Abstract
FoxO3 is a member of the forkhead box O (FoxO) transcription factor subfamily, which regulates the expression of target genes not only through DNA-binding as a transcription factor but also through protein-protein interaction. Although FoxO3 is a well-known transcription factor involved in diverse biological processes, the role of FoxO3 in cigarette smoke (CS)-induced lung inflammation and injury has not been studied. It is, therefore, hypothesized that deficiency of FoxO3 leads to increased susceptibility to CS-induced lung inflammatory responses and airspace enlargement. Here, we showed that the levels of FoxO3 are significantly decreased in lungs of smokers and patients with chronic obstructive pulmonary disease (COPD), as well as in lungs of mice exposed to CS. Genetic ablation of FoxO3 led to pulmonary emphysema and exaggerated inflammatory cell influx and response in lungs of mice exposed to CS. We further showed that CS induced the translocation of FoxO3 into the nucleus where FoxO3 interacted with NF-κB and disrupted NF-κB DNA-binding ability leading to inhibition of its activity. Targeted disruption of FoxO3 also resulted in down-regulation of antioxidant genes in mouse lung in response to CS. These results suggest that FoxO3 plays a pivotal role in regulation of lung inflammatory response and antioxidant genes, and deficiency of FoxO3 results in development of COPD/emphysema.
Keywords: FoxO3, Oxidant, Cigarette smoke, NF-κB, Inflammation, Emphysema
INTRODUCTION
Cigarette smoke (CS) is the major risk factor for the development of chronic obstructive pulmonary disease (COPD)/emphysema via inducing an abnormal and persistent inflammatory response with an increase of inflammatory cells, such as neutrophils, macrophages, dendritic cells and T cells in the lung (1-3). COPD is a highly prevalent and debilitating lung disease, but the molecular and cellular mechanisms responsible for its pathogenesis are not clear. This is due to lack of understanding of the specific cellular and molecular pathways triggered in the lung by CS/oxidants. It is currently thought that increased lung oxidative stress, abnormal immune-inflammatory response, and cellular senescence play an important role in development of COPD/emphysema (1, 4-6). It has also been proposed that abnormal inflammatory responses are associated with increased cell senescence incited by CS in the development of COPD/emphysema (6-7).
FoxO3 is a member of the forkhead box class O (FoxO) subfamily, identified as transcription factors with forkhead DNA-binding domains, which regulate the expression of several genes involved in diverse biological processes, such as apoptosis, cell cycle progression, vascular remodeling, development, senescence, oxidative stress resistance, innate immune homeostasis and inflammation (8-17). FoxO3 controls T cell response and regulates neutrophil function during inflammation (18-19). Although CS induces pulmonary inflammation and modulates innate immune response (20), the role of FoxO3 in CS-mediated inflammation is not known. FoxO3 functions as a transcription factor through its direct DNA-binding, and through interaction with other proteins (21-24). In addition, the function of FoxO3 is modulated by post-translational modifications, such as phosphorylation and acetylation. Translocation into nucleus and degradation of FoxO3 in the proteasome are regulated by its phosphorylation via divergent cell signaling (11, 25-26). Furthermore, acetylation of FoxO3 by cAMP-response-element-binding protein-binding protein (CBP) can alter its DNA binding and transcription activity, which can be restored by Sirtuin 1 (SIRT1) (27-31). We and others have shown that SIRT1 levels/activity is decreased in response to CS exposure in vitro in macrophages and epithelial cells as well as in vivo in lungs of patients with COPD (32-35). Hence, CS-mediated SIRT1 reduction will result in acetylation of FoxO3 and alter its transactivation ability, thereby regulating antioxidant gene transcription. Furthermore, since FoxO3 regulates RelA/p65 (25, 36), it is possible that FoxO3 deficiency in lungs can lead to exaggerated lung inflammatory response. We hypothesized that FoxO3 plays an important role in regulating CS-induced lung inflammation and airspace enlargement (emphysema) through the modification of NF-κB activity and antioxidant genes in mouse lung as well as in patients with COPD. We, therefore, determined the abundance and localization of FoxO3 in lungs of smokers and patients with COPD, and investigated whether deficiency of FoxO3 would lead to increased susceptibility to CS-induced lung inflammation and emphysema in mouse lung. We further studied the potential mechanisms of FoxO3-mediated protection from CS-induced lung inflammatory response by determining the ability of FoxO3 to interact with RelA/p65 and dampen NF-κB activation.
MATERIALS AND METHODS
Human lung tissues and sputum cells
Lung tissue specimens from 37 subjects/patients including 10 life-long nonsmokers, 10 current smokers with normal lung function, and 17 patients with COPD (11 former and 6 current smokers; 10 patients had been prescribed inhaled and/or low-dosage oral corticosteroids) undergoing resection for suspected lung tumor (either malignant or nonmalignant-local carcinoma or hamartoma), or lung transplantation from the Department of Medicine and Pathology, Helsinki University Hospital as described in our previous study (33). None of the patients had suffered from acute exacerbation for 2 months. Tumor-free peripheral lung tissues were immediately stored at -80°C for immunoblot analysis and/or embedded in paraffin for immunohistochemistry. Sputum samples were obtained from all subjects/patients as previously described (37). The use of the tissues and sputum was approved by the ethics committee of the Helsinki University Hospital, Helsinki, Finland. All subjects/patients provided informed consent. The clinical characteristics of the subjects/patients used are described in detail previously (33, 37).
Animals
Heterozygous FoxO3 (FoxO3+/-) mice (FVB;129S6-Foxo3atm1.1Rdp) were obtained from the Mutant Mouse Regional Resource Centers, the University of California at Davis (Davis, CA; stock number 016132-UCD). The generation and genotyping of mice was performed as previously described (38). Wild-type (FoxO3+/+; on FVB;129S6 mixed background) and knockout (FoxO3-/-) littermates were housed in the Inhalation Core Facility at the University of Rochester before being exposed to air or CS. All experimental protocols were approved by the University Committee on Animal Research at the University of Rochester.
Generation of chimeras by bone marrow transplantation
Bone marrow transplantation (BMT) chimeras were generated by radioablation of recipient bone marrow followed by reconstitution with donor bone marrow cells as previously described (39). Briefly, the bone marrow was extracted from a minimum of three donors of the appropriate mouse strain by flushing femurs and tibias into HBSS with 1% FCS, dispersing through a 21-gauge needle, and pooling. Erythrocytes were removed by hypotonic lysis. The cells were counted, resuspended in media at 5 × 107 cells/ml, and delivered to the recipient mice by tail vein injection (1 × 107 cells per mouse). Following BMT, animals were allowed to reconstitute for 8 weeks under the microisolator conditions. The mortality rate by a failure of the engraftment after post-transplantation was only 31.4% (16 dead mice/ 51 BMT trial mice), suggesting that the survived mice (68.6%) after post-transplantation were transplanted by donor bone marrow cells. The reconstituted mice (donor to recipient; WT to WT, WT to KO, KO to WT, and KO to KO) were exposed to air or CS for 3 days. Chimerism was confirmed by FoxO3 mRNA expression in peripheral blood cells and lung tissue using quantitative real-time PCR (Table S1).
Quantification of FoxO3 expression in peripheral blood and lung tissues
Blood was collected by cardiac puncture from the air-exposed BMT chimeric mice. To perform cardiac puncture, mice were anesthetized by sodium pentobarbital (50 mg/kg, intraperitoneally), then the right ventricle was accessed with a 23 gauge needle and 400–500 μl blood was aspirated into a 3 ml syringe. Blood was immediately discharged into a 2 ml microfuge tube preloaded with 1.3 ml RNAlater® Tissue Collection:RNA Stabilization Solution (Ambion, Austin, TX), mixed by inversion, and stored at -20°C. Mouse RiboPure™-Blood RNA Isolation kit (Ambion, Austin, TX) was used for extraction of RNA. Briefly, the samples were centrifuged and the RNAlater Solution removed prior to disruption of the blood pellet in a guanidinium-based lysis solution, followed by organic extraction and purification of total RNA fraction. RNA yields were determined by UV absorbance using a Nanodrop instrument (ND-1000 Spectrophotometer, NanoDrop Technologies). To validate the expression of FoxO3 in blood cells and lung tissues, a quantitative real-time PCR was performed by Bio-Rad iCycler real-time system using the SYBR Green qPCR Master mix from SABioscience (Fredrick, MD). Specific primers against FoxO3 (product no. PPM03393E) and 18S rRNA (PPM57735E) were purchased from SABioscience. Expression of FoxO3 was normalized to 18s rRNA levels. RNA relative abundance was quantified by the comparative 2-ΔΔCt methods.
