Abstract
Oncolytic viruses consist of a diverse range of DNA and RNA viruses traditionally thought to mediate their effects by exploiting aberrations in tumor pathways, allowing preferential viral replication in, and killing of, tumor cells. Clinical development has progressed to late phase trials, potentially heralding their introduction into clinical practice. However, despite this promise, the activity of oncolytic viruses has yet to achieve the potential suggested in preclinical models. To address this disparity, we need to recognise the complex interaction between oncolytic viruses, tumor, chemotherapy, host immune system, and appreciate that direct oncolysis may not be the only factor to play an important role in oncolytic virus-mediated anti-tumor efficacy.
Although key in inactivating viruses, the host immune system can also act as an ally against tumors, interacting with oncolytic viruses under the right conditions to generate useful and long-lasting anti-tumor immunity.
Preclinical data also suggest that oncolytic viruses demonstrate synergy with standard therapies, which may offer improved clinical response rates. Here we explore clinical and preclinical data on clinically relevant oncolytic viruses, highlighting areas of progress, uncertainty and translational opportunity, with respect to immune recruitment and therapeutic synergy.
Keywords: Oncolytic virus, oncolysis, anti-tumor immune response, chemotherapy, synergy
Introduction
The notion of using of replicating viruses as potential anti-cancer agents goes back over a century, with occasionally dramatic regressions of cancers following viral infections (1–6). Clinical responses were observed in preliminary studies using replicating wild-type viruses such as adenovirus (1) and mumps (5), However, progress faltered for a number of reasons: fears over safety; the lack of objective response criteria; lack of randomized trials; and the absence of Good Manufacturing Practice standards (1, 5, 6).
Despite these reservations, oncolytic viruses (OVs) remain exciting prospective anti-cancer agents, because of reports of selective killing of tumor cells, (7, 8). There has been a recent resurgence of interest in OVs, based not only on fundamental advances in tumor and viral biology, but also the ability to scale-up manufacture of clinical grade viruses, and improved clinical trial designs (9, 10).
Clinical development of oncolytic viruses
Modern trials commenced in the mid 1990s, administering OVs by a variety of routes, including intra-tumoral (IT), locoregionally and, more recently, intravenous (IV) routes (Table 1).
Table 1.
Oncolytic viruses in clinical development
| Virus (clinical example) | Tumor type | Status | Refs |
|---|---|---|---|
| E1B deleted Adenovirus (Onyx-015, H-101) | SCCHN SCCHN, |
H-101 licensed as combination therapy for SCCHN (China only) Phase II/III trials SCCHN, HCC, CRC, Hepatobilliary |
27,107 15,16,17,19,24 |
| HSV (OncoVEXGMCSF) | Melanoma | Phase III registration trial in Melanoma Phase III in SCCHN |
23, 33, 46 |
| Reovirus (Reolysin) | SCCHN | Phase I, Phase II melanoma, lung sarcoma Phase III in SCCHN |
14, 25, 30, 34, 35 |
| Vaccinia (JX-594) | HCC | Phase I/II HCC, SCCHN Phase III trial planned in Liver |
22 |
| NDV (PV-701) | CRC | Phase I/II | 12, 21 |
| Measles (MV-CEA) | Ovary | Phase I in Ovary | 13 |
| VSV (VSV-hIFNbeta) | HCC | Phase I HCC | N/A |
Abbreviations: SCCHN, squamous cell cancer of the head and neck; CRC, colorectal cancer; HCC, hepatocellular cancer; Refs, references.
Concerns over the safety of replicating OVs have eased, given the satisfactory treatment of several hundred patients within multiple early phase trials of RNA (reovirus, Newcastle Disease Virus (NDV), measles) and DNA (adenovirus, vaccinia, and Herpes Simplex Virus (HSV)) OVs (11–25). Typical local response rates observed after IT administration range from ~10–60% (14, 16, 17, 20, 23), with the best objective radiological responses lower, at just under 30%, at best (20, 23). Single agent IV treatment offers even lower objective responses, at <10% (12, 19, 21, 25).
Commonly-observed side-effects include local reactions within injected tumor masses, following IT administration, and `flu-like syndromes, following intravenous infusion. Edema, precipitating billiary tract obstruction and jaundice (22), or bronchial obstruction and respiratory compromise (21), represent serious adverse events and have led to trial protocols excluding patients where disease has the potential to cause critical obstruction (23).
A closer look at the reasons behind the difference between preclinical studies and the clinical experience may be the first step in realising the full anti-tumor potential of OVs. The clinical development of dl-1520 (Onyx-015) (26), a well-characterised oncolytic adenovirus, which was first used over a decade ago, illustrates some of the challenges in developing OVs clinically.
Multiple clinical trials were completed in multiple tumor types and using various routes of administration (Table 1). Objective local response rates were improved to >50% by combining Onyx-015 with chemotherapy in squamous cell cancer of the head and neck (SCCHN), hinting at synergy (15). However an unreported, incomplete Phase III trial halted Onyx-015 clinical development (27).
H-101, a closely related virus, has since found use as a licensed cancer therapy in China for SCCHN in combination with radiotherapy. Unfortunately H-101 approval is based on limited controlled trial evidence (27), and a corruption scandal over the drugs approvals process in China (involving unrelated agents) appears to have discouraged widespread use (28).
