Abstract
The endogenous opioid system is involved in modulating a number of behavioral and physiological systems, including the hypothalamic-pituitary-adrenal (HPA) axis. In humans, a functional variant in the OPRM1 gene (OPRM1 A118G) is associated with a number of outcomes, including attenuated HPA axis responses to stress. A nonsynonymous variant (OPRM1 C77G) in the rhesus macaque has been shown to have similar effects in vivo to the human variant. The current study investigated whether OPRM1 C77G influences HPA axis response to stress in rhesus macaques. We analyzed plasma adrenocorticotropic hormone (ACTH) and cortisol levels measured in response to three different stressors: 1) maternal separation in infant subjects at 6 months of age, 2) acute ethanol administration in adolescent subjects at 4 years of age, and 3) postpartum HPA axis function in adult rhesus macaque females. For the maternal separation paradigm, ACTH and cortisol levels were determined at baseline as well as peak levels during each of 4 consecutive separation episodes. For the acute ethanol administration paradigm, hormone levels were determined at baseline and again at 5 minutes, 10 minutes, and 60 minutes following the ethanol infusion. For postpartum sampling, hormone levels were determined at postpartum days 7, 14, 21, 30, 60, 90, 120, and 150. Infants carrying the 77G allele exhibited lower levels of cortisol across all 4 separation episodes. Furthermore, adolescents carrying the 77G allele exhibited lower cortisol levels at 5 and 10 minutes following acute ethanol administration. Adult females with prior reproductive experience and who carry the 77G allele exhibited lower cortisol levels across the postpartum period. No significant genotype effects were found for ACTH, although there were some trends for lower ACTH levels in 77G allele carriers. These data are consistent with human studies that have demonstrated attenuated cortisol responses to stress among carriers of the OPRM1 118G allele, lending further support to the argument that the rhesus and human allelic variants are functionally similar. Our results also suggest that OPRM1 variation may influence coping style, as well as alcohol-induced and postpartum levels of HPA axis activity and, as such, may modify vulnerability to alcohol use disorders and postpartum depression.
Keywords: Opioids, Hypothalamic-Pituitary-Adrenal (HPA) Axis, Cortisol, Stress, Nonhuman Primate, Separation, Alcohol, Postpartum Depression
Introduction
The endogenous opioids modulate a number of vital physiological systems and associated processes and behavioral outcomes, including anti-nociception, reward, immune function, gastrointestinal function, social attachment, and stress response (Panksepp et al., 1994; Van Ree et al., 2000; Drolet et al., 2001; Bodnar, 2004; Ribeiro et al., 2005; Frew and Drummond, 2007; Sher and Stanley, 2008; Stefano and Kream, 2008). The hypothalamic-pituitary-adrenal (HPA) axis is a key mediator of stress response and is of particular interest in neuroendocrine and psychological research, since dysregulated HPA axis activity is associated with a number of neuropsychological disorders and other health problems (Chrousos and Gold, 1992; Heim et al., 2000; Sapolsky, 2000; Gianoulakis, 2004). In nonhuman primates and in humans, β-endorphin, which preferentially binds the μ-opioid receptor, promotes tonic restraint of the HPA axis through inhibitory neurons providing input to corticotropin releasing hormone (CRH)-releasing neurons in the paraventricular nucleus of the hypothalamus (Wand et al., 1998). Consequently, pharmacological blockade of μ-opioid receptors results in an increase in adrenocorticotropin hormone (ACTH) and cortisol release, even in the absence of an external stressor (Volavka et al., 1979; Kreek, 1996; Wand et al., 1998).
In humans, a single nucleotide polymorphism (SNP) in the μ-opioid receptor gene (OPRM1) results in an amino acid substitution (asparagine to aspartate) in the extracellular N-terminal arm of the receptor (Bond et al., 1998). Although the precise molecular mechanism remains unclear (Befort et al., 2001; Beyer et al., 2004; Kroslak, 2007), it is well established that the A118G variant has functional consequences in vivo. Specifically, studies have shown A118G variation to modulate nociception (Fillingim et al., 2005; Lotsch and Geisslinger, 2006; Janicki et al., 2006; Sia et al., 2008), response to opioid analgesia (Klepstad et al., 2004; Romberg et al., 2005; Oertel et al., 2006; Chou et al., 2006a; Chou et al., 2006b), response to alcohol and nicotine (Ray and Hutchison, 2004; Lerman et al., 2004; Ray et al., 2006), and the therapeutic efficacy of opioid antagonists in alcohol-dependence (Oslin et al., 2003; Garbutt et al., 2005; Anton et al., 2008; Kim et al., 2009). Individuals carrying the 118G allele also show an enhanced cortisol response to μ-opioid receptor blockade (Wand et al., 2002; Hernandez-Avila et al., 2003; Chong et al., 2006) and, conversely, a reduced cortisol response to psychosocial stress (Chong et al., 2006; Pratt and Davidson, 2009). While these latter two findings initially may appear contradictory, both could be linked to a potential association between the 118G allele and increased inhibitory opioid tone directed at CRH neurons, such that G allele carriers would experience a dampened cortisol response to psychological stress but, upon μ-opioid receptor blockade, would experience a more dramatic rebound in cortisol levels (Chong et al., 2006; Pratt and Davidson, 2009).