Cigarette smoke exposure
Mice were exposed to CS for 3 days and 8 weeks using Baumgartner-Jaeger CSM2082i cigarette smoking machine (CH Technologies, Westwood, NJ), and for 4 months using Teague TE-10 smoking machine (Teague Enterprises, Davis, CA) in the Inhalation Core Facility at the University of Rochester. For 3 days and 8 weeks of CS exposure, mice were placed in individual compartments of a wire cage, which was placed inside a closed plastic box connected to the smoke source. The smoke was generated from 2R4F research cigarettes containing 11.7 mg of total particulate matter (TPM), 9.7 mg of tar and 0.76 mg of nicotine per cigarette (University of Kentucky, Lexington, KY). Mice received two 1-h exposures per day, 1 h apart, according to the Federal Trade Commission protocol (1 puff/min of 2 second duration and 35 ml volume) for 3 days to 8 weeks (40-42). Mainstream CS was diluted with filtered air and directed into the exposure chamber. The smoke exposure (TPM per cubic meter of air) was monitored in real-time with a MicroDust Pro-aerosol monitor (Casella CEL, Bedford, UK) and verified daily by gravimetric sampling. The smoke concentration was set at a nominal value of ~300 mg/m3 TPM by adjusting the number of cigarettes used to produce smoke and the flow rate of the dilution air. Control groups were exposed to filtered air in an identical manner for the same duration of time. Carbon monoxide concentration in the chamber was 290 ~ 300 ppm. For 4 months of CS exposure, mice received a total of 5 h exposures per day, 5 days a week for 4 months. Each lighted cigarette was puffed for 2 seconds and once every minute for a total of 8 puffs with the flow rate of 1.05 l/min, a standard puff of 35 cm3. The smoke machine was adjusted to produce a mixture of side stream smoke (89%) and mainstream smoke (11%) by smoldering five cigarettes at one time and the smoke chamber atmosphere was monitored for total suspended particulates (90 mg/m3) and carbon monoxide (350 ppm) (40, 43).
Mean linear intercept analyses
Mouse lungs (which had not been lavaged) were inflated by 1% low-melting agarose at a pressure of 25 cm H2O, and then fixed with 4% neutral buffered formalin. Tissues were embedded in paraffin and sectioned (4 μm). Lung sections were deparaffinized and rehydrated by passing through a series of xylene and graded alcohol, were stained with hematoxylin and eosin (H&E). Alveolar size was estimated from the mean linear intercept (Lm) of the airspace which is a measure of airspace enlargement/emphysema (40, 42). Lm was calculated for each sample based on 10 random fields observed at a magnification of × 200 using a cross-line.
Measurements of lung mechanical properties
Lung mechanical properties of mice were determined using Scireq Flexivent apparatus (Montreal, Canada). Quasi-static compliance (QsC), lung resistance (R), and tissue elastance (E) were measured in mice, anesthetized by sodium pentobarbital (50 mg/kg, intraperitoneally) and paralyzed by pancuronium (0.5 mg/kg, intraperitoneally). A tracheotomy was performed, and an 18-gauge cannula was inserted 3 mm into an anterior nick in the exposed trachea and connected to a computer controlled rodent ventilator. Initially, the mice were ventilated with room air (150 breaths/min) at a volume of 10 ml/kg body mass. After 3 min of ventilation, measurement of lung mechanical properties was initiated by a computer-generated program to measure quasi-static compliance, lung resistance, and tissue elastance (40, 44-46). These measurements were repeated three times for each animal.
Exercise performance tests
Exercise performance of air- and CS-exposed mice at 4 months was assessed using a motorized rodent treadmill (Columbus Instruments, Columbus, OH). Briefly, mice were placed on a motorized rodent treadmill with an electric grid at the rear to adapt and run on a rodent treadmill 2 days before conducting an exercise performance test. Familiarization runs were 10 min in duration with a treadmill incline of 10°. Treadmill speed on the first day was 10 m/min and 12 m/min on the second day. For the performance test, mice were placed on the treadmill and allowed to adapt to the surroundings for 3–5 min before starting. The treadmill was started at a speed of 8.5 m/min with a 0° incline. After 9 min, the speed and incline were raised to 10 m/min and 5°, respectively, for the second stage of the test. Speed was then increased by 2.5 m/min every 3 min to a maximum of 40 m/min, and the incline progressively increased 5° every 9 min to a maximum of 15°. Exercise was continued until exhaustion, defined as an inability to maintain running speed despite repeated contact with the electric grid. Each mouse was immediately removed from the treadmill when exhaustion has been determined, and returned to its home cage. Running time and running distance were measured for each mouse at the end of exercise performance test (47).
Immunohistochemistry
Lung sections were deparaffinizied in xylene, rehydrated through graded alcohols to PBS, and then treated with 3% H2O2 for 10 min to quench endogenous peroxidase activity. To identify the localization of FoxO3 in human lung sections, PictureTM-Double Staining Kit (Invitrogen, Camarillo, CA) was used according to the manufacturer's instructions. Immunohistochemistry for FoxO3 was performed using a rabbit polyclonal anti-FoxO3 antibody (Abnova, Taipei, Taiwan). Omitting the primary antibody served as negative control and resulted without any tissue staining. The assessment of immunostaining intensity was performed semiquantitatively (10 random microscopic fields per lung section in 3 different sections) and in a blinded fashion as described previously (33, 48). Macrophages in mouse lung sections were indentified using a rat anti-mouse Mac-3 antibody (BD Pharmingen, Franklin Lakes, NJ), Vectastain ABC kit (Rat IgG) and DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA) following the manufacturer's instructions. The number of Mac-3-positive cells in lung sections (6 random microscopic fields per lung section in 3 different sections) was counted manually at × 200 magnification and averaged (42).
Bronchoalveolar lavage (BAL)
Mice were anesthetized at 24 h after the last exposure by an intraperitoneal injection of pentobarbital sodium (100 mg/kg; Abbott Laboratories, Abbott Park, IL) followed by exsanguination. The lungs were lavaged three times with 0.7 ml of saline via a cannula inserted into the trachea. The aliquots were combined and centrifuged, and the BAL inflammatory cell pellet was resuspended in saline. The cells were stained with trypan blue (Invitrogen, Carlsbad, CA) and the total cell number was counted using a hemocytometer. Cytospin slides (Thermo Shandon, Pittsburgh, PA) were prepared using 50,000 cells per slide, and differential cell counts (~500 cells/slide) were performed on cytospin-prepared slides stained with Diff-Quik (Dade Behring, Newark, DE).
Proinflammatory mediators analysis
The levels of proinflammatory mediators, such as monocyte chemotatic protein 1 (MCP-1), keratinocyte derived chemokine (KC) and interferon γ inducible protein 10 (IP-10) in lung homogenates were measured by enzyme-linked immunosorbent assay (ELISA) using respective duo-antibody kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The results were expressed in the samples as pg/mg protein.
Assay of NF-κB DNA-binding activity
NF-κB DNA-binding activity in nuclear extracts was measured using the Trans-AM RelA/p65 transcription factor assay kit (Active Motif, Carlsbad, CA) following the manufacturer's instructions. In brief, 2.5 μg of nuclear extracts was incubated with already plate-coated NF-κB consensus oligonucleotide. Plates were then washed before addition of anti-RelA/p65 antibody. A horseradish peroxidase antibody was used for signal detection and quantification. The absorbance was determined on a spectrophotometer (Microplate reader model 680, Bio-Rad) at 450 nm.
IKK2 inhibitor administration
IKK2 inhibitor IMD-0354 was purchased from Tocris (Ellisville, MO). Vehicle (0.5% carboxymethylcellulose) or IMD-0354 was administered to mice (30 mg/kg) by intraperitoneal injection for 3 days daily at 2 h before CS exposure.
Immunoblotting
Cytoplasmic and nuclear proteins (20 μg) from mouse lung were separated on a 6.5% to 12% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis (SDS-PAGE). Separated proteins were transferred onto nitrocellulose membranes (Amersham, Arlington Heights, IL), and blocked for 1 h at room temperature with 5% bovine serum albumin (BSA) (Sigma-Aldrich). The membranes were then probed with a specific primary antibodies (1:1000 dilution in 5% BSA in PBS containing 0.1% Tween 20) of acetylated lysine, GAPDH (Cell Signaling Technology, Beverly, MA), FoxO3, MnSOD (Upstate, Temecula, CA), catalase, β-actin (Sigma), and CuZnSOD (Assay Designs, Ann Arbor, MI) at 4 °C for overnight. After three washing steps (10 min each), the levels of protein were detected by probing with secondary anti-rabbit or anti-mouse antibody (1:10,000 dilution in 5% BSA) linked to horseradish peroxidase for 1 h, and bound complexes were detected using the enhanced chemiluminescence method (Perkin Elmer, Waltham, MA). Equivalent loading of the gel was determined by quantitation of protein as well as by reprobing the membranes for β-actin or GAPDH. The ImageJ densitometry software (Version 1.41, National Institutes of Health, Bethesda, MD) was used for gel band quantitative densitometric analysis.