Despite these setbacks, anticipation remains high, with recently reported phase 2 trials, with HSV and reovirus OVs, underpinning current randomised phase 3 trials in melanoma (23, 29) and head & neck cancers (30). Clinical observations with these OVs, as outlined below, suggest that recruitment of a host anti-tumor immune response, or synergy with other anti-cancer agents, may represent important factors in optimizing OV efficacy.
The DNA herpes simplex virus OV, JS1/34.5-/47-/GM-CSF (“OncoVEXGMCSF”) represents a clinically-advanced OV candidate, designed to invoke an anti-tumor immune response by oncolytic release of tumor antigens, which, if “presented” appropriately to immune cells, may provoke an anti-tumor immune response. This aim is enhanced by deletion of the ICP 47 gene, promoting greater presentation of tumor antigen on the infected cell surface. Further to this, expression of Granulocyte-Macrophage colony stimulating factor (GM-CSF), a protein that stimulates antigen-presenting dendritic cell (DC) activity, increases the likelihood of successful “recognition” of tumor antigen and a therapeutic anti-tumor immune response (31).
In a phase two trial, intratumoral JS1/34.5-/47-/GM-CSF elicited thirteen (8 complete and 5 partial) objective RECIST (Response Evaluation Criteria In Solid Tumors) responses in 50 patients with unresectable metastatic melanoma (23). Treated patients had a 58% one-year survival rate (23), comparing favourably to historical Phase II survival rates of 25.5% (32). Data from a Phase III trial in metastatic malignant melanoma patients are keenly awaited (33).
The dsRNA reovirus (Type 3 Dearing),is safe and effective in early phase clinical trials employing IT (14, 34), IV (25, 35) and combination (30, 36) approaches (Table 1). An on-going, randomised Phase III trial of reovirus, in combination with chemotherapy, in refractory SCCHN follows a recent phase 1/2 study where 8 of 19 HNSCC patients achieved an objective partial response (42%) (30). Reovirus therefore represents another advanced contender in the race to enter the clinic (30, 36).
Oncolytic viruses and selective replication
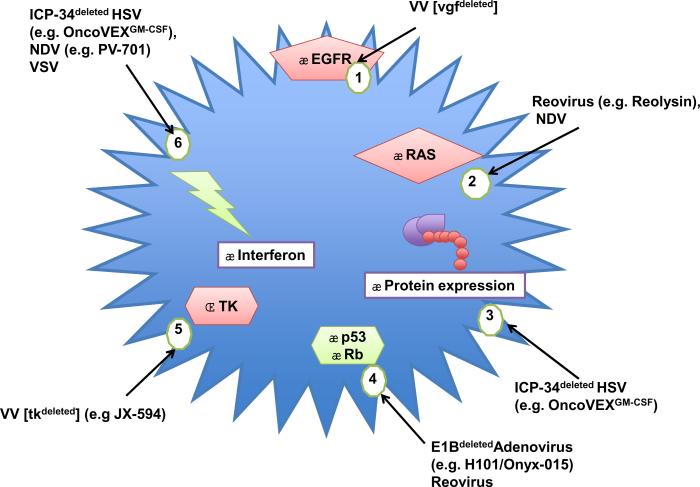
Appreciating the rationale for the action of OV may help to put the role of the immune response and synergy into perspective. Preclinical evidence regarding oncolytic efficacy concentrates on the exploitation of dysregulated signalling pathways in tumor cells, which may attenuate anti-viral responses or support viral replication (Figure 1).
Fig. 1.
Tumor selective replication of oncolytic viruses: The figure illustrates aberrant tumor pathways that offer redundancy to oncolytic viral genes contributing to tumor selectivity: [1] Activation of EGFR abrogates vgf that normally stimulates EGFR, in readiness for vaccinia infection; [2] Activation of RAS induces an inhibitor of PKR, that would normally prevent translation of RNA viral (reovirus or NDV) genes, to control infection; [3] Aberrantly activated protein expression compensates for ICP-34 absence (ICP-34 normally induces protein expression) restricting replication to dividing (tumor) cells; [4] Tumor suppressor inactivation can compensate for absent viral proteins, e.g. E1B is normally required to inactivate p53; [5] Up-regulated cellular TK in tumor compensates for absent TK in tk deleted VV (tk-); [6] Interferon responses are powerful mediators of anti-viral responses, and often impaired in tumors especially benefitting RNA viruses NDV and VSV. Downstream attenuation of PKR also benefits the replication of reovirus and ICP34 deleted HSV. Clinically relevant OV examples are given in brackets.
RNA viruses benefit from disruption of anti-viral immune responses. Reovirus, benefits from attenuation of the dsRNA-sensing, protein kinase receptor (PKR), via RAS activation (37, 38). Deficiencies in cellular interferon responses in tumors allow NDV and vesicular stomatitis virus (VSV) to replicate selectively (39, 40) (Figure 1).
Other viruses have been engineered to enhance anti-tumor activity or improve safety (Figure 1). The function of selectively-inactivated, replicative genes may be redundant due to abnormalities in tumor cells, but equally can enhance safety, by preventing OV replication in normal cells. Figure 1 outlines mechanisms, including: inactivation of the ICP 34.5 gene controlling neurovirulence and late protein synthesis in HSV OVs e.g. JS1/34.5-/47-/GM-CSF used in melanoma (31); deletion of thymidine kinase (TK), required for vaccinia replication e.g. JX-594 used in melanoma and liver tumors (41); and deletion of the adenoviral E1B gene, the product of which normally binds to and inactivates p53 e.g. Onyx-015/H101 used in SCCHN (26).