Similar to the human A118G variant, a nonsynonymous OPRM1 C77G SNP in the rhesus macaque results in an amino acid substitution in the N-terminal arm of the μ-opioid receptor (Arg26Pro) In vivo, the 77G allele was associated with significantly lower basal and ACTH-stimulated plasma cortisol levels and increased aggressive threat behavior (Miller et al., 2004). Recent investigations from our laboratory have demonstrated a role for this SNP in increased ethanol-induced stimulation, naltrexone response, and social attachment, findings that translate to humans and are consistent with a gain-of-function role for this polymorphism (Barr et al., 2007; Barr et al., 2008; Barr et al., 2009). Given the effects of the 118G allele on the neuroendocrine stress response in humans, we here investigated the effects of the rhesus macaque 77G allele on the response to different stressors in rhesus macaques.
Across a wide variety of species, maternal separation induces HPA axis activity (Coe et al., 1985; Kalin et al., 1988; Bayart et al., 1990; Wiener et al., 1990; Higley et al., 1992; Dettling et al., 1998; Nelson and Panksepp, 1998; Dettling et al., 2002; Barr et al., 2004b). Similarly, acute ethanol administration stimulates the HPA axis in both animals and humans (Schuckit et al., 1987; Piazza and Le, 1997; Laszlo et al., 2001; Barr et al., 2004a; Richardson et al., 2008). Dysregulated activity of the HPA axis is also observed during the postpartum period. Here, we measured HPA axis activation in rhesus macaques in response to two acutely stressful situations, maternal separation and acute ethanol administration, as well as HPA axis function in mothers during the postpartum period leading up to weaning. We predicted that 77G allele carriers would show attenuated cortisol responses to both maternal separation and acutely administered alcohol. While not an acute stressor, the postpartum period is accompanied by important changes in HPA axis functioning, including suppression of CRH secretion and, typically, reduced cortisol responses to stress (Schuckit et al., 1987; Piazza and Le, 1997; Laszlo et al., 2001; Mastorakos and Ilias, 2003; Barr et al., 2004a; Richardson et al., 2008). Consequently, we examined whether OPRM1 variation was associated with differences in plasma cortisol levels in adult female rhesus macaques during the postpartum period. We extended this analysis up through when the infants were 5 months of age in order to capture the additional stress of the weaning period. Consistent with our previous predictions, we expected to observe lower cortisol levels in mothers carrying the 77G allele.
Methods
Subjects
The subjects were rhesus macaques maintained at the National Institutes of Health Animal Center (NIHAC). Table 1 presents the sample size for each analysis performed as part of this investigation. For the maternal separation study, data from a total of 81 infants studied from 1994 to 2000 were analyzed. The subjects were 6-month old infants that had been reared in large indoor-outdoor enclosures with their mothers in mixed-sex social groups containing two adult males and six to eight adult females with their infant offspring. For the acute ethanol administration study, data from a total of 51 monkeys, studied from 1992 to 1997, were analyzed. These subjects were adolescent/young adult monkeys (average age: 36.4 months; age range: 20.9 – 48.6 months) living in peer groups. The analysis of postpartum HPA axis function in adult female rhesus macaques included both primiparous (n = 58) and multiparous mothers (n = 47). The average age for primiparous mothers was 5.6 years (range: 3.6 – 10.8 years), and for multiparous mothers the average age was 9.8 years (range: 4.0 – 18.1 years).
Table 1.
Subject sample
| Maternal Separation |
Acute Alcohol Administration |
Postpartum | ||||||
|---|---|---|---|---|---|---|---|---|
| ‘ | Primiparous | Multiparous | ||||||
| C/C | C/G | C/C | C/G | C/C | C/Ga | C/C | C/Ga | |
| Total (n) |
61 | 20 | 33 | 13 | 39 | 19 | 29 | 18 |
| F | 28 | 12 | 21 | 10 | ||||
| M | 33 | 8 | 12 | 3 | ||||
There were 2 individuals with a genotype of G/G (both were included in primiparous and multiparous data sets); these individuals were combined with C/G individuals for analysis.
Maternal Separation Procedures
At 6 months of age, infants were separated from their mothers for 4 days, with 3 days of reunion between each of four repetitions of the separation procedure. To avoid potential confounds of stress resulting from exposures to a novel environment or social isolation, infants remained in the home cage with the social group (consisting of adult females, males, and infants) for the duration of study, and their mothers were removed from the enclosure. No maternal siblings or older juveniles were present in any infant’s social group. Separation was initiated between 12:00 and 12:30 p.m. on Monday of each week. Baseline blood samples were obtained from the femoral vein in non-anesthetized animals immediately prior to separation (hour 0). The infants were not anesthetized so that behavioral observations could be conducted immediately following collection of the blood sample. Mother-infant pairs were captured together and, if necessary, the mother was lightly anesthetized in order to remove the infant. Pairs were typically captured within 5–10 minutes, and blood samples were obtained within about 5 minutes of capture. Additional blood samples were obtained from the infants at hour 1 and hour 2 of separation. All blood samples were immediately placed on ice and then centrifuged at 4°C for 20 minutes. The plasma was aliquoted and frozen in liquid nitrogen, after which the plasma samples were stored in a freezer at −70°C until assayed. Plasma samples were assayed for ACTH and cortisol using standard radioimmunoassay.
Acute Ethanol Administration
Acute ethanol administration procedures have been described in detail previously (Barr et al., 2007). Briefly, monkeys were removed from their home enclosure and restrained on a flat surface while ethanol (16.8%[v/v] USP; 2.2 g/kg for males, 2.0 g.kg for females) was infused into the saphenous vein at a constant rate over a 15 minute period. Blood samples for baseline cortisol levels were obtained under ketamine anesthesia (15mg/kg, IM) prior to the first administration session, as well as at 5, 10, and 60 minutes following initiation of the infusion. Behavioral testing (data not reported here) took place between the 10 and 60 minute blood samples (see (Barr et al., 2007) for details). Blood samples for analysis of ACTH and cortisol were handled and processed as described above for the maternal separation samples. Each subject was given two administrations of ethanol, with each session, on average, less than one month apart. Repeated outcome measures for each individual were then averaged in order to reduce the effect of random measurement error (Martin and Kraemer, 1987).