Cell culture
The human bronchial epithelial cell line H292 was purchased from American Type Tissue Culture Collection (Manassas, VA). H292 cells were grown in 100 mm dishes (4 × 106 cells), containing RPMI-1640 supplemented with 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT), 2 mM L-glutamine, 100 μg/ml penicillin and 100 U/ml streptomycin in humidified atmosphere under 7.5% CO2 at 37 °C.
Preparation of CS extract
We used the same cigarettes which were used in exposure of mice to CS. Cigarette smoke extract (CSE) was prepared by bubbling smoke from one cigarette into 10 ml serum-free RPMI 1640 media at a rate of 1 cigarette/minute as described previously (49-51). The pH of the CSE was adjusted to 7.4, and it was sterile-filtered through a 0.45 μm filter (25 mm Acrodisc; Pall Corporation, Ann Arbor, MI). CSE preparation was standardized by measuring the absorbance (OD: 1.00 ± 0.05) at a wavelength of 320 nm. The pattern of absorbance (spectrogram) observed at 320 nm showed very little variation between different preparations of CSE. CSE was freshly prepared for each experiment and diluted with culture media supplemented with 10% FBS immediately before use. Control medium was prepared by bubbling air through 10 ml serum-free RPMI 1640 media, adjusting pH to 7.4, and sterile filtering as described above.
Immunocytochemistry
The cells treated with or without indicated concentration of CSE for 24 h or H2O2 for 1 h were fixed in acetone/methanol (1:1, v/v) at -20 °C for 20 min. The cells were incubated with 10% normal goat serum and then stained with FoxO3 antibody (1:500; Upstate, Temecula, CA) followed by 1 h incubation with Alexa 594-labelled secondary antibody (1:1000; Molecular Probes, Eugene, OR). Anti-fade DAPI Fluoromount-G (Southern Biotech, Birmingham, AL) was used for nuclei staining and mounting. Images were captured using a fluorescent microscope (BX51, Olympus Optical, Tokyo, Japan).
Immunoprecipitation (IP)
IP was performed with 2 μg of antibodies in 300 μg nuclear extracts or 1 mg whole tissue lysates. Antibodies were incubated with protein overnight at 4 °C on a rocker. Protein-A/G agarose beads (20 μl) (Santa Cruz Biotechnology, Santa Cruz, CA) were added to each sample at 4 °C on a rocker for 1 h. The samples were then centrifuged at 13,000 g at 4 °C for 5 min. The supernatant was discarded, and the beads were washed five times and then subjected to immunoblot as described above.
Reverse transcriptase polymerase chain reaction
Total RNA was isolated from non-lavaged lung tissue specimens (stored in RNAlater; Ambion, Austin, TX) using RNeasy kit (Qiagen, Valencia, CA). Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using oligo(dT) primers and superscript reverse transcriptase (Invitrogen Life Sciences) following the manufacturer's instructions. The PCR conditions and the primer pairs were as described. MnSOD and CuZnSOD, 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds during 30 cycles; catalase, 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds during 28 cycles; GAPDH, 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds during 32 cycles with a final extension for 10 minutes at 72°C in a PTC-200 DNA thermal cycler (MJ Research, Waltham, MA). The primer pairs were as follows (forward and reverse, respectively): MnSOD, 5'-AGCGGTCGTGTAAACCTCA-3' and 5'-AGACATGGCTGTCAGCTTC-3'; CuZnSOD, 5'-ATCCACTTCGAGCAGAAG-3' and 5'-TTCCACCTTTGCCCAAGT-3'; catalase, 5'-AATCCTACACCATGTCGGACA-3' and 5'-CGGTCTTGTAATGGAACTTGC-3'; GAPDH, 5'-ACGACCCCTTCATTGAC-3' and 5'-CCACGACATACTCAGCAC-3' (52). The amplified products were resolved by 1.5% agarose gel electrophoresis, stained with SYBR safe dye (Invitrogen), visualized and scanned by a white/UV transilluminator and quantified by densitometry.
Statistical analysis
Data were presented as mean ± SEM. Statistical analysis of significance was calculated using one-way Analysis of Variance (ANOVA) followed by Tukey's post-hoc test for multigroup comparisons using StatView software. P < 0.05 was considered as significant.
RESULTS
FoxO3 is down-regulated in lungs of patients with COPD and in lungs of mouse exposed to CS
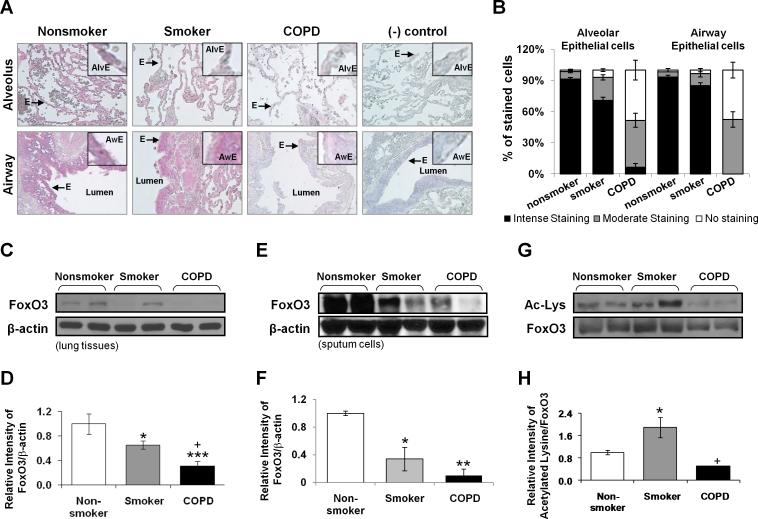
To determine the possible involvement of FoxO3 in pathogenesis of COPD, we determined the abundance of FoxO3 in peripheral lung samples from nonsmokers, smokers, and patients with COPD by immunohistochemistry and immunoblot analysis. Immunohistochemical staining of fixed peripheral lung tissues showed that FoxO3 was predominantly localized in airways/alveolar epithelium in nonsmokers, which was decreased both in lungs of smokers and patients with COPD (Fig. 1, A and B). The levels of FoxO3 were significantly decreased in peripheral lung tissues of smokers, and were further reduced in patients with COPD as compared to nonsmokers as shown by immnoblotting (Fig. 1, C and D). In sputum cells, which mainly consist of macrophages, the levels of FoxO3 were also significantly reduced in smokers and patients with COPD (Fig. 1, E and F). Similar result for FoxO3 staining was also confirmed in tissue macrophages by immunohistochemistry staining in serial lung sections of patients with COPD (93%) and smokers (84%) versus nonsmokers (11%) (data not shown). By immunoprecipitation assay, we found that acetylation of FoxO3 was increased in lungs of smokers (Fig. 1, G and H).
FIGURE 1. FoxO3 is down-regulated in lungs of smokers and patients with COPD.
A, Abundance and localization of FoxO3 in lung alveolar/airway epithelial cells of nonsmokers, smokers and patients with COPD. Red color represents the presence of FoxO3 (indicated with arrow), which was decreased in lungs of smokers and patients with COPD. (-) control = negative control which was stained without primary antibody Alv = alveoli; Aw = airway; E = epithelial cells. Original magnification, × 200. B, Immunostaining scores for FoxO3 per cell-type in alveolar and airway regions of the lung. The assessment of immunostaining intensity was performed semiquantitatively and in a blinded fashion. Immunoblot analysis of FoxO3 in whole tissue lysates extracted from the lung tissue (C) and sputum cells (E) of nonsmokers, smokers, and patients with COPD. After densitometric analysis, the values of FoxO3 in lung tissue (D) and sputum cells (F) were normalized against β-actin (loading control). The relative levels of FoxO3 were significantly decreased in lung tissues of smokers and patients with COPD compared to nonsmokers. G, Whole tissue lysates extracted from the lung tissues of nonsmokers, smokers, and patients with COPD were immunoprecipitated with anti-FoxO3 antibody, and immunoprecipitates were subjected to immunoblot and probed with anti-acetylated lysine antibody. H, Relative intensity of acetylated lysine/FoxO3 represents the increased acetylation of FoxO3 in lungs of smokers. Data are shown as mean ± SEM (n= 3 to 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared to nonsmokers. +P < 0.05, significant compared with smokers.