Strategies for maximising the efficacy of OV therapy: exploiting the host immune system
A comprehensive review of the immune system in the context of OV therapy is beyond the scope of this article and has been summarised elsewhere (42, 43). Briefly, the two arms of the immune system are: adaptive (antigen-specific), which creates immunological memory via B and T-cells; and innate (non-antigen-specific), involving macrophages, dendritic cells (DC), and Natural-Killer (NK) cells.
Potential interactions of the host immune system with OVs and tumors are summarised in Tables 2 and 3. These interactions are complex and illustrate how the host immune response can be focused on the virus (anti-viral immunity), or the tumor (anti-tumor immunity). The development of anti-tumor immunity depends on the interplay between tumor and immune system and it is well recognised that tumors employ multiple mechanisms to avoid anti-tumor immunity, including decreased immunogenicity, resistance to immune-mediated killing, and immune subversion (Table 2).
Table 2.
Key interactions between tumors, the host immune system, and oncolytic viruses, resulting in enhanced anti-tumor effects
| Immune Effect | Innate mechanism | Adaptive mechanism |
|---|---|---|
| Decreased anti-tumor immunity | Improved lung cancer prognosis, related to NK infiltration of tumor [C]*87 Tumor killing, associated with induction of anti-tumor cytokines [P]55,88 |
Melanoma regression, following adoptive transfer and tumoricidal effect of TS-CTL [C]89 |
| OV stimulated anti-tumor immunity | Improved tumor killing, associated with increased innate neutrophil infiltration & vascular shutdown (VV, VSV) [P]61 Improved tumor killing, due to induction of intra-tumoral anti-tumor cytokines (reovirus) [P]54 Enhanced release and presentation of tumor associated antigens [P]91 |
Tumor regression in non-OV injected sites, associated with ATI (HSV, reovirus, measles) [C]14,23,29 [P]62 Tumor regression, associated with generation of TS-CTL, and fall in immune-suppressive cell levels (HSV) [C]29 Protection from tumor rechallenge, via TS-CTL (reovirus, VSV) [P]47–49,55 |
| Manipulation of anti-viral or anti-tumor immunity | Increased tumor killing with enhanced OV replication after attenuation of the host innate response (HSV, VSV, VV & reovirus) [P]63–65,81 | Tumor regression, associated with GMCSF induced TS-CTL (VV, HSV) [C]23,29 [P]41,55 |
The complex interactions between tumor and host immune system, are further altered by the introduction of OV. Understanding and exploiting the conditions that result in anti-tumor effects may help to maximise OV therapy.
Abbreviations: [C], clinical evidence; [P], preclinical evidence; NK, natural killer cells; TS-CTL, tumor specific cytotoxic T-lymphocyte; GMCSF, granulocyte-macrophage colony stimulating factor.
References in superscript.
Table 3.
Interactions between tumor, the host immune system, and oncolytic viruses attenuating anti-cancer effects, or increasing toxicity.
| Immune Effect | Innate mechanism | Adaptive mechanism |
|---|---|---|
| Decreased anti-tumor immunity | ↓Tumor cell Immunogenicity, due to ↓caspase expression [P]*97 ↓ Antitumor NK cell activity, due to sustained NKG2D ligand expression [P]98 |
Tumor resistance to immune killing, via ↓death receptors [P]94 Suppression of ATI, due to increased immune-suppressive Treg numbers [C]68 [P]95 |
| OV stimulated anti-viral immunity | OV inactivation with potential reduced oncolysis due to: | OV inactivation with potential reduced oncolysis due to: |
| Manipulation of anti-viral immunity | Decreased tumor killing following loss of bystander effect, due to attenuation of innate response (VSV) [P]61 | Increased in vivo toxicity from OV, due to ablation of nAb response (reovirus) [P]100 |
The complex interplay between tumor, host immunity and OV may be detrimental to anti-tumor therapy. Direct loss of immune control of tumors may be observed, oncolytic therapy may be attenuated by the host immune response to OVs, and OV toxicity may be increased. Understanding and avoiding these interactions may serve to enhance OV mediated therapy.
Abbreviations: [C], clinical evidence; [P], preclinical evidence; NKG2D, activating receptors for NK cells; Tregs, T regulatory cells.
References in superscript.
Theories of immune activation suggest effective immunity requires an appropriate “danger signal” indicating cellular or tissue distress (44), or stimulation of “pattern recognition receptors” (PRR) on immune activating cells (45). The immune premise of OVs is the provision of these activating functions, by oncolytic killing of tumor cells (“danger”) and release of tumor antigens (stimulation of PRR), thereby engaging effective anti-tumor immunity.
Preclinical adaptive immune response data
Pre-clinical work suggests that OVs may promote immune responses, which outweigh direct oncolysis in mediating anti-tumor efficacy (Table 2). Long-term immune control may arise from OV-infected tumor cells boosting both innate and subsequent adaptive tumor specific immune responses. The clinical OVs JS1/34.5-/47-/GM-CSF and JX-594 express a GMCSF transgene in order to enhance adaptive anti-tumor immunity (41, 46). GMCSF improves antigen presentation through activation of DC, consequent immune recognition of released tumor antigens, eventually stimulating an increase in tumor specific cytotoxic T-lymphocytes (TS-CTL), which have been associated with long-term tumor control in both clinical and pre-clinical studies (Table 2). .