Postpartum Blood Sampling
In order to conduct standard neonatal behavioral testing as part of a separate research project in the laboratory, mother-infant pairs were caught up on postpartum days 7, 14, 21, and 30, and at month 2, 3, 4, and 5. Mothers were immediately anesthetized with ketamine and blood samples were drawn from the femoral vein. All samples were handled and assayed as described above.
Genotyping
Using standard extraction methods, DNA was isolated from whole blood, collected from the femoral vein under ketamine anesthesia (15 mg/kg, IM). Genotyping was performed using the procedure modified from Miller et al. (Miller et al., 2004), which has been described previously (Barr et al., 2007; Barr et al., 2008). Genotyping accuracy was determined by genotyping of 10% of the samples in duplicate. Genotyping completion was 95% and accuracy was 98%. In all cases, inaccurate calls were for C/G vs. G/G genotypes. For any ambiguous calls or failed genotyping, genotyping was repeated until completion and accuracy were 100%.
Data Analysis
All data on ACTH and cortisol levels were examined for outliers and tested for deviation from normality (Kolmogorov-Smirnov test, all p > 0.05). For the maternal separation study, peak ACTH and cortisol levels on the Monday of each separation week were determined for each subject and analyzed along with levels measured at baseline of the first separation using repeated measures analysis of variance (ANOVA). ACTH levels were not available for subjects in the earliest cohort (1994) and this, combined with different patterns of missing data, resulted in a reduced sample size for the analysis of ACTH in response to separation compared to that available for cortisol. For the analysis of HPA axis response to acute alcohol administration, ACTH and cortisol levels at baseline, 5 minutes, 10 minutes, and 60 minutes were averaged between the two testing sessions and also analyzed with repeated measures ANOVA. In both of the above analyses, genotype and sex were included as between-subjects factors and time point (baseline and peak measures at weeks 1–4 for separation study; baseline and 5, 10, and 60 minutes measures for the alcohol study) was the within-subjects factor. In addition, age was included as a covariate in the analysis for the acute alcohol administration paradigm.
ACTH and cortisol levels during the postpartum period were analyzed separately for primiparous and multiparous mothers primarily for two reasons: 1) studies have shown that mothers with reproductive experience have altered sensitivity to opioids (Mann et al., 1989; Bridges and Hammer, 1992), suggesting that effects of OPRM1 variation on HPA axis function may differ between primiparous and multiparous mothers, and 2) data were collected from a number of subjects (n = 27) under both primiparous and multiparous conditions, so it was not appropriate to analyze both groups together. Slightly more than half of the multiparous mothers had data collected across multiple births (each during a separate year). Preliminary analyses revealed no significant differences in maternal cortisol levels between consecutive infants, thus the data were averaged across infants for each postpartum time point. For both primparous and multiparous mothers, data were analyzed with repeated measures ANOVA with time point as the within subjects factor. Initial analysis included mothers’ age as a covariate, but because age did not significantly contribute to the model it was dropped from the final analysis. Analyses were performed using the general linear models (GLM) procedure in Statistica (Statsoft, Tulsa, Oklahoma). Effect sizes were calculated using G*Power 3 (Institut für Experimentelle Psychologie, Heinrich Hein Universität, Düsseldorf, Germany).
In the subject sample as a whole, the frequency of the 77G allele was 15%, and genotype frequencies did not deviate from Hardy-Weinberg equilibrium (X2 = 0.62, p = 0.43). The frequency of the minor G allele is consistent with previous reports from our laboratory (Barr et al., 2007; Barr et al., 2008) as well as with reports of 118G allele frequencies in humans (excepting African Americans) (LaForge et al., 2000). Because the G/G genotype was rare (only two individuals, both adult females), G/G and C/G animals were collapsed together into one group for all analyses.