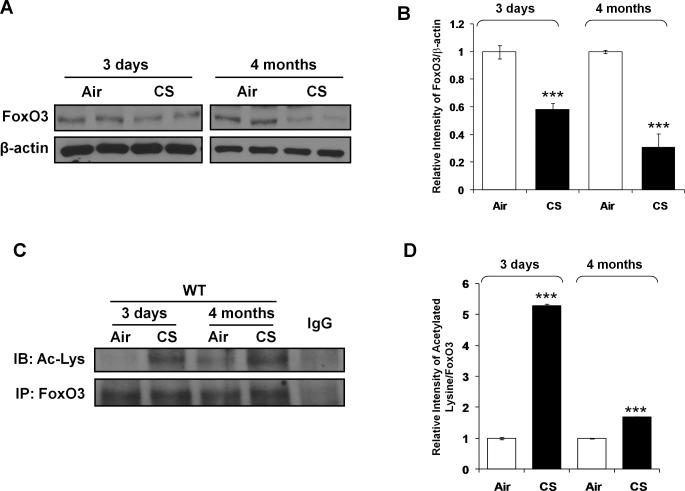
The levels of FoxO3 were significantly lower in lungs of CS-exposed WT mice than that of air-exposed WT mice (Fig. 2, A and B). The reduction in levels of FoxO3 was more pronounced in CS-exposed WT mice after 4 months of CS exposure compared to those of at 3 days of CS exposure. Furthermore, CS caused acetylation of FoxO3 both at 3 days and 4 months of CS exposures in mouse lungs (Fig. 2, C and D). These results suggest that FoxO3 is down-regulated and acetylated in response to CS.
FIGURE 2. FoxO3 is down-regulated in lungs of mouse in response to CS.
A, Immunoblot analysis of FoxO3 in whole tissue lysates extracted from lungs of mouse exposed to CS for 3 days and 4 months. B, After densitometric analysis, the values of FoxO3 were normalized against β-actin (a loading control), respectively. C, Nuclear fraction from lungs of mouse exposed to CS for 3 days and 4 months were immunoprecipitated with anti-FoxO3 antibody, and immunoprecipitates were subjected to immunoblot analysis and probed with anti-acetyl-lysine antibody. IgG was used as an isotype control. D, The relative density of acetylated FoxO3 showed increased acetylation of FoxO3 in CS exposed mice lung at 3 days and 4 months. A representative blot is shown. Data are shown as mean ± SEM (n= 3 to 4 per group). ***P < 0.001, significant compared to air-exposed WT mice.
FoxO3 deficiency results in airspace enlargement/emphysema and alters lung mechanical properties in mice in response to 4 months of CS exposure
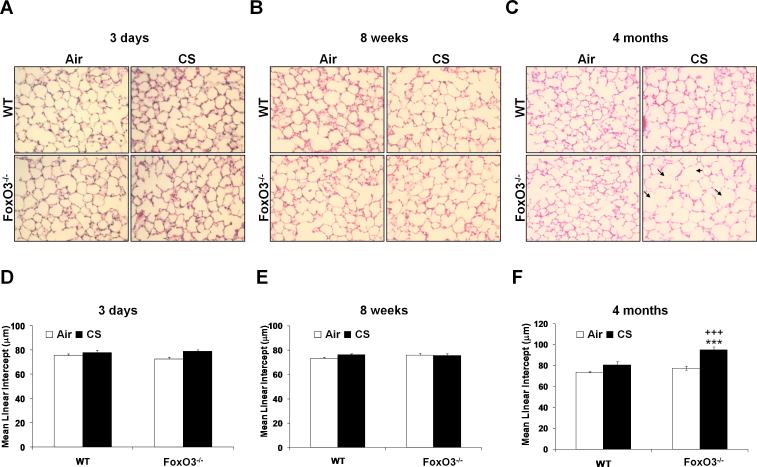
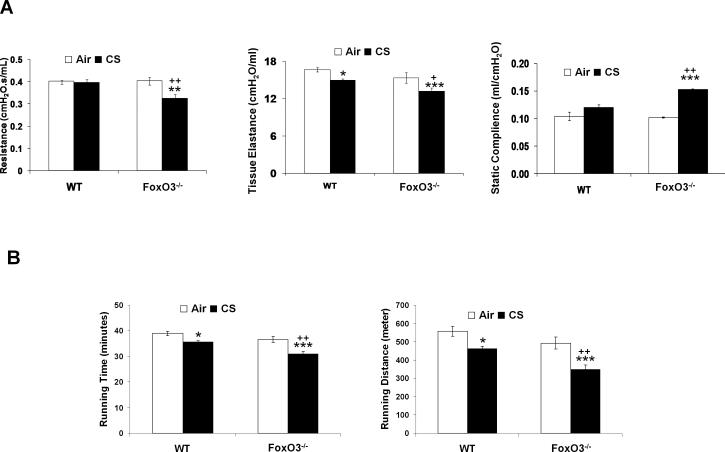
To investigate the potential effect of FoxO3 on CS-induced airspace enlargement/emphysema, FoxO3-/- and WT littermates were subjected to CS exposures for 3 days, 8 weeks and 4 months. We then determined the susceptibility of FoxO3-/- mice for CS-induced airspace enlargement/emphysema by lung histopathological and functional measurements, and exercise performance test. There was no significant difference in lung histopathology by H&E staining between air- or CS-exposed WT and FoxO3-/- mice lungs at 3 days (Fig. 3A) and 8 weeks (Fig. 3B) of CS exposures, which was assessed by determining the mean linear intercept (Lm) (Fig. 3, D and E). However, 4 months of CS exposure led to significantly increased airspace enlargement as measured by Lm in the lung of CS-exposed FoxO3-/- mice as compared to CS-exposed WT mice (Fig. 3, C and F). CS exposure at 4 months did not cause airspace enlargement in WT mice. Similarly, the lung resistance and elastance were significantly decreased in FoxO3-/- mice exposed to CS for 4 months, whereas the static lung compliance was significantly increased in these mice as compared to 4 months of CS-exposed WT mice (Fig. 4A). Similar trend in lung mechanical properties was observed in 6 months of CS-exposed FoxO3-/- mice (data not shown). In addition, FoxO3-/- mice exposed to CS for 4 months showed a significant reduction in exercise performance (i.e. running time and distance) compared to CS-exposed WT mice (Fig. 4B). WT mice exposed to CS for 4 months also showed a slight reduction in exercise performance. The exercise performance in the CS-exposed FoxO3-/- mice was persistently decreased at 6 months of CS exposure (data not shown). There was no statistical significant difference in body weight or mortality between air-exposed WT and FoxO3-/- mice up to 6 months of CS exposure (data not shown). These data are consistent with previous report which showed no significant differences in body weight and mortality up to 48 weeks of age in FoxO3-/- mice (38). Taken together, these data suggest that FoxO3 deficiency leads to an increased susceptibility to chronic CS-induced airspace enlargement/emphysema.
FIGURE 3. Increased airspace enlargement in lungs of FoxO3-/- mice exposed to CS for 4 months.
The pictures shown are H&E stained lung sections from air- or CS-exposed WT and FoxO3-/- mice for 3 days (A), 8 weeks (B) and 4 months (C). Arrows indicate airspace enlargement. Original magnification is × 200. Mean Linear Intercept (Lm) was analyzed in H&E stained slides (D, 3 days; E, 8 weeks; F, 4 months). Lung sections from CS-exposed FoxO3-/- mice for 4 months show an increased airspace enlargement when compared with CS-exposed WT mice. Data are shown as mean ± SEM (n=3 per group). ***P < 0.001, significant compared with corresponding air-exposed mice; +++P < 0.001, significant compared with CS-exposed WT mice.
FIGURE 4. Alterations in lung mechanical properties and reduction in exercise performance indicate emphysema in FoxO3-/- mice exposed to CS.
A, Tissue resistance, static lung compliance and tissue elastance were measured by FlexiVent after 4 months of CS exposure. B, Mice exposed to air or CS for 4 months were subjected to running on the treadmill at 24 h after the last exposure. Running time and running distance were measured. Data are shown as mean ± SEM (n=4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with corresponding air-exposed mice; +P < 0.05, ++P < 0.01, significant compared with CS-exposed WT mice.