We have demonstrated, in immunocompetent mice carrying B16 melanoma cells, that reovirus and VSV can enhance tumor clearance and induce specific long-term protection from tumor re-challenge, via generation of melanoma antigen specific lymphocytes (47–49).
However, our studies also demonstrate an important pitfall: Viral delivery method (i.v. versus carriage on immune cells), or increasing dose of virus, induces anti-viral immunity, rather than anti-tumor immunity, with loss of long-term tumor-control (Table 3) (48). This is unsurprising, as adaptive responses, and, in particular, neutralising antibodies (nAb) prove a common and powerful inhibitory end-response to infection, involving a variety of oncolytic viruses including VSV (50), reovirus (51), measles virus (52) and HSV-1 (53). The difficult task of understanding how to stimulate profitable anti-tumor immunity, rather than (or alongside) anti-viral immunity, will be key in mediating successful OV-immunotherapy (Tables 2,3).
Preclinical innate immune response data
Data using clinically-relevant OVs demonstrate an intriguing relationship with the host innate system. OVs may be inactivated preventing direct oncolysis (Table 3), but also show a potentially productive inflammatory anti-tumor response (Table 2). Reovirus can boost a variety of innate anti-tumor functions, including NK recruitment, alongside activation and induction of DC maturation (54).
Cytokines such as the interleukins (IL) and interferons (IFN) are proteins which regulate the growth and function of immune cells, thereby potentially having either positive or negative effects on anti-tumor immunity. Whilst reovirus can directly influence the balance of tumor cytokines from immunosuppressive to inflammatory, increasing cytokines associated with tumor rejection (55), several attempts have been made to directly incorporate cytokine transgenes into OVs. Interferons are cytokines which enhance tumor antigen presentation and cytotoxicity. Interferon 1-beta (IFN-1-β) transgene expression from an oncolytic VSV vector enhances overall anti-tumor activity in a murine mesothelioma model, through a T-cell activating mechanism. In addition, SCID mice were protected from lethal neurotoxicity associated with wild-type VSV by IFN-1-β production in non-tumor tissue (56).
IL-12 is another cytokine of interest which shows pleiotrophic effects, including stimulation of T helper cells, increased tumor infiltration and cytotoxicity by CTLs and NK cells, and stimulation of IFN-γ production, resulting in anti-angiogenic effects (57–59). IL-12 expression from the clinically-relevant HSV virus, NV1020, led to increased IFN-γ production, induction of antiangiogenic proteins, and an enhanced therapeutic effect, when assessed in vivo (60).
Innate neutrophil infiltration also enhances therapeutic efficacy of measles and vaccinia viruses, the latter by triggering endothelial collapse, anti-vascular effects and bystander apoptosis of tumor (61, 62). In this instance, interfering with the neutrophil response increased direct oncolytic killing, but decreased bystander anti-vascular therapy and overall anti-tumor efficacy (61) (Table 3). However other studies have shown the opposite effect, with inhibition of the innate response improving replication and therapeutic efficacy of HSV, vaccinia, and reovirus (53, 63–65) (Table 2). These differences underline the influence of experimental conditions. Ultimately, complex OV, tumor and immune interactions may not be adequately represented in present preclinical models, and clinical relevance may be best sought in a translational setting.
Clinical Immune data with OVs
Immune response data on OVs in clinical practice are limited, but give an indication of the host response to tumor and to OV alike. Available data relate to the phase-2 trial of GMCSF-expressing JS1/34.5-/47-/GM-CSF, described earlier (23). This trial is notable for the clinically significant proportion of complete responses (16%).
The authors utilised novel immune assessment criteria, allowing a limited degree of tumor progression, prior to response (considered clinically insignificant and not requiring alternative treatment intervention), permitting the development of an immune-mediated response (66). These guidelines were developed for immune-stimulating therapies, such as the monoclonal antibody ipilimumab, which has recently shown a ground-breaking 3-month survival advantage over a peptide vaccine in metastatic melanoma (67). Six of the 13 patients showing an objective response also showed characteristics in keeping with immune response criteria, with limited progression in soft tissue and visceral sites, followed by four complete and two partial RECIST, responses (23).
Despite an emphasis on JS1/34.5-/47-/GM-CSF's “cancer-vaccine” properties, the only immune study on tumor and blood samples reported includes just eleven of the 50 trial patients recruited in total (29). Nevertheless, indicative observations were made, with the generation of cytotoxic T-cells against a melanoma-associated antigen (MART-1) found in the tumor and peripheral blood of responding patients. In addition, comparatively low levels of immune-suppressive, T-regulatory (T-reg) cells, linked to poorer outcomes in other clinical studies, were found within injected tumors (29, 68) (Table 3).
Another well-studied cytokine, IL-2, offers the potential to increase NK cell and CD8+ T-cell function, and the ability to increase vascular permeability (69). When expressed from a vaccinia virus vector in 6 patients with malignant pleural mesothelioma, IL-2 expression was detectable and associated with T-cell infiltration in half of biopsied tumors, obtained from all six patients. No systemic toxicity was observed, but nor were any objective clinical responses seen, or further studies reported (70).
Clinical evidence of both, radiological, and immune-mediated, anti-tumour responses have been observed in trials employing vaccinia, JS1/34.5-/47-/GMCSF, JX-594 and Onyx-015. These responses were seen at sites distant from those injected, in keeping with preclinical observations of systemic, immune-mediated effects (15, 20, 22, 23) (Table 2). Biopsies of non-injected tumor sites have demonstrated immune cell infiltration consistent with this (20). However, another study observed vaccinia virus (JX-594) in biopsies from non-injected sites, suggesting systemic dissemination of virus and direct oncolysis as a viable alternative mediator of tumor responses (22).