Results
Maternal Separation
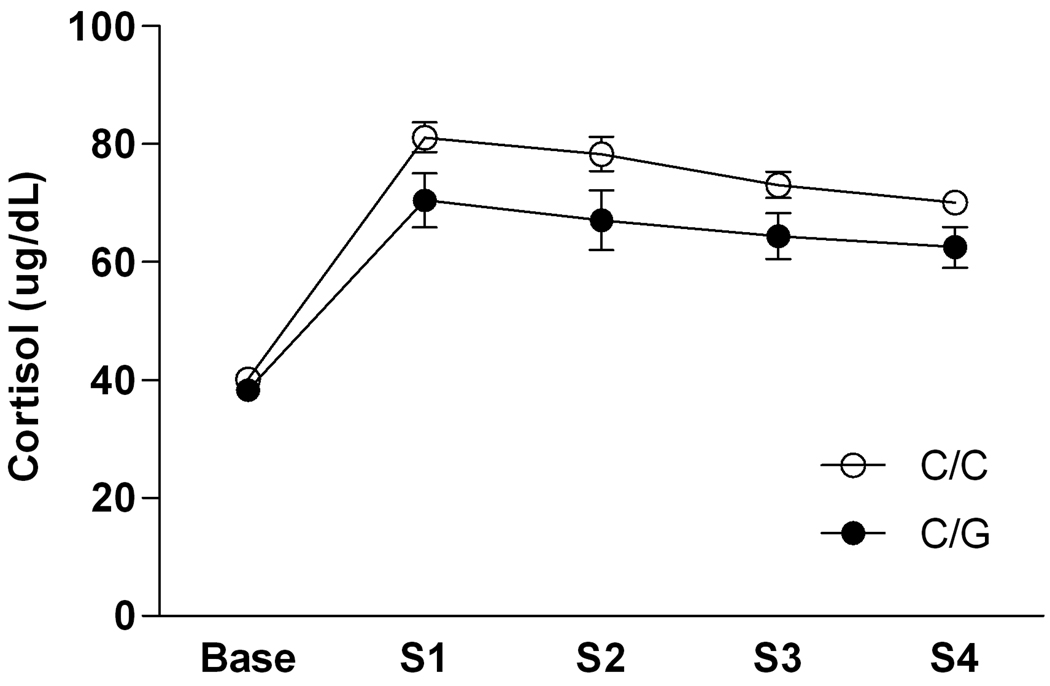
There was a main effect of time point [F(4, 308) = 81.04, p < 0.0001] and a main effect of genotype [F(1, 77) = 5.34, p = 0.02] on cortisol levels during maternal separation (Figure 1). As expected, cortisol levels increased significantly over baseline levels during all four separations. Overall, peak cortisol levels during weeks 3 and 4 were lower compared to peak cortisol levels during weeks 1 and 2 (Newman-Keuls post hoc tests, all p < 0.05), indicating some habituation to the stressor. On average, however, cortisol levels in response to maternal separation were significantly lower in carriers of the 77G allele. The Cohen’s f for this effect was 0.27, indicating a small to medium effect size.
Figure 1.
Peak cortisol response to maternal separation as a function of OPRM1 genotype in six month old rhesus macaque infants. Values are given as mean ± SEM. Peak cortisol responses were determined by comparing values at hour 1 and hour 2 of separation on the Monday of each separation week, When data were analyzed using only the hour 2 values for all subjects (the time point for which most subjects exhibited the peak response), the results were the same. S1 = week 1 separation, S2 = week 2 separation, S3 = week 3 separation, S4 = week 4 separation.
There was a significant effect of time point for ACTH [F(4, 244) = 17.98, p < 0.0001] but no main or interactive effects of genotype. There was, however, a non-significant trend for lower ACTH levels in carriers of the 77G allele [F(1, 61) = 2.97, p = 0.09; Supplemental Figure 1a].
Acute Alcohol Administration
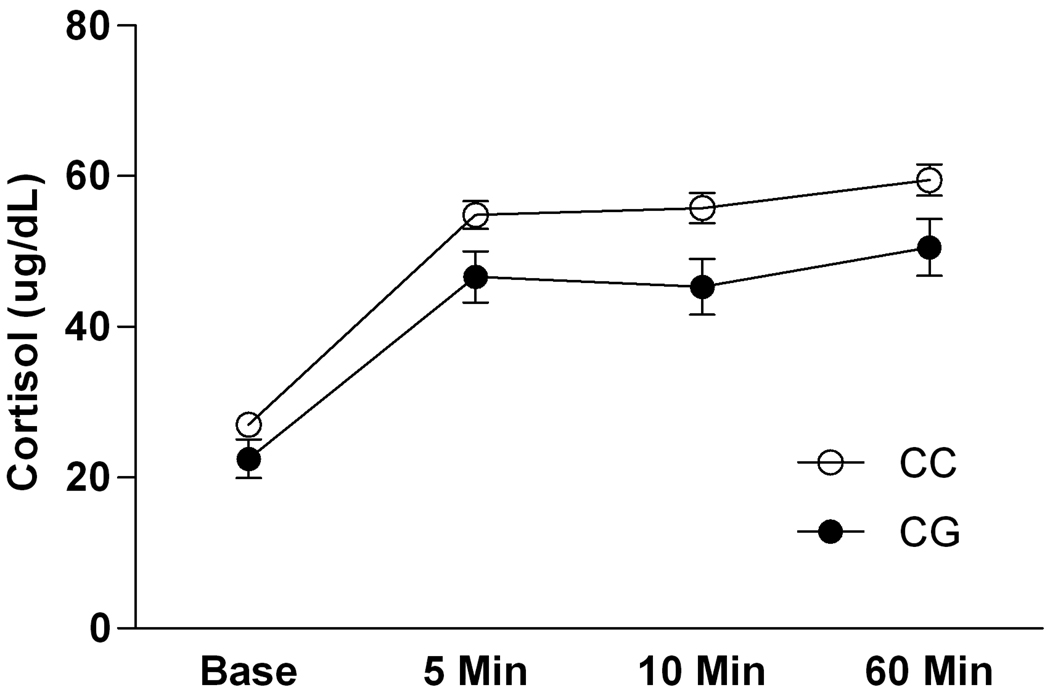
There was a main effect of time point [F(3, 123) = 14.67, p <0.0001] and a main effect of genotype [F(1, 41) = 6.26, p = 0.02] on cortisol levels in response to acute alcohol administration (Figure 2). On average, cortisol levels were significantly increased over baseline following alcohol administration at 5 and 10 minutes; a further increase in cortisol was seen at 60 minutes, following the behavioral testing (Newman-Keuls post hoc tests, all p < 0.05). Overall, carriers of the 77G alleles had lower cortisol levels compared to subjects homozygous for the C allele. The Cohen’s f for this effect was 0.39, indicating a medium effect size.