FoxO3-deficient mice show an increased susceptibility to lung inflammation in response to CS exposure
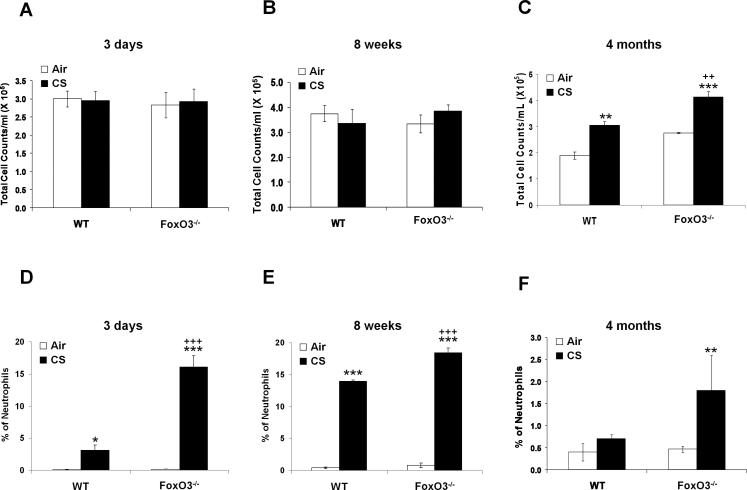
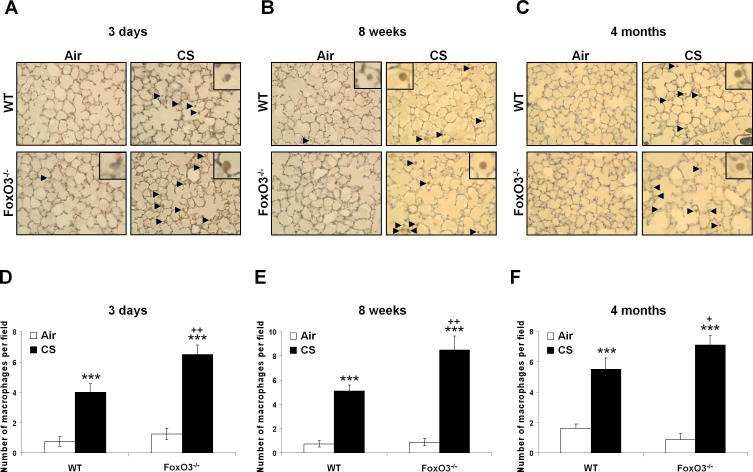
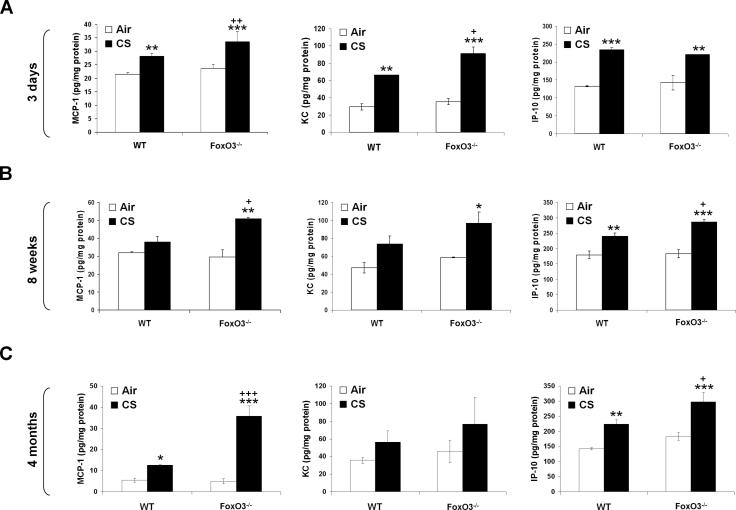
CS induces the lung inflammatory response, which is associated with development of emphysema in mouse (1, 49, 53). To determine the susceptibility of FoxO3-/- mice to CS-induced lung inflammation, we assessed the inflammatory cell influx into the bronchoalveolar lavage (BAL) fluid and lung tissue using the Diff-Quik and immunohistochemical stainings, respectively. There was no significant change in total cell numbers in BAL fluids after 3 days and 8 weeks of CS exposures in any group (Fig. 5, A and B), but CS exposure for 4 months resulted in a significant increase in total cell numbers in BAL fluid of WT mice, which was further augmented in FoxO3-/- mice (Fig. 5C). In both 3 days and 8 weeks of CS exposures, increased neutrophil influx in BAL fluid was observed (Fig. 5, D and E), whereas no change in neutrophil influx in BAL fluid was seen in WT mice exposed to CS for 4 months (Fig. 5F). Nevertheless, CS-exposed FoxO3-/- mice showed a significant increase in neutrophil influx in BAL fluid as compared to WT mice exposed to CS for 3 days, 8 weeks and 4 months (Fig. 5, DF). Similarly, CS exposures for 3 days (Fig. 6, A and D), 8 weeks (Fig. 6, B and E), and 4 months (Fig. 6, C and F) increased the macrophage infiltration in lungs of WT mice, which was further augmented in corresponding CS-exposed FoxO3-/- mice. The increased inflammatory cell recruitment in lungs of CS-exposed FoxO3-/- mice was persistently increased at 6 months of CS exposure (data not shown). In addition, the deficiency in FoxO3 intensified the release of proinflammatory mediators, such as MCP-1, KC, and IP-10, triggered by CS exposure for 3 days (Fig. 7A), and 8 weeks (Fig. 7B), and 4 months (Fig. 7C). These data suggest that FoxO3-deficient mice show an increased susceptibility to lung inflammatory responses in response to CS exposure, which may contribute to enhanced airspace enlargement/emphysema in these mice.
FIGURE 5. Increased neutrophil influx in BAL fluid of FoxO3-/- mice in response to CS exposure.
The number of total cells in BAL fluid from air- or CS-exposed mice for 3 days (A), 8 weeks (B) and 4 months (C) was determined. Lavaged neutrophil numbers were counted in Diff-Quik stained cytospin slides, which were prepared using BAL fluid. Quantification of neutrophils expressed as % of total cells in BAL fluid from air- or CS-exposed mice for 3 days (D), 8 weeks (E) and 4 months (F). Data are shown as mean ± SEM (n=3 to 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with corresponding air-exposed mice; ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice.
FIGURE 6. Increased macrophage influx into the lungs of FoxO3-/- mice exposed to CS.
Mac-3 positive cells were identified by dark brown immunohistochemical staining, which were indicated by arrows in lung sections from air- or CS-exposed mice for 3 days (A), 8 weeks (B) and 4 months (C). The representative pictures shown are from at least three separate experiments. Quantification results are presented as the number of Mac-3-positive cells in lung sections from 3 days (D), 8 weeks (E) and 4 months (F) air- or CS-exposed mice. Original magnification, × 200. Data are shown as mean ± SEM (n=3 per group). ***P < 0.001, significant compared with corresponding air-exposed mice; +P < 0.05, ++P < 0.01, significant compared with CS-exposed WT mice.
FIGURE 7. Increased levels of proinflammatory mediators in lungs of FoxO3-/- mice in response to CS exposure.
The level of proinflammatory mediators, such as MCP-1, KC and IP-10 are measured in lung homogenates obtained from air- or CS-exposed WT and FoxO3-/- mice for 3 days (A), 8 weeks (B) and 4 months (C). Data are shown as mean ± SEM (n= 3 to 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared to corresponding air-exposed mice. +P < 0.05, ++P < 0.01, +++P < 0.001 significant compared with CS-exposed WT mice.
Both hematopoietic and non-hematopoietic cells are responsible for enhanced lung inflammatory response in FoxO3-deficient mice
To identify the cells responsible for the altered response to CS exposure in FoxO3-/- mice, bone marrow transplantation (BMT) chimeras were generated by radioablation, and the reconstituted mice (donors to recipients; WT to WT, WT to KO, KO to WT, and KO to KO) were exposed to air or CS for 3 days at 8 weeks after BMT. All CS-exposed chimeras showed an increased susceptibility to CS-induced lung inflammation compared to air-exposed chimeras, and the effect was augmented in CS-exposed KO to KO (FoxO3-deficient in both hematopoietic and structural cells) chimeras (Table 1). WT to KO (FoxO3-deficient in only lung structural cells) and KO to WT (FoxO3-deficient in only hematopoietic cells) chimeras showed increased inflammatory response to CS exposure as compared to WT to WT (FoxO3-expressed in both hematopoietic and structural cells) chimeras, but it was lower than KO to KO chimeras, suggesting that both the structural and hematopoietic cells are responsible for the altered responses seen by CS exposure in FoxO3-/- mice.
