treated cells may be mediated by AP-2α, additional work is needed to substantiate this possibility. Nonetheless, the present study indicates that E-cadherin is necessary, at least in part, for BITC-mediated inhibition of EMT because E-cadherin knockdown confers partial protection against anti-cell migration effect of BITC.
During EMT, cells undergo cytoskeletal reorganization into actin stress fibers to acquire spindle shape morphology and increased cellular motility (29,33). The TGFβ/TNFα treated MCF-10A cells display higher propensity for migration and cytoskeleton remodeling with actin reorganization. The BITC treatment is able to inhibit cytoskeletal remodeling and formation of actin stress fibers leading to suppression of TGFβ/TNFα-stimulated cell migration.
In conclusion, the present study is the first published report to document inhibition of EMT by BITC that is characterized by up-regulation of adherens junction proteins (E-cadherin and occludin), down-regulation of mesenchymal markers such as vimentin and fibronectin, and suppression of transcriptional repressors of E-cadherin (snail and slug).
Supplementary Material
Acknowledgments
Grant Support: This study was supported in part by the USPHS grants RO1 CA129347-04 and RO1 CA142604-01 awarded by the National Cancer Institute.
Footnotes
Conflict of Interest: None.
References
- 1.DeSantis C, Jemal A, Ward E, Thun MJ. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 2008;19:537–45. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.van de Ven SM, Elias SG, van den Bosch MA, Luijten P, Mali WP. Optical imaging of the breast. Cancer Imaging. 2008;8:206–15. doi: 10.1102/1470-7330.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz M, Estevez LG, Alvarez I, et al. Evaluation of international treatment guidelines and prognostic tests for the treatment of early breast cancer. Cancer Treat Rev. 2008;34:701–9. doi: 10.1016/j.ctrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 6.Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–7. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 10.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–51. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 11.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 12.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104:339–56. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 13.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010;7:347–56. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowke JH, Chung FL, Jin F, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–6. [PubMed] [Google Scholar]
- 15.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 16.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 17.Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69:9473–80. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol Carcinog. 2010;49:500–7. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–45. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–52. [PubMed] [Google Scholar]
- 21.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood) 2004;229:835–42. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- 22.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–63. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SH, Singh SV. p53-Independent apoptosis by benzyl isothiocyanate in human breast cancer cells is mediated by suppression of XIAP expression. Cancer Prev Res. 2010;3:718–26. doi: 10.1158/1940-6207.CAPR-10-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cellular Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 25.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 27.Xiao D, Lew KL, Kim YA, et al. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836–43. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 28.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–62. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–60. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 30.Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blick T, Widodo E, Hugo H, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–42. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 32.Gotte M, Kersting C, Radke I, Kiesel L, Wülfing P. An expression signature of syndecan-1 (CD138), E-cadherin and c-met is associated with factors of angiogenesis and lymphangiogenesis in ductal breast carcinoma in situ. Breast Cancer Res. 2007;9:R8. doi: 10.1186/bcr1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 34.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 35.Arima Y, Inoue Y, Shibata T, et al. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer Res. 2008;68:5104–12. doi: 10.1158/0008-5472.CAN-07-5680. [DOI] [PubMed] [Google Scholar]
- 36.Derksen PW, Liu X, Saridin F, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–31. doi: 10.1016/s0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 38.Chu Q, Ling MT, Feng H, et al. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinog. 2006;11:2180–9. doi: 10.1093/carcin/bgl054. [DOI] [PubMed] [Google Scholar]
- 39.Tang FY, Chiang EP, Chung JG, Lee HZ, Hsu CY. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J Nutr Biochem. 2009;20:1013–20. doi: 10.1016/j.jnutbio.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LL, Li L, Wu DP, et al. A novel anti-cancer effect of genistein: reversal of epithelial mesenchymal transition in prostate cancer cells. Acta Pharmacol Sin. 2008;29:1060–8. doi: 10.1111/j.1745-7254.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 41.Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinog. 2009;30:331–9. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 42.Belguise K, Guo S, Yang S, et al. Green tea polyphenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007;67:11742–50. doi: 10.1158/0008-5472.CAN-07-2730. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Moreno M, Jamora C, Fuchs E. Sticky buisiness: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–48. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 44.Agiostratidou G, Hulit J, Phillips GR, Hazan RB. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia. 2007;12:127–33. doi: 10.1007/s10911-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 45.Mohammadizadeh F, Ghasemibasir H, Rajabi P, Naimi A, Eftekhari A, Mesbah A. Correlation of E-cadherin expression and routine immunohistochemistry panel in breast invasive ductal carcinoma. Cancer Biomar. 2009;5:1–8. doi: 10.3233/CBM-2009-0551. [DOI] [PubMed] [Google Scholar]
- 46.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–22. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giroldi LA, Bringuier PP, de Weijert M, et al. Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun. 1997;241:453–8. doi: 10.1006/bbrc.1997.7831. [DOI] [PubMed] [Google Scholar]
- 48.Whipple RA, Balzer EM, Cho EH, et al. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68:5678–88. doi: 10.1158/0008-5472.CAN-07-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.