Figure 2.
Cortisol response to acute alcohol administration as a function of OPRM1 genotype in the adolescent/young adult rhesus macaques. Values are given as mean ± SEM.
There was a significant effect of time point for ACTH [F(3, 123) = 4.47, p = 0.005; Supplemental Figure 1b] but no main or interactive effects of genotype.
Postpartum Cortisol
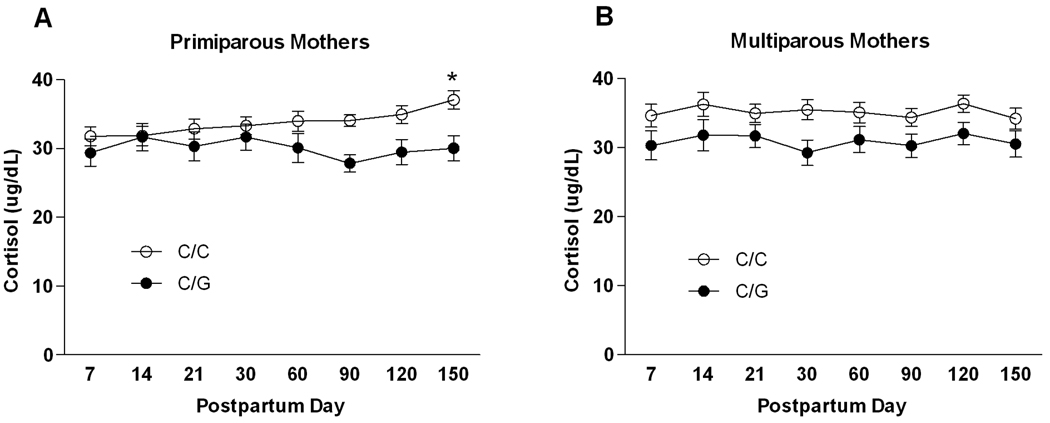
There was a main effect of genotype [F(1, 56) = 4.61, p = 0.04] and an interaction of genotype and postpartum time point [F(7, 392) = 2.10, p = 0.04] on cortisol levels in primiparous mothers (Figure 3a). On average, cortisol levels were lower among 77G allele carriers (Cohen’s f = 0.29 indicating a small to medium effect size). However, among the C/C subjects there was a gradual increase in cortisol such that levels at month 5 were significantly higher compared to day 7 and day 14 (Newman-Keuls post hoc tests, both p < 0.05). This increase was not observed in the 77G allele carriers. There was a main effect of postpartum time point on ACTH [F(7, 385) = 2.18, p = 0.03; Supplemental Figure 2a] but no significant effects of genotype.
Figure 3.
Cortisol levels in rhesus macaque mothers as a function of OPRM1 genotype. Values are given as mean ± SEM. (A) Primiparous (first-time) mothers. * indicates a significant difference (p < 0.05) from day 7 and day 14 in primaparous mothers according to Newman-Keuls post-hoc tests. (B) Multiparous mothers.
The analysis of cortisol in multiparous mothers revealed a main effect of genotype only [F(1, 45) = 5.48, p = 0.02] (Figure 3b). On average across the postpartum period, carriers of the 77G allele had lower cortisol levels than subjects homozygous for the C allele. The Cohen’s f for this effect was 0.29, indicating a small to medium effect size.
There were no significant effects of postpartum time point or genotype on ACTH, although there was a non-significant trend for lower ACTH levels in carriers of the 77G allele [F(1, 45) = 3.31, p = 0.08; Supplemental Figure 2b].
Discussion
The results of this study demonstrate that OPRM1 gene variation modulates cortisol responses across various life stages, in response to both emotional and physical stressors. Among six month old infants, carriers of the 77G allele showed a blunted cortisol response across multiple exposures to emotional stress (ie, maternal separation). Similarly, a blunted cortisol response was observed among adolescents and young adults carrying the 77G allele when they were given an acute dose of alcohol, a known physiological stressor. Lastly, adult females with infants exhibited lower cortisol levels across the postpartum period if they carried the 77G allele, an effect seen primarily in mothers with prior reproductive experience, but which was also observed in first-time mothers during the latter stages on the postpartum period. The effect sizes were small to medium in each case, and in general the magnitude of differences is consistent with earlier findings of reduced cortisol levels in response to stress in adult male rhesus macaques with the 77G allele (Miller et al., 2004). The observation of lower cortisol levels in response to maternal separation in 77G allele carriers is consistent with findings in humans, which show that OPRM1 variation predicts individual differences in HPA axis responses to social/emotional stress. The current finding is noteworthy in light of previous reports from our lab, showing that 77G allele carriers exhibit stronger attachment to their mothers than infants homozygous for the C allele (Barr et al., 2008). This strength of attachment was exemplified by higher attachment scores during the latter stages of the postpartum period (18–24 weeks) as well as elevated levels of distress vocalizations during repeated, protracted maternal separation exposures at 6 months and increased social preference for the mother during reunion periods. The coupling of higher levels of vocalizations during protracted periods of maternal separation with a blunted cortisol response is particularly interesting, as it suggests 77G allele carriers may be more likely to adopt an active coping mechanism to this type of social stressor. Among nonhuman primates, protracted periods of maternal separation are typically accompanied by “despair”, as evidenced by behavioral withdrawal and self-directed activities (Mineka and Suomi, 1978; Barr et al., 2008), responses indicative of a passive coping style. As such, increased vocalizations during this period could be interpreted as an active coping mechanism, which in studies of other mammalian species is usually associated with a dampened neuroendocrine response to stress (Olff, 1999; Veenema et al., 2003; Olff et al., 2005; Ebner et al., 2005). Of interest, both passive and active coping styles, especially when expressed in the extreme (e.g., internalizing vs. externalizing behaviors), have been linked to stress-related pathology including alcohol use disorders (Korte et al., 2005; Dawson et al., 2010), as have blunted cortisol responses to stress (Wand et al., 2002; McNally et al., 2003; Vungkhanching et al., 2004; Schuckit et al., 2004) In this context, our findings of both an active coping style and a blunted cortisol response to separation stress early in life suggests a suite of characteristics influenced by a common underlying genetic mechanism, OPRM1, and which may indicate a vulnerability to addictive behavior later in life.