Table 1.
Release of proinflammatory cytokines in lungs of bone marrow transplantation chimeras
| Air |
CS |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT to WT | WT to KO | KO to WT | KO to KO | WT to WT | WT to KO | KO to WT | KO to KO | |
| KC | 70.8 ± 4.01 | 71.3 ± 0.82 | 65.4 ± 4.86 | 87.7 ± 5.88 | 103.2 ± 4.91** | 129.6 ± 2.16***,## | 129.2 ± 10.78***,# | 165.6 ± 11.93***,### |
| MCP-1 | 9.86 ± 0.52 | 10.5 ± 0.58 | 8.7 ± 1.75 | 9.2 ± 0.66 | 14.2 ± 1.24* | 17.4 ± 1.77** | 20.1 ± 1.68**,# | 25.9 ± 4.07***,### |
n = 3, mean ± SE
P < 0.05
P < 0.01
< 0.001, significant compared to corresponding air controls
P < 0.05
P < 0.01
P < 0.001, significant compared to CS-exposed WT to WT chimer.
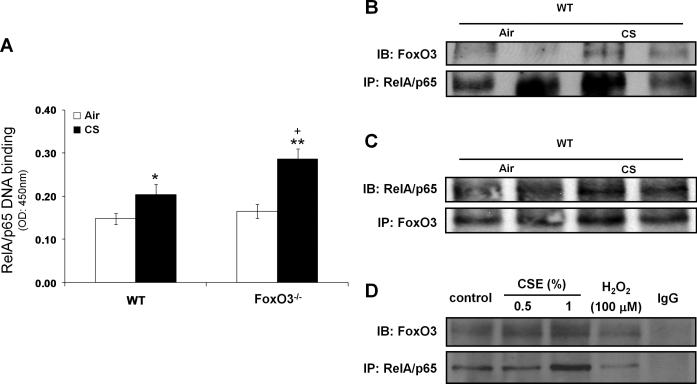
Deficiency of FoxO3 leads to augmented NF-κB activation due to loss of its interaction with RelA/p65 in lungs of mouse in response to CS
Given that NF-κB plays a crucial role in expression of proinflammatory genes in response to diverse stimuli (40, 42), we speculated that FoxO3-/- mice might show augmented NF-κB activity in response to CS. To test this contention, we performed the Trans-AM RelA/p65 DNA binding assay, and found that 3 days of CS-exposed FoxO3-/- mice showed 1.4-fold increase in RelA/p65 DNA binding activity as compared to CS-exposed WT mice (Fig. 8A). However, there was no significant difference in RelA/p65 DNA binding activity between air-exposed WT and FoxO3-/- mice. We further evaluated the total and acetylated levels of RelA/p65 at Lys310 residue in lung nuclear fractions, but there was no detectable difference seen between CS-exposed WT and FoxO3-/- mice exposed to CS for 3 days (Figs. S1, A and B).
FIGURE 8. Deficiency of FoxO3 leads to augmented NF-κB-DNA binding activity due to loss of its interaction with RelA/p65 in lungs of mouse exposed to CS.
A, NF-κB-DNA binding was measured by the Trans-AM transcription factor RelA/p65 ELISA kit in nuclear proteins from CS-exposed mice lung for 3 days. Data are shown as mean ± SEM (n= 3 to 4 per group). *P < 0.05, **P < 0.01, significant compared to corresponding air-exposed mice. +P < 0.05, significant compared with CS-exposed WT mice. B, Nuclear fraction from mouse lung exposed to CS for 3 days were immunoprecipitated (IP) with anti-RelA/p65 antibodies. C, Reciprocal IP of nuclear fraction from mouse lung exposed to CS 3 days was performed with anti-FoxO3 antibodies, followed by immunoblot analysis. D, H292 epithelial cells were treated with CSE (1%), and nuclear fractions from cell lysates were used for IP with anti-RelA/p65 antibody. IgG was used as an isotype control. Immunoprecipitates were subjected to immunoblot analysis and probed with anti-FoxO3 or anti-RelA/p65 antibody.
To examine the possibility that FoxO3 might inhibit RelA/p65 DNA binding activity through their interaction in the nucleus, we determined whether FoxO3 and RelA/p65 could form a complex in the nucleus. By immunoprecipitation assay, we found that CS exposure increased the interaction of FoxO3 with RelA/p65 (Fig. 8B), which was further confirmed by reciprocal immunoprecipitation (Fig. 8C). Consistent with these results, this interaction was also observed in the nuclear fractions of CSE-treated human bronchial epithelial cells (Fig. 8D). Furthermore, we found CS induced nuclear translocation of FoxO3 in vivo in mouse lungs and in vitro in lung epithelial cells (Fig. S2, A-C). Taken together, these results indicate that FoxO3 is translocated into the nucleus where it interacts with RelA/p65 in response to CS, resulting in inhibition of NF-κB DNA binding activity.
To further validate the role of increased NF-κB activity in enhanced lung inflammation of FoxO3-/- mice, both WT and FoxO3-/- mice were administered with an IKK2 inhibitor, IMD-0354 (Tocris, Ellisville, MO) as previously described (40). The administration of IKK2 inhibitor resulted in attenuation of CS-mediated the proinflammatory cytokine release in WT and FoxO3-/-mice (Fig. S3). Interestingly, there was a 14% reduction in KC levels of WT mice lung in response to CS, but a marked reduction (34%) was seen in FoxO3-/- mice (p < 0.05). These data suggest that the CS-induced heightened lung inflammation in FoxO3-/- mice is, at least in part, due to increased NF-κB activity.
Antioxidant genes are regulated by FoxO3
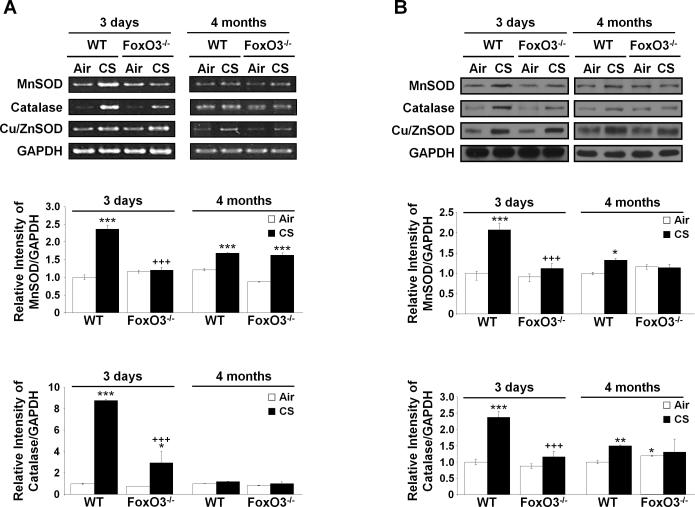
FoxO3 plays a role in detoxification of cellular ROS levels through the regulation of antioxidants gene transcription in response to oxidative stress (22). We hypothesized that deficiency of FoxO3 leads to transcriptional suppression of antioxidant genes, resulting in reduction in detoxification of ROS in response to CS. We determined the mRNA and protein expression levels of MnSOD and catalase, which are well-known target stress genes that are transcriptionally regulated by FoxO3. After 3 days of CS exposure, both mRNA expression and protein abundance of MnSOD and catalase were increased in CS-exposed WT mice as compared to air-exposed WT mice, whereas CS-exposed FoxO3-/-mice showed a significant reduction in mRNA and protein levels of MnSOD and catalase as compared to CS-exposed WT mice (Fig. 9, A and B). At 4 months of CS exposure, the upregulated levels of MnSOD and catalase were reduced in CS-exposed WT mice as compared to 3 days of CS exposure to WT mice. There were no significant changes in MnSOD and catalase mRNA levels between WT and FoxO3-/- mice at 4 months of CS exposure (Fig. 9, A and B). Expression levels of CuZnSOD which was used for non-target control showed no difference between WT and FoxO3-/- mice even though the level was increased in response to CS exposure. These data suggest that the absence of FoxO3 reduces the expression of antioxidant genes in response to CS, resulting in imbalance between oxidants and antioxidants in the lung.
FIGURE 9. Antioxidant genes are regulated by FoxO3.
The levels of MnSOD and catalase were measured by RT-PCR (A) and immunoblot (B) at the indicated time points of CS exposures. CuZnSOD was used as a non-specific target protein of FoxO3, and GAPDH was used for loading controls. After densitometric analysis, the values of MnSOD and catalase were normalized against GAPDH, respectively. Gel pictures shown are representative of at least three separate experiments. Data are shown as mean ± SEM (n= 3 to 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared to corresponding air-exposed mice. +++P < 0.001, significant compared with CS-exposed WT mice.