Pre-clinical work with OVs raises questions of whether the traditional dose-escalation approach is appropriate for early phase trials of OVs. Our own pre-clinical studies in murine melanoma models, suggest it may actually be counterproductive to administer the Maximum Tolerated Dose of OV, as this approach may encourage anti-viral, rather than anti-tumor, immunity (48, 49) (Table 3). It is well-established that current clinical doses and modes of administration result in clinically robust, protective neutralising antibody (nAb) responses to reovirus, NDV and vaccinia virus, even in heavily pre-treated patients (21, 22, 71) (Table 3). Levels of nAb do not directly relate to initial clinical response, i.e. pre-existing anti-viral immunity does not necessarily prevent direct oncolytic therapy, perhaps reflecting the immune suppressive local tumor environment, allowing OV replication (21, 22, 71). However, it would be of interest to establish whether the nAb response occurs at the expense of an eventual adaptive anti-tumor immune response and, more importantly, whether this impacts on long-term outcome.
Overall, present clinical data support, to a limited extent, preclinical observations that anti-tumor immune responses are important in long-term OV efficacy. It would seem desirable that primary immune end-points are explored and validated in future trials of OV therapy.
Maximising OV therapy: combination therapy for synergy
Pre-clinical: OV combination with chemotherapy
Multiple preclinical studies indicate a highly-desirable synergistic effect when combining chemotherapy with OV. Table 4 lists OVs showing synergy in combination with chemotherapy and some possible mechanisms involved. A common pre-clinical method for assessing synergy is the Chou Talalay Combination Index (CI). This commonly-used analysis involves plotting dose-effect curves for each therapy and multiplying diluted combinations of the therapies, using the “median effect” equation, to obtain a combination index (CI). CI values of <1, 1, and >1 indicate synergy, additive effect and antagonism respectively (72).
Table 4.
Synergy between oncolytic viruses and chemotherapy agents
| Virus | Agent (tumor model) | Putative Mechanisms |
|---|---|---|
| HSV (G207) * | Temozolamide (Glioma)†103 Taxane (docetaxel) (Prostate)75 |
Temozolamide induced increase in stress response genes, with ICP-34 homology102,103 Mitotic slippage with ↑apoptosis through combined G2-M and G1 arrest75 |
| Reovirus (Reolysin) | Taxane (paclitaxel), cisplatin gemcitabine, vinblastine (Lung)79 Cisplatin, paclitaxel (Melanoma)78 |
Prolonged mitotic arrest, with ↑apoptosis78,79 ↑Caspase dependent apoptosis78 |
| Vaccinia Virus | Taxane (paclitaxel) (Ovary, Colorectal)76 | Chemosensitisation by:
|
| Adeno Virus (Onyx-015) | Taxane (paclitaxel), cisplatin, (Lung)‡101 Paclitaxel (Ovary)104 |
E1a induced cell cycle activation106 E1a sensitization to chemotherapy105 Mitotic slippage, and apoptosis104 |
Preclinical therapeutic synergy with OV is recognised across a range of tumor types and chemotherapies, and is ascribed when the Chou-Talalay combination index (CI) <1, (see main text). Underlying putative mechanisms of synergy are outlined.
Examples of clinically assessed oncolytic viruses are given in brackets
references in superscript
this study used an alternative method to CI, to attribute synergy.
The taxane chemotherapies (docetaxel and paclitaxel) consistently demonstrate strong synergistic activity (CI <1) in pre-clinical combination studies with a variety of OVs including adenovirus, reovirus and HSV (see table 4). There are various suggested mechanisms of synergy, perhaps reflecting the complex biology of OV and broad effects of chemotherapy. The microtubule-stabilizing action of taxanes appears to be important in facilitating reoviral and adenovirus replication (73, 74). Induction of apoptosis may be a common pathway for OV synergy with taxanes. Reovirus-induced, caspase-dependent apoptosis is synergistically enhanced by the prolonged G2-M arrest induced by paclitaxel, in lung cancer cell lines. Similarly synergistic apoptotic cell death results from the combination of HSV induced G1 arrest, and taxane G2-M arrest, in prostate cancer cells (75).
Paclitaxel sensitivity is also synergistically enhanced by vaccinia-induced release of type I IFN following viral infection, and high-mobility group protein B1 following cell lysis (76). Finally, physical effects may play a part in synergy, as shown in pre-clinical studies in which the combination of oncolytic HSV and taxane chemotherapy resulted in cell lysis and breakdown of tumor, with improved ingress and replication of virus in tumor cells (77). Other chemotherapies also show synergy, via similar mechanisms, e.g. cisplatin, which potentiates apoptosis in melanoma lines (78, 79), however the wide-ranging, high-level, synergy observed between various OVs and taxanes, would support such combinations being explored clinically (Table 4).
A recent report describes a systematic attempt to maximise synergy whilst retaining oncolytic ability (80). Diallo and colleagues describe a “pharmacoviral” screen, in which the impact of each of over 12,000 chemical compounds on viral oncolysis was assessed in a cell-based assay, using a high throughput screening method. The cytotoxicity of low titers of the VSV mutant, VSV-Δ51, which is highly sensitive to the interferon response, was assessed, with and without drug, in a partially-resistant cell-line (81). Their approach identified a number of potential compounds demonstrating synergy for the replication and spread of VSV-Δ51 in vitro.