In the present study, we also demonstrate that 77G allele carriers exhibit a blunted cortisol response to acutely administered alcohol in adolescence and young adulthood. Of note, we see lower levels of cortisol among 77G allele carriers, despite the fact that this allele predicts higher levels of alcohol-induced stimulation as measured by locomotor behavior, which can also activate the HPA axis (Barr et al., 2007). While it has been suggested that modulation of alcohol reinforcement by genetic variation at the OPRM1 locus may relate to μ-opioid receptor control of HPA axis function, due to the fact that suppression of alcohol self-administration and craving by opioid receptor blockade is accompanied by activation of the HPA axis (O'Malley et al., 2002), our findings suggest that acute alcohol reinforcement and HPA axis activity may not be directly related. This is not to say that alterations in HPA axis functioning have no bearing on other alcohol-related phenotypes. In humans, a blunted HPA axis response to stress and alcohol administration is associated with increased alcohol craving, tolerance, and the propensity to relapse (O'Malley et al., 2002; Adinoff et al., 2005; Morrow et al., 2006). Typically, a blunted neuroendocrine state has been viewed as more of a consequence of chronic alcohol consumption leading to dependence, rather than as a precursor (Richardson et al., 2008). However, human subjects with a family history of alcohol dependence exhibit blunted cortisol responses to alcohol, and this blunting is believed to be predictive of future alcohol-related problems (Schuckit, 1984; Schuckit et al., 1987). Our data indicate that a blunted HPA axis may predate chronic alcohol use among individuals carrying functional OPRM1 variants. It may be that OPRM1 variation simultaneously predicts blunted HPA axis activity and increased alcohol preference and that further blunting of the HPA axis would occur more rapidly or to a greater degree as a function of genotype.
The neuroendocrine changes that take place during pregnancy and after birth are fairly consistent across mammalian species and appear to be beneficial for both the mother and the infant (Slattery and Neumann, 2008). Attenuated hormonal stress responses during the postpartum period are the norm as the physiological stress circuits gradually return to their normal state after parturition. However, disruption of these processes can have negative effects on the mother’s health, such as the development of postpartum depression (PPD) as well as various autoimmune disorders (Mastorakos and Ilias, 2000; Mastorakos and Ilias, 2003; Jolley et al., 2007), which in turn can have negative consequences for the infant as well. While dysregulation of the HPA axis in postpartum women has been associated with PPD, it remains unclear whether vulnerability for PPD is linked to hyperactivity or suppression of the HPA axis (Slattery and Neumann, 2008). Major depression (MDD) has been repeatedly associated with hyperactivity of the HPA axis (Pariante and Lightman, 2008); however the subtype of atypical depression is actually associated with down-regulation of the HPA axis (Gold and Chrousos, 2002). Recent evidence suggests that PPD may be more similar to atypical depression in this respect, in that blunted cortisol levels have been observed in women with PDD compared to those without (Jolley et al., 2007; Jolley and Betrus, 2007; Groer and Morgan, 2007). Among rhesus macaque mothers with prior reproductive experience, we observed consistently diminished cortisol levels during the extended postpartum period in females carrying the 77G allele. Although speculative, this suggests that variation at the OPRM1 locus is a potential marker for individual susceptibility to PPD, as reflected in the observed effects on HPA axis function. As of yet, no study has shown a specific association between the OPRM1 gene and major depressive disorders. However, reduced mu-opioid receptor binding potential has been observed in women with major depression compared to controls (Kennedy et al., 2006), and OPRM1 gene variation has been shown to influence the response to citalopram therapy for depression (Garriock et al., 2010). Interestingly, the effects of the 77G allele on cortisol levels in first-time mothers were only apparent during the latter stages of the postpartum period, around the time when infants become more independent and the weaning process is initiated. The difference in findings for primiparous and multiparous mothers is most likely due to differences in opioid sensitivity as a result of reproductive experience. Increased OPRM1 gene expression, as well as increased opioid receptor expression, has been demonstrated in multiparous female rats (Teodorov et al., 2010), suggesting a lesser role of the endogenous opioids in mothers without prior reproductive experience. However, our data suggest that with the potential added stress of the weaning process, effects of OPRM1 variation on HPA axis function may become apparent in first time mothers.