DISCUSSION
FoxO3 has been shown to regulate expression of several genes involved in inflammation, oxidative stress resistance, and senescence which are intertwined in the pathogenesis of COPD. However, the role of FoxO3 in lung inflammation and pathogenesis of COPD/emphysema is not known. We found that the abundance of FoxO3 was decreased in lungs of smokers, and even more prominently reduced in patients with COPD compared to nonsmokers. FoxO3 was also reduced in mouse lungs in response to CS exposure, which is a major risk factor in development of COPD. Furthermore, the deficiency in FoxO3 increased the susceptibility to CS-induced emphysema, which was reflected by airspace enlargement, impaired lung function, and decreased exercise performance. All these findings suggest CS-induced reduction of FoxO3 is a key contributing factor in development of COPD/emphysema. The mechanism underlying CS-induced reduction of FoxO3 is unclear, but it may be associated with its post-translational modifications, such as acetylation and phosphorylation. This is corroborated by findings that increased acetylation of FoxO3 occurred in lungs of smokers and mouse exposed to CS.
Sustained inflammation is a characteristic feature in pathogenesis of COPD/emphysema, and FoxO3 is known to be involved in augmented inflammatory responses (18, 54). Therefore, we determined whether susceptibility of FoxO3-/- mice to development of emphysema is associated with increased lung inflammatory response. CS exposure increased inflammatory cell infiltration into the lung accompanied with proinflammatory cytokines release, including MCP-1, KC and IP-10 in WT mice, which was more pronounced in lungs of FoxO3-/- mice. This finding is consistent with a previous study showing that an enhanced inflammatory response occurred in intestinal tissue of FoxO3-/- mice challenged with dextran sulfate sodium as compared to WT mice (55). Hence, increased inflammatory response may contribute to susceptibility in development of emphysema in FoxO3-/- mice.
FoxO3 was predominantly localized in both lung airway/alveolar epithelium and macrophages in nonsmokers, and significantly decreased in all locations in lungs of smokers and patients with COPD. These findings raise a question as to which specific cell-type is responsible for CS-induced pathological changes (e.g. inflammation) in lungs of FoxO3-/- mice. We, therefore generated the BMT chimeric mice in order to distinguish the role of FoxO3 in lung epithelial cells (radioresistant non-hematopoietic-derived structural cells) and inflammatory cells (radiosensitive hematopoietic-derived cells) in CS-induced lung inflammation. We found that CS-induced release of proinflammatory cytokines was increased in FoxO3-deficient mice in hematopoietic or non-hematopoietic cells as compared to the mice expressing FoxO3 in these cells. Deficiency in FoxO3 in both the cells further increased the release of proinflammatory mediators in response to CS exposure, suggesting that both structural cells (mainly epithelial cells) and hematopoietic cells (mainly macrophages and neutrophils) are responsible for the altered responses to CS exposure seen in FoxO3-/- mice. Although it has been reported that simultaneous conditional deletion of FoxO1, FoxO3 and FoxO4 results in long-term defect of hemotopoietic stem cell (56), Miyamoto et al. has demonstrated that young adult FoxO3-/- mice (8- to 12 week-old) show normal proliferation and differentiation of hematopoietic progenitors, which is supported by normal morphology and cell numbers in lymphoid and myeloid cells in peripheral blood and bone marrow (57). Consistent with the latter observation, we did not find any differences in differential cell counts from myeloid cell-derived neutrophils and macrophages in air-exposed FoxO3-/- and BMT chimeric mice. A recent study has also shown that the loss of FoxO3 alone does not alter intrinsic phenotype and activation of T cell (18).
Although we did not investigate the lymphoid cell population in BMT chimeric mice, CD8+ T cell product, IP-10 levels were similar in between air-exposed chimeric mice (unpublished observations), possibly suggesting that lymphoid-lineage cells of hematopoiesis is not impaired in FoxO3-/- mice. As evidenced by a significant increase of IP-10 levels (a specific chemoattractant for activated T cells), shown in chronic CS exposure, BMT chimeric mice exposed to chronic CS may broaden the understanding of the role of FoxO3 in regulation of immune response by CS exposure and in pathogenesis of COPD.
It has been shown that CS induces lung inflammation which is accompanied by NF-κB activation in experimental mouse model, and IκB kinase which is implicated in activation of NF-κB regulates FoxO3 activity (25, 40, 42, 58). Therefore, we postulated that increased susceptibility to CS-induced inflammation in FoxO3-/- mice was due to exaggerated increase in NF-κB activity. Interestingly, we found that CS exposure led to increased NF-κB RelA/p65 DNA binding activity in WT mice, which was augmented in FoxO3-/- mice. These data suggest that NF-κB activity is inhibited by FoxO3, which is consistent with a previous report that FoxO3 can function as NF-κB antagonist and inhibit its activation (36). In addition, we demonstrated the FoxO3-RelA/p65 interaction in response to CS in lung in vivo and in epithelial cells in vitro. It may be possible that FoxO3-RelA/p65 interaction specifically affects RelA/p65 activity by blocking its DNA binding in response to CS. Recent data showed that N-terminal region of FoxO4 interacts with Rel-homology domain of NF-κB, which was confirmed by a series of deletion mutants of FoxO4 and NF-κB (59). Since forkhead DNA-binding domain was included in the N-terminal region of FoxO4 as well as FoxO3, FoxO3 may bind directly with NF-κB through its forkhead domain. However, further studies are required to identify the interactive domain between FoxO3 and NF-κB, and their involvement in control of inflammatory processes.
Since it has been known that RelA/p65 translocated into the nucleus in response to oxidative stress and CS, we speculated that CS also induced nuclear translocation of FoxO3 to interact with RelA/p65. Brunet et al. reported that FoxO3 was localized in cytoplasm when growth factors were present, and translocated into the nucleus in response to oxidative stress (30). Consistent with this observation, we found that CS/oxidative stress promoted FoxO3 translocation into the nucleus in vivo in the lung and in vitro in bronchial epithelial cells. Although the phosphorylation of FoxO3 causes proteolysis of FoxO3 via ubiqutin-proteasome pathway in the cytoplasm (25), a recent study reported that oxidative stress-induced de-phosphorylation of FoxO3 promoted nuclear translocation of FoxO3 without affecting the total FoxO3 levels (60). Similarly, we also found that CSE induced de-phosphorylation of FoxO3 in a time-dependent fashion in bronchial epithelial cells (data not shown), which was accompanied with nuclear translocation of FoxO3. The mechanism underlying CS-induced de-phosphorylation and translocation of FoxO3 into the nucleus is not known. However, it may be possible that CS-induced de-phosphorylation of FoxO3 is closely related with its translocation into the nucleus. Oxidative stress due to imbalance between oxidants and antioxidants is involved in pathogenesis of chronic pulmonary diseases (61). It has been reported that FoxO3 plays a role in regulation of cellular ROS levels through modulation of transcription of antioxidant genes (22, 62-63). In this study, we showed that the mRNA and protein expression of MnSOD and catalase, which are well-known specific target genes of FoxO3, were increased in CS-exposed WT mice, whereas the levels were significantly reduced in CS-exposed FoxO3-/- mice at 3 days. Despite reduction of FoxO3 in lungs of CS-exposed WT mice, the expression of its target MnSOD and catalase was increased in CS-exposed WT mice. This discrepancy may be due to increased acetylation of FoxO3 against acute lung inflammation. We showed that acetylation of FoxO3 was increased in lungs of smokers, as well as in lungs of mice exposed to CS suggesting alteration in transactivation of FoxO3 for target genes, such as MnSOD and catalase. CS-induced oxidant stress may potentially modify FoxO3 via CBP-mediated acetylation which is shown to be the case for FoxO4 (64) and SIRT1-mediated deacetylation (30). We have recently shown that the levels of SIRT1 are reduced in lungs of smokers and patients with COPD as well as in mouse lung (33, 35), which can result in acetylation of FoxO3. Transcriptional activity of Foxo3 is modified mainly by post-translational modifications and association with many different cofactors (65). Although many studies have described that post-translational modifications, such as phosphorylation and acetylation, of FoxO3 lead to the repression of its transcriptional activity, some suggested that acetylation of FoxO3 increases the target gene transcription (30, 66). Therefore, it is possible that CS-induced acetylation of FoxO3 enhances the transcription of antioxidants genes to counteract oxidative stress. In 4 months of CS exposure, however, the expression of antioxidant genes was reduced both in CS-exposed WT and FoxO3-/- mice due to the loss of FoxO3 levels per se. Altogether, our data suggest that acetylation of FoxO3 may enhance transcription of antioxidant genes but further study is required to assess the role of acetylation and deacetylation of FoxO3 in regulation of MnSOD and catalase under the condition of oxidative stress.