One of the chemical compounds assessed in this way, VSe1 (3,4-dichloro-5-phenyl 2,5-dihydrofuran-2-one), was shown to suppress the interferon response to VSV, conferring on VSV a temporary and apparently tumor-selective replication advantage in vivo. Their discovery of a specific compound complementing the known biology of the mutant VSV-Δ51 suggests this screening approach could be replicated with other viruses. Although this is an attractive prospect, it has not yet been realised in clinical practice (81).
This synergy of VSV-Δ51and VSe-1 is in keeping with other preclinical observations that suppression of the innate anti-viral immune response can improve oncolysis and efficacy (Table 2) (63–65). There is further preclinical evidence, in melanoma, that reoviral synergy with cisplatin accompanies ablation of the local innate inflammatory response (78), shown preclinically to boost innate anti-tumor immunity (54, 82). An important question to resolve is therefore whether the improvement in direct oncolysis accompanying selective suppression of the innate anti-viral response may be offset by the potential loss of anti-tumor immunity? A reasonable hypothesis would be to expect synergy to be reflected in greater, immediate, tumor shrinkage (compared to chemotherapy alone). In contrast, development of an anti-tumor response may be expected to correspond to longer duration of response, prior to subsequent progression. These transtionally relevant questions could be addressed, for example, in the ongoing trial of chemotherapy ± reovirus in SCCHN.
Clinical OV and chemotherapy combination data
None of the currently available data clearly indicate whether pre-clinical synergy between OVs and chemotherapy can be translated into improved clinical outcomes, but there are signals suggesting improved response from the combination of OV and cytotoxic drugs in clinical trials. Although not developed commercially, a combination Phase 2 trial of IT Onyx-015, combined with cisplatin and 5-fluourouracil chemotherapy, in patients with recurrent SCCHN, demonstrated notable complete (8/37) and partial response rates (19/37) in injected nodules (15). These results compared favorably to historical data obtained with either virus alone or chemotherapy alone (22–33%) (17, 83), and were consistent with preclinical models showing synergy with the same agents (26).
Reovirus combined with docetaxel has proven safe in a Phase I trial of 16 patients, with one objective complete response, 3 partial responses and 7 patients with stable disease observed (84). Reovirus was detectable in tumor biopsies, and docetaxel did not compromise the neutralising antibody response to reovirus (NARA). In contrast, a similar early phase trial of gemcitabine combined with reovirus led to liver toxicity, and reduced NARA. Only one objective response was seen amongst 16 patients treated, plus 6 patients with stable disease (85). Reovirus/taxane and reovirus/platinum combinations have also featured in a phase 2 study in relapsed SCCHN patients. Nineteen patients, most of whom were refractory to previous platinum-based chemotherapy, were treated with intravenous reovirus, along with carboplatin and paclitaxel chemotherapy with partial response rates over 40% and stable disease in a further 30% (30). However, in contrast to the JS1/34.5-/47-/GM-CSF phase 2 data in melanoma (23), no complete responses were observed, and though promising in terms of response rate, the small sample size and current lack of information regarding duration of response, do not immediately predict the success of chemo-virotherapy in this setting according to predictive algorithms (30, 86). The result of a key ongoing randomised phase-3 trial using the same chemotherapy combination ± reovirus in SCCHN patients is therefore awaited with genuine interest.
Conclusions
OVs represent a diverse group of viruses with the ability to selectively kill tumor cells, and thus represent attractive anti-cancer agents. Pre-clinical oncolytic activity has not, thus far, been translated into routine clinical, which may reflect the inability of pre-clinical models to replicate the complexity of diverse interactions between virus tumor, and intact host immune system.
It is clear that embracing existing knowledge, by encouraging anti-tumor immunity (OncoVEXGMCSF) or exploiting synergy with chemotherapy (reovirus, ONYX-015), to enhance OV efficacy has already contributed to emerging promise, leading to late phase OV clinical trials (23, 30, 33). Robust late phase data is required before we can accept OVs as legitimate alternatives to current therapies, however current approaches appear to be on the cusp of offering the genuine prospect of improved clinical outcomes.
Questions still remain: Is it possible, or even desirable, to overcome anti-viral immunity? Is anti-tumor immunity really more important than direct oncolysis? If so, is it possible to quantify this, and consistently to manipulate the host immune response against tumors? Synergy may offer improved response rates, but will it also lead to long-term tumor control? Is synergy also compatible with productive anti-tumor immunity? These questions may well be too complex to resolve using current preclinical models and further highlight the continuing need for in-depth translational studies, ideally in the context of OV trials. Deriving clear answers will help direct future approaches, offer enhanced therapy and could ultimately lead to improved survival for patients.
Translational relevance.
Oncolytic viruses (OVs) are biologically targeted agents, with the ability to potently, and selectively replicate in and kill tumor cells. There is undoubted promise with late phase trials using both DNA and RNA viruses underway, however their clinical efficacy remains to be proven.
Increasingly there is recognition of a potentially productive, rather than inhibitory, relationship between OVs and the host immune response, with fresh approaches encouraging host anti-tumor immunity showing clinical promise.