Similar to the previous studies on HPA activity in humans (Wand et al., 2002; Hernandez-Avila et al., 2003; Chong et al., 2006), we did not find significant effects of OPRM1 genotype on ACTH levels. This absence of significant effects of the 118G and 77G allele on ACTH raises the question of what specific mechanism or process is involved in the effect we do see on cortisol levels. If variation at this locus exerts influence at the level of CRH neurons in the hypothalamus, as has been argued, then a similar reduction in ACTH levels might be expected. As suggested by Chong et al. (2006), it is possible that geneotype effects on cortisol release could occur independently of ACTH through opioid receptors located in the adrendal cortex (Lymangrover et al, 1981). Furthermore, the decrease in cortisol levels may have altered the negative feedback loop whereby cortisol inhibits further release of ACTH. We do note that trends for reduced ACTH levels in 77G allele carriers were observed in some cases, and this coupled with a reduced sample size in some cases for analysis of ACTH suggests that we may simply have been underpowered to detect signficant effects on ACTH levels. In any case, the consistent finding of blunted cortisol levels in 77G allele carriers across different contexts in the rhesus macaque, combined with similar findings in humans with the 118G allele, provide a strong argument for variation at the OPRM1 locus playing an significant role in stress physiology.
Individual differences in the functioning of the endogenous opioid system have been proposed to influence susceptibility to addictive disorders and other psychopathology through effects on reward processing and the response to stress (Ribeiro et al., 2005; Gianoulakis, 2009). Genetic variation is undoubtedly one factor underlying these individual differences. We have shown that rhesus macaques carrying the OPRM1 77G allele show a reduced cortisol response to maternal separation in infancy and to acute alcohol exposure later in life. Furthermore, attenuated cortisol levels were observed in 77G allele carriers during the maternal postpartum period. These findings are in general agreement with those for adult male rhesus macaques and for humans exposed to a psychological stressor, lending further support to the theory that the human and rhesus OPRM1 variants are functionally similar. Moreover, the current study suggests roles for OPRM1 in alcohol-induced HPA axis dysregulation and HPA function in the postpartum period.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of General Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2004a;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004b;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc.Natl.Acad.Sci.U.S.A. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta) Behav Neurosci. 1990;104:98–107. [PubMed] [Google Scholar]
- Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. Journal of Biological Chemistry. 2001;276:3130–3137. doi: 10.1074/jbc.M006352200. [DOI] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. Journal of Neurochemistry. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Bond A, O'Neill MJ, Hicks CA, Monn JA, Lodge D. Neuroprotective effects of a systemically active Group II metabotropic glutamate receptor agonist LY354740 in a gerbil model of global ischaemia. Neuroreport. 1998;9:1191–1193. doi: 10.1097/00001756-199804200-00042. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Hammer RP. Parity-associated alterations of medial preoptic opiate receptors in female rats. Brain Research. 1992;578:269–274. doi: 10.1016/0006-8993(92)90257-a. [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The Micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006a;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- Chou WY, Yang LC, Lu HF, Ko JY, Wang CH, Lin SH, Lee TH, Concejero A, Hsu CJ. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006b;50:787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Coe CL, Wiener SG, Rosenberg LT, Levine S. Endocrine and immune responses to separation and maternal loss in nonhuman primates. 1985:165–199. [Google Scholar]
- Dawson DA, Goldstein RB, Moss HB, Li TK, Grant BF. Gender differences in the relationship of internalizing and externalizing psychopathology to alcohol dependence: Likelihood, expression and course. Drug and Alcohol Dependence. 2010 doi: 10.1016/j.drugalcdep.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettling A, Pryce CR, Martin RD, Dobeli M. Physiological Responses to parental separation and a strange situation are related to parental care received in juvenile Goeldi's monkeys (Callimico goeldii) Dev Psychobiol. 1998;33:21–31. doi: 10.1002/(sici)1098-2302(199807)33:1<21::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacolgy, Biochemistry and Behavior. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Hormones and Behavior. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J.Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Frew AK, Drummond PD. Negative affect, pain and sex: the role of endogenous opioids. Pain. 2007;132 Suppl. 1:S77–S85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Garriock HA, Tanowitz M, Kraft JB, Dang VC, Peters EJ, Jenkins GD, Reinalda MS, McGrath PJ, von Zastrow M, Slager SL, Hamilton SP. Association of mu-opioid receptor variants and response to citalopram treatment in major depressive disorder. Am J Psychiatry. 2010;167:565–573. doi: 10.1176/appi.ajp.2009.08081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top.Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Molecular Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32:133–139. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am.J.Med.Genet.B Neuropsychiatr.Genet. 2003;118:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Janicki PK, Schuler G, Francis D, Bohr A, Gordin V, Jarzembowski T, Ruiz-Velasco V, Mets B. A genetic association study of the functional A118G polymorphism of the human mu-opioid receptor gene in patients with acute and chronic pain. Anesthesia and Analgesia. 2006;103:1011–1017. doi: 10.1213/01.ane.0000231634.20341.88. [DOI] [PubMed] [Google Scholar]
- Jolley SN, Betrus P. Comparing postpartum depression and major depressive disorder: issues in assessment. Issues in Mental Health Nursing. 2007;28:765–780. doi: 10.1080/01612840701413590. [DOI] [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol.Res.Nurs. 2007;8:210–222. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Archives of General Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Kim JG, Choi YS, Kim HO, Kim SY, Oslin DW. A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Rakvag TT, Kaasa S, Holthe M, Dale O, Borchgrevink PC, Baar C, Vikan T, Krokan HE, Skorpen F. The 118 A > G polymorphism in the human mu-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiologica Scandinavica. 2004;48:1232–1239. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav.Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Opiates, opioids and addiction. Molecular Psychiatry. 1996;1:232–254. [PubMed] [Google Scholar]