In conclusion, we show a novel role of FoxO3 in regulation of lung inflammation and in pathogenesis of COPD/emphysema. The abundance of FoxO3 was decreased in lungs of patients with COPD. Furthermore, genetic ablation of FoxO3 in mice led to increased susceptibility to airspace enlargement/emphysema in response to CS, in association with exaggerated lung inflammatory response and decreased antioxidant genes due to FoxO3 deficiency. We further provide a novel mechanistic link between FoxO3 and NF-κB both in vivo and in vitro, and suggest that FoxO3 acts as a fine tuner that modulates CS-induced lung inflammatory response and COPD/emphysema. In addition, it may be possible that cellular senescence/accelerated lung aging which are important events in pathogenesis of COPD are regulated by FoxO3 via SIRT1 deacetylase. Hence, further studies are required to expand our understanding for the role of FoxO3 in pathogenesis of COPD/emphysema particularly with respect to regulation of antioxidant defense mechanisms and cellular senescence in the lung.
Supplementary Material
Acknowledgments
This study was supported by the NIH R01-HL085613, NIH R01-HL097751, NIH R01-HL092842 and NIEHS P30-ES01247, and NIEHS Environmental Health Science Center grant P30-ES01247. VLK was partly supported by a governmental subsidy for health science research (EVO) in Helsinki University Hospital and Finnish Antituberculosis Association Foundation. We also thank Suzanne Bellanca and Stephanie Uhrinek for their technical assistance.
ABBREBIATIONS
Abbreviations used in this paper
- FoxO3
forkhead box class O 3a
- CS
cigarette smoke
- COPD
chronic obstructive pulmonary disease
- MnSOD
manganese superoxide dismutase
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- CBP
cAMP-response-element-binding protein-binding protein
- Lm
mean linear intercept
- BMT
bone marrow transplantation
REFERENCES
- 1.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118:394–402. doi: 10.1172/JCI31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bracke KR, D'Hulst A I, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 3.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 5.MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc. 2009;6:527–531. doi: 10.1513/pats.200905-027DS. [DOI] [PubMed] [Google Scholar]
- 6.Karrasch S, Holz O, Jorres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102:1215–1230. doi: 10.1016/j.rmed.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 9.Arden KC. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene. 2008;27:2345–2350. doi: 10.1038/onc.2008.27. [DOI] [PubMed] [Google Scholar]
- 10.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 12.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 13.Birkenkamp KU, Coffer PJ. FOXO transcription factors as regulators of immune homeostasis: molecules to die for? J Immunol. 2003;171:1623–1629. doi: 10.4049/jimmunol.171.4.1623. [DOI] [PubMed] [Google Scholar]
- 14.Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Schultze JL, Hoch M. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 15.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronnebaum SM, Patterson C. The FoxO Family in Cardiac Function and Dysfunction. Annu Rev Physiol. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui M, Huang Y, Zhao Y, Zheng J. Transcription factor FOXO3a mediates apoptosis in HIV-1-infected macrophages. J Immunol. 2008;180:898–906. doi: 10.4049/jimmunol.180.2.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejean AS, Beisner DR, Ch'en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 20.Vernooy JH, Bracke KR, Drummen NE, Pauwels NS, Zabeau L, van Suylen RJ, Tavernier J, Joos GF, Wouters EF, Brusselle GG. Leptin Modulates Innate and Adaptive Immune Cell Recruitment after Cigarette Smoke Exposure in Mice. J Immunol. 2010 doi: 10.4049/jimmunol.0900963. [DOI] [PubMed] [Google Scholar]
- 21.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 22.Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, Monsalve M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czymai T, Viemann D, Sticht C, Molema G, Goebeler M, Schmidt M. FOXO3 modulates endothelial gene expression and function by classical and alternative mechanisms. J Biol Chem. 2010;285:10163–10178. doi: 10.1074/jbc.M109.056663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene. 2008;27:2289–2299. doi: 10.1038/onc.2008.22. [DOI] [PubMed] [Google Scholar]
- 25.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 26.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 28.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 29.Nasrin N, Ogg S, Cahill CM, Biggs W, Nui S, Dore J, Calvo D, Shi Y, Ruvkun G, Alexander-Bridges MC. DAF-16 recruits the CREB-binding protein coactivator complex to the insulin-like growth factor binding protein 1 promoter in HepG2 cells. Proc Natl Acad Sci U S A. 2000;97:10412–10417. doi: 10.1073/pnas.190326997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 31.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 33.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 35.Caito S, Hwang JW, Chung S, Yao H, Sundar IK, Rahman I. PARP-1 inhibition does not restore oxidant-mediated reduction in SIRT1 activity. Biochem Biophys Res Commun. 2010;392:264–270. doi: 10.1016/j.bbrc.2009.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Rytila P, Rehn T, Ilumets H, Rouhos A, Sovijarvi A, Myllarniemi M, Kinnula VL. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD. Respir Res. 2006;7:69. doi: 10.1186/1465-9921-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 39.Pryhuber GS, Huyck HL, Bhagwat S, O'Reilly MA, Finkelstein JN, Gigliotti F, Wright TW. Parenchymal cell TNF receptors contribute to inflammatory cell recruitment and respiratory failure in Pneumocystis carinii-induced pneumonia. J Immunol. 2008;181:1409–1419. doi: 10.4049/jimmunol.181.2.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajendrasozhan S, Chung S, Sundar IK, Yao H, Rahman I. Targeted disruption of NF-{kappa}B1 (p50) augments cigarette smoke-induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling. Am J Physiol Lung Cell Mol Physiol. 2010;298:L197–209. doi: 10.1152/ajplung.00265.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–1186. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- 42.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172:1222–1237. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, Hegab AE, Hosoya T, Nomura A, Sakamoto T, Yamamoto M, Sekizawa K. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175:6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 45.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170:974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 46.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D'Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp Lung Res. 2005;31:547–562. doi: 10.1080/019021490951522. [DOI] [PubMed] [Google Scholar]
- 47.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1006–1013. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- 48.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 49.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 50.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol. 2008;38:689–698. doi: 10.1165/rcmb.2007-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 53.Couillin I, Vasseur V, Charron S, Gasse P, Tavernier M, Guillet J, Lagente V, Fick L, Jacobs M, Coelho FR, Moser R, Ryffel B. IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema. J Immunol. 2009;183:8195–8202. doi: 10.4049/jimmunol.0803154. [DOI] [PubMed] [Google Scholar]
- 54.Hinman RM, Nichols WA, Diaz TM, Gallardo TD, Castrillon DH, Satterthwaite AB. Foxo3-/- mice demonstrate reduced numbers of pre-B and recirculating B cells but normal splenic B cell sub-population distribution. Int Immunol. 2009;21:831–842. doi: 10.1093/intimm/dxp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snoeks L, Weber CR, Wasland K, Turner JR, Vainder C, Qi W, Savkovic SD. Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation. Lab Invest. 2009;89:1053–1062. doi: 10.1038/labinvest.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Valenca SS, Castro P, Pimenta WA, Lanzetti M, Silva SV, Barja-Fidalgo C, Koatz VL, Porto LC. Light cigarette smoke-induced emphysema and NFkappaB activation in mouse lung. Int J Exp Pathol. 2006;87:373–381. doi: 10.1111/j.1365-2613.2006.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA, Liu ZP. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology. 2009;137:1403–1414. doi: 10.1053/j.gastro.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Derijard B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol. 2010;30:470–480. doi: 10.1128/MCB.00666-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chintapalli J, Yang S, Opawumi D, Goyal SR, Shamsuddin N, Malhotra A, Reiss K, Meggs LG. Inhibition of wild-type p66ShcA in mesangial cells prevents glycooxidant-dependent FOXO3a regulation and promotes the survival phenotype. Am J Physiol Renal Physiol. 2007;292:F523–530. doi: 10.1152/ajprenal.00215.2006. [DOI] [PubMed] [Google Scholar]
- 63.Sengupta A, Molkentin JD, Paik JH, Depinho RA, Yutzey KE. FoxO Transcription Factors Promote Cardiomyocyte Survival upon Induction of Oxidative Stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, Szypowska A, Meppelink A, Brenkman AB, Yodoi J, Holstege FC, Burgering BM. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- 65.Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–86. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.