Preclinical synergy with chemotherapy is reflected in increased clinical response rates, and may represent another way to optimise OV therapy. However preclinical evidence also suggests chemotherapy may impact on the host immune response, with uncertain effects on long-term outcome. Clinical data corresponding to the complex interactions between OV, tumor, chemotherapies, and the host immune system is lacking. Therefore high quality translational studies are required to enhance our understanding of the biology of OVs, in order to improve outcomes further.
Acknowledgments
Richard G Vile is supported by NIH grants CA107082, CA130878, and CA132734, the Mayo Foundation, and the Richard M. Schulze Family Foundation.
R.G.V. has received research grants from Oncolytics Biotech (Calgary, AB, Canada).
Abbreviations
- EGFR
epidermal growth factor receptor
- vgf
vaccinia growth factor
- TK
Thymidine kinase
- tkdeleted
thymidine kinase deleted
- Rb
Retinoblastoma gene
- ICP34deleted
ICP34 deleted virus
- VV
vaccinia virus
- PKR
protein kinase receptor
References
- 1.Georgiades J, Zielinski T, Cicholska A, Jordan E. Research on the oncolytic effect of APC viruses in cancer of the cervix uteri; preliminary report. Biul Inst Med Morsk Gdansk. 1959;10:49–57. [PubMed] [Google Scholar]
- 2.Bluming AZ, Ziegler JL. Regression of Burkitt's lymphoma in association with measles infection. Lancet. 1971;2:105–6. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 3.Pasquinucci G. Possible effect of measles on leukaemia. Lancet. 1971;1:136. doi: 10.1016/s0140-6736(71)90869-5. [DOI] [PubMed] [Google Scholar]
- 4.Hansen RM, Libnoch JA. Remission of chronic lymphocytic leukemia after smallpox vaccination. Arch Intern Med. 1978;138:1137–8. [PubMed] [Google Scholar]
- 5.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34:1907–28. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Southam CM, Moore AE. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–34. doi: 10.1002/1097-0142(195209)5:5<1025::aid-cncr2820050518>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 8.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nature medicine. 2001;7:781–7. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Uchida E. Regulatory aspects of oncolytic virus products. Curr Cancer Drug Targets. 2007;7:203–8. doi: 10.2174/156800907780058790. [DOI] [PubMed] [Google Scholar]
- 10.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature clinical practice. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 11.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221–8. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–32. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 15.Khuri FR, Nemunaitis J, Ganly I, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nature medicine. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 16.Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308–15. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 17.Nemunaitis J, Ganly I, Khuri F, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–66. [PubMed] [Google Scholar]
- 18.Nemunaitis J, Tong AW, Nemunaitis M, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther. 2009;18:429–34. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid O, Varterasian ML, Wadler S, et al. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:1498–504. doi: 10.1200/JCO.2003.09.114. [DOI] [PubMed] [Google Scholar]
- 20.Mastrangelo MJ, Maguire HC, Jr., Eisenlohr LC, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–22. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 21.Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20:2251–66. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. The lancet oncology. 2008;9:533–42. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 23.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–71. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 24.Vasey PA, Shulman LN, Campos S, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–9. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 25.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 26.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature medicine. 1997;3:639–45. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 27.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. Journal of the National Cancer Institute. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 28.Jia H. China syndrome--a regulatory framework in meltdown? Nat Biotechnol. 2007;25:835–7. doi: 10.1038/nbt0807-835. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–30. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 30.Karapanagiotou JDC EM, Pandha HS, Gill GM, Coffey MC, Mettinger K, Harrington KJ. A phase I/II study of oncolytic reovirus plus carboplatin/paclitaxel in patients with advanced solid cancers with emphasis on squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2010;28:15s. suppl; abstr 3080. [Google Scholar]
- 31.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 32.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6:941–9. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 34.Morris DGFP, Paterson AH, Fonseca K, Difrancesco LM, Thompson BG, Coffey MC. A phase I clinical trial evaluating intralesional Reolysin (reovirus) in histologically confirmed malignancies. 2002 ASCO Annual Meeting; 2002.2002. [Google Scholar]
- 35.Mita AC, Sankhala K, Sarantopoulos J, et al. A phase II study of intravenous (IV) wild-type reovirus (Reolysin) in the treatment of patients with bone and soft tissue sarcomas metastatic to the lung. 2009 ASCO Annual Meeting J Clin Oncol; 2009. p. suppl. abstr 10524. [Google Scholar]
- 36.Rowan K. Oncolytic viruses move forward in clinical trials. Journal of the National Cancer Institute. 2010;102:590–5. doi: 10.1093/jnci/djq165. [DOI] [PubMed] [Google Scholar]
- 37.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 38.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. The EMBO journal. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nature medicine. 2000;6:821–5. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy S, Takimoto T, Scroggs RA, Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006;80:5145–55. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GMCSF. Mol Ther. 2006;14:361–70. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Prestwich RJ, Errington F, Diaz RM, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–32. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 44.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 45.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–47. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 47.Qiao J, Kottke T, Willmon C, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nature medicine. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 48.Qiao J, Wang H, Kottke T, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–16. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ilett EJ, Prestwich RJ, Kottke T, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–99. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Power AT, Wang J, Falls TJ, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–30. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 51.Hirasawa K, Nishikawa SG, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–53. [PubMed] [Google Scholar]