- Kroslak T. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. Journal of Neurochemistry. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur.J Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- Laszlo FA, Varga C, Pavo I, Gardi J, Vecsernyes M, Galfi M, Morschl E, Laszlo F, Makara GB. Vasopressin pressor receptor-mediated activation of HPA axis by acute ethanol stress in rats. Am.J.Physiol Regul.Integr.Comp Physiol. 2001;280:R458–R465. doi: 10.1152/ajpregu.2001.280.2.R458. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, udrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. PharmacogenomicsJournal. 2004;4:184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Geisslinger G. Relevance of frequent mu-opioid receptor polymorphisms for opioid activity in healthy volunteers. Pharmacogenomics Journal. 2006;6:200–210. doi: 10.1038/sj.tpj.6500362. [DOI] [PubMed] [Google Scholar]
- Lymangrover JR, Dokas LA, Kong A, Martin R, Saffran M. Naloxone has a direct effect on the adrenal cortex. Endocrinology. 1981;109:1132–1137. doi: 10.1210/endo-109-4-1132. [DOI] [PubMed] [Google Scholar]
- Mann PE, Kinsley CH, Ronsheim PM, Bridges RS. Long-term effects of parity on opioid and nonopioid behavioral and endocrine responses. Pharmacology, Biochemistry and Behavior. 1989;34:83–88. doi: 10.1016/0091-3057(89)90357-2. [DOI] [PubMed] [Google Scholar]
- Martin P, Kraemer HC. Individual differences in behavior and their statistical consequences. Animal Behaviour. 1987;35:1366–1375. [Google Scholar]
- Mastorakos G, Ilias I. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period. Postpartum-related disorders. Annals of the New York Academy of Sciences. 2000;900:95–106. doi: 10.1111/j.1749-6632.2000.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Annals of the New York Academy of Sciences. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- McNally AM, Palfai TP, Levine RV, Moore BM. Attachment dimensions and drinking-related problems among young adults - The mediational role of coping motives. Addictive Behaviors. 2003;28:1115–1127. doi: 10.1016/s0306-4603(02)00224-1. [DOI] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Molecular Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psychological Bulletin. 1978;85:1376–1400. [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues.Clin.Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl.) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Schmidt R, Schneider A, Geisslinger G, Lotsch J. The mu-opioid receptor gene polymorphism 118A>G depletes alfentanil-induced analgesia and protects against respiratory depression in homozygous carriers. Pharmacogenet.Genomics. 2006;16:625–636. doi: 10.1097/01.fpc.0000220566.90466.a2. [DOI] [PubMed] [Google Scholar]
- Olff M. Stress, depression and immunity: the role of defense and coping styles. Psychiatry Research. 1999;85:7–15. doi: 10.1016/s0165-1781(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BP. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neuroscience and Biobehavioral Reviews. 2005;29:457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S. Brain opioids and mother-infant social motivation. Acta Paediatr.Suppl. 1994;397:40–46. doi: 10.1111/j.1651-2227.1994.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le MM. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Research - Brain Research Reviews. 1997;25(3):359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol and Alcoholism. 2009;44:358–365. doi: 10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin.Exp.Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser A, Rukstalis M, Perkins K, Lynch KG, O'Malley S, Berrettini WH, Lerman C. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology (Berl) 2006;188:355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg RR, Olofsen E, Bijl H, Taschner PE, Teppema LJ, Sarton EY, van Kleef JW, Dahan A. Polymorphism of mu-opioid receptor gene (OPRM1:c.118A>G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102:522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Differences in plasma cortisol after ingestion of ethanol in relatives of alcoholics and controls: preliminary results. Journal of Clinical Psychiatry. 1984;45:374–376. [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Archives of General Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcoholism: Clinical & Experimental Research. 2004;28(10):1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Sher L, Stanley BH. The role of endogenous opioids in the pathophysiology of self-injurious and suicidal behavior. Arch.Suicide Res. 2008;12:299–308. doi: 10.1080/13811110802324748. [DOI] [PubMed] [Google Scholar]
- Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, Teo YY, Tan EC. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J.Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Kream R. Endogenous opiates, opioids, and immune function: evolutionary brokerage of defensive behaviors. Seminars in Cancer Biology. 2008;18:190–198. doi: 10.1016/j.semcancer.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Teodorov E, Bernardi MM, Ferrari MF, Fior-Chadi DR, Felicio LF. Plasticity of Opioid Receptors in the Female Periaqueductal Gray: Multiparity-Induced Increase in the Activity of Genes Encoding for Mu and Kappa Receptors and a Post-Translational Decrease in Delta Receptor Expression. Journal of Molecular Neuroscience. 2010 doi: 10.1007/s12031-010-9407-0. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Niesink RJ, Van WL, Ramsey NF, Kornet MM, Van Furth WR, Vanderschuren LJ, Gerrits MA, Van den Berg CL. Endogenous opioids and reward. European Journal of Pharmacology. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Meijer OC, De Kloet ER, Koolhaas JM, Bohus BG. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Horm Behav. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Volavka J, Cho D, Mallya A, Bauman J. Naloxone increases ACTH and cortisol levels in man. New England Journal of Medicine. 1979;300:1056–1057. doi: 10.1056/nejm197905033001817. [DOI] [PubMed] [Google Scholar]
- Vungkhanching M, Sher KJ, Jackson KA, Parra GR. Relation of attachment style to family history of alcoholism and alcohol use disorders in early adulthood. Drug and Alcohol Dependence. 2004;75:47–53. doi: 10.1016/j.drugalcdep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Archives of General Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wiener SG, Bayart F, Faull KF, Levine S. Behavioral and physiological responses to maternal separation in squirrel monkeys (Saimiri sciureus) Behavioral Neuroscience. 1990;104:108–115. doi: 10.1037//0735-7044.104.1.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.