- 52.Iankov ID, Blechacz B, Liu C, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–22. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 54.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 55.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willmon CL, Saloura V, Fridlender ZG, et al. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–20. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavallo F, Signorelli P, Giovarelli M, et al. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. Journal of the National Cancer Institute. 1997;89:1049–58. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 58.Portielje JE, Gratama JW, van Ojik HH, Stoter G, Kruit WH. IL-12: a promising adjuvant for cancer vaccination. Cancer Immunol Immunother. 2003;52:133–44. doi: 10.1007/s00262-002-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. Journal of the National Cancer Institute. 1995;87:581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 60.Wong RJ, Chan MK, Yu Z, et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 2004;10:4509–16. doi: 10.1158/1078-0432.CCR-04-0081. [DOI] [PubMed] [Google Scholar]
- 61.Breitbach CJ, Paterson JM, Lemay CG, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–93. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 62.Grote D, Cattaneo R, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–8. [PubMed] [Google Scholar]
- 63.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–88. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 65.Smakman N, van der Bilt JD, van den Wollenberg DJ, Hoeben RC, Borel Rinkes IH, Kranenburg O. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13:815–8. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]
- 66.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 67.Hodi FS, O'Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 69.Gansbacher B, Zier K, Daniels B, Cronin K, Bannerji R, Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990;172:1217–24. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mukherjee S, Haenel T, Himbeck R, et al. Replication-restricted vaccinia as a cytokine gene therapy vector in cancer: persistent transgene expression despite antibody generation. Cancer Gene Ther. 2000;7:663–70. doi: 10.1038/sj.cgt.7700133. [DOI] [PubMed] [Google Scholar]
- 71.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–20. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 72.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 73.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–30. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 74.AbouEl Hassan MA, Braam SR, Kruyt FA. Paclitaxel and vincristine potentiate adenoviral oncolysis that is associated with cell cycle and apoptosis modulation, whereas they differentially affect the viral life cycle in non-small-cell lung cancer cells. Cancer Gene Ther. 2006;13:1105–14. doi: 10.1038/sj.cgt.7700984. [DOI] [PubMed] [Google Scholar]
- 75.Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–60. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang B, Sikorski R, Kirn DH, Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2010 doi: 10.1038/gt.2010.121. [DOI] [PubMed] [Google Scholar]
- 77.Nagano S, Perentes JY, Jain RK, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pandha HS, Heinemann L, Simpson GR, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–66. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 79.Sei S, Mussio JK, Yang QE, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 81.Diallo JS, Le Boeuf F, Lai F, et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 2010;18:1123–9. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358–66. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nemunaitis J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 84.Comins C, Spicer J, Protheroe A, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–72. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lolkema MP, Arkenau HT, Harrington K, et al. A phase I study of the combination of intravenous reovirus type 3 dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2010;17:581–8. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 86.Chan JK, Ueda SM, Sugiyama VE, et al. Analysis of phase II studies on targeted agents and subsequent phase III trials: what are the predictors for success? J Clin Oncol. 2008;26:1511–8. doi: 10.1200/JCO.2007.14.8874. [DOI] [PubMed] [Google Scholar]
- 87.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121:1058–63. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- 88.Forni G, Lollini PL, Musiani P, Colombo MP. Immunoprevention of cancer: is the time ripe? Cancer Res. 2000;60:2571–5. [PubMed] [Google Scholar]
- 89.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greiner S, Humrich JY, Thuman P, Sauter B, Schuler G, Jenne L. The highly attenuated vaccinia virus strain modified virus Ankara induces apoptosis in melanoma cells and allows bystander dendritic cells to generate a potent anti-tumoral immunity. Clinical and experimental immunology. 2006;146:344–53. doi: 10.1111/j.1365-2249.2006.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 92.Apostolidis L, Schirrmacher V, Fournier P. Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int J Oncol. 2007;31:1009–19. [PubMed] [Google Scholar]
- 93.So T, Takenoyama M, Mizukami M, et al. Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005;65:5945–52. doi: 10.1158/0008-5472.CAN-04-3787. [DOI] [PubMed] [Google Scholar]
- 94.Ochsenbein AF. Immunological ignorance of solid tumors. Springer Semin Immunopathol. 2005;27:19–35. doi: 10.1007/s00281-004-0192-0. [DOI] [PubMed] [Google Scholar]
- 95.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 96.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–53. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 97.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oppenheim DE, Roberts SJ, Clarke SL, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 99.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–75. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.You L, Yang CT, Jablons DM. ONYX-015 works synergistically with chemotherapy in lung cancer cell lines and primary cultures freshly made from lung cancer patients. Cancer Res. 2000;60:1009–13. [PubMed] [Google Scholar]
- 102.Adusumilli PS, Chan MK, Chun YS, et al. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol Ther. 2006;5:48–53. doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. Journal of the National Cancer Institute. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 104.Ingemarsdotter CK, Baird SK, Connell CM, Oberg D, Hallden G, McNeish IA. Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene. 2010 doi: 10.1038/onc.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez-Prieto R, Quintanilla M, Cano A, et al. Carcinoma cell lines become sensitive to DNA-damaging agents by the expression of the adenovirus E1A gene. Oncogene. 1996;13:1083–92. [PubMed] [Google Scholar]
- 106.Tiainen M, Spitkovsky D, Jansen-Durr P, Sacchi A, Crescenzi M. Expression of E1A in terminally differentiated muscle cells reactivates the cell cycle and suppresses tissue-specific genes by separable mechanisms. Mol Cell Biol. 1996;16:5302–12. doi: 10.1128/mcb.16.10.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23:1666–70. [PubMed] [Google Scholar]