Abstract
Adaptive tolerance is a hyporesponsive state in which lymphocyte antigen receptor signaling becomes desensitized following prolonged in vivo encounter with antigen. The molecular mechanisms underlying this hyporesponsive state in T cells are not fully understood, although a major signaling block has been shown to be present at the level of ZAP70 phosphorylation of LAT (linker for activation of T cells). In this study, we investigated the ability of adaptively tolerant mouse T cells to form conjugates with Ag-bearing APCs and to translocate signaling molecules into the interface between the T cells and APCs. Compared with naïve or pre-activated T cells, adaptively tolerant T cells showed no dramatic impairment in their formation of conjugates with APCs. In contrast, there was a large impairment in immunological synapse formation. Adaptively tolerant T cells were defective in their translocation of signaling molecules, such as ZAP70, LAT, and PLCγ1 (phospholipase Cγ1), into the T cell-APC contact sites. Although Ag-induced activation of VAV1 was normal, VAV’s recruitment into the synapse was also impaired. Interestingly, expressions of both ITK (interleukin-2-inducible T-cell kinase) and GADS (growth-factor-receptor-bound protein-2-related adaptor downstream of SHC) were decreased by 60 to 80% in adaptively tolerant T cells. These decreases, in addition to the impairment in LAT phosphorylation by ZAP70, appear to be the major impediments to the phosphorylation of SLP76 (SRC homology-2-domain-containing leukocyte protein of 76 kDa) and the recruitment of VAV1, which are important for stable immunological synapse formation.
Keywords: T cells, anergy, transgenic mice, spleen and lymph nodes
Introduction
T cell clonal anergy was first described as an in vitro model of tolerance using both human and mouse T cell clones (1–3). These studies revealed a hyporesponsive state induced by TCR occupancy in the absence of costimulation. After such a suboptimal T cell activation stimulus, IL-2 production and proliferation were impaired following a subsequent rechallenge of the T cells under optimum stimulation conditions. Anergy was induced in several different ways (4). All of them were characterized by a failure to produce IL-2 and sustain proliferation following T cell activation (5). However, as each of these models has been explored in more detail, it has become clear that many of them are quite unique and probably represent different biological states of unresponsiveness (4). In our laboratory we have compared T cell clonal anergy with an in vivo process called adaptive tolerance, and found these two states to be quite different (6). Biologically, clonal anergy represents a growth arrest state in which the production of only selective cytokines (IL-2 and IL-3) is significantly impaired in response to TCR stimulation. It does not require antigen to maintain the state and it can be reversed by stimulation with IL-2 (7). In contrast, adaptive tolerance results in the down-regulation of all TCR-induced cytokine production, requires antigen persistence to maintain the state, and is not reversed by the addition of IL-2 (5, 8, 9). A recent biochemical comparison revealed that adaptive tolerance involved a block in TCR stimulation at the level of ZAP70’s phosphorylation of LAT, while clonal anergy was confirmed to be a block further downstream in the RAS-MAP (mitogen-activated protein) kinase pathway without any impairment of ZAP70 activity (6).
To facilitate the biochemical analysis of CD4+ T cell activation, Ab-mediated cross-linking of TCRs is commonly used to strongly signal the cell. In contrast, upon stimulation with peptide/MHCII complexes on Ag-pulsed APC, a more physiological ligand for the TCR, T cells have to first form conjugates with the APCs (10). LFA1(lymphocyte function-associated antigen-1), a β2 integrin expressed on T cells, plays a crucial role in initiating and maintaining stable conjugates in order for sustained TCR signaling to occur (11). However, this process is first initiated by microcluster formation of engaged TCRs (12, 13) and this activates the β2 integrins through a process known as “inside-out signaling” (14). Subsequent integrin conformational change and clustering then increases LFA1’s avidity for its ICAM (intercellular adhesion molecule) ligand on the APC to form the stable conjugates (14–16). Several cytoplasmic signaling molecules have been implicated in inside-out signaling even though the exact mechanism is not fully understood. The small GTPase RAP1 has emerged as a crucial regulator (17) and requires VAV1 (18) and PLCγ1 for activation (19). RAP1 interacts with Protein Kinase D (PKD) (20), RAP1 ligand (RAPL) (21) and RAP1-interacting adaptor molecule (RIAM) (22) to propagate the signal to MST1 (mammalian sterile twenty-like-1) (23) and the actin-regulatory proteins MENA (mammalian enabled) (22), VASP (vasodilator-stimulated phosphoprotein) (22) and profilin (22), which facilitate the binding of talin to the cytoplasmic tail of the β-integrin (24). In addition, the molecule ADAP (adhesion- and degranulation-adaptor protein) is known to couple the TCR to integrin activation (25, 26) with its binding partner SKAP55 (SRC-kinase-associated protein of 55kDa) (27), possibly by localizing RAP1 to the plasma membrane (28).
Following initial conjugate formation, the TCRs and signaling molecules are recruited to the interface between the T cell and the APC (29–31), where they arrange into a complex known as the cSMAC (central supramolecular activation cluster) (29). The β2 integrins also accumulate at the immune synapse in a ring surrounding the TCR signaling core (peripheral SMAC) (29). The purpose of this overall structure is still not clearly defined. Initially it was thought to be the main mechanism for sustained signaling of the T cell, in order to insure full downstream activation of the transcription factors required for differentiation and cell cycle entry. Some more recent studies, however, have suggested that its main function may be to terminate the signaling of TCR microclusters (32, 33). Nonetheless, a failure to form these synapses is usually correlated with a reduced or abortive T cell activation process (34, 35).
The VAV family of proteins plays a key role in stabilizing the immunological synapse. They do this in two different ways (36, 37). One is through their contribution to the assembly of the TCR signaling complex involving LAT, SLP76, ITK and PLCγ1. Without VAV1 present, this complex is not stably formed and down stream activation of calcium flux and ERK (early response kinase) activation are both impaired (38). Second is by recruiting molecules such as HS1 (haematopoietic-cell-specific protein-1) (39), DNM2 (dynamin-2) (40), NCK (non-catalytic region of tyrosine kinase) (41), and ABI1/2 (abelson-interacting protein 1 or 2) (42) to help activate the small RHO GTPases such as RAC1 and CDC42, which in turn help to catalyze the polymerization of actin filaments (F-actin) at the contact site by facilitating the proteins WAVE2 (WASP-family verprolin-homologous protein-2) (43) and WASP (Wiskott-Aldrich syndrome protein) (41) to activate ARP2/3 (actin-related protein 2 and 3) (41). To function, VAVs must first be activated by tyrosine phosphorylation following TCR engagement (36). This phosphorylation is mediated by SRC-family kinases (44, 45) and can be enhanced by costimulation through the CD28 receptor (46). Mice deficient in VAV family proteins show major defects in both T cell responses and development (47). Polymerization of F-actin is also accompanied by polarization of the Golgi apparatus and the T cell microtubule-organizing center (MTOC) toward the APC (48, 49). The molecular pathways that are directly involved in the movement of the MTOC following TCR engagement are poorly defined, but a role for the motor protein dynein interacting with ADAP has been implicated. (50)
Our previous studies using antibodies against TCR, CD3, CD4, and CD28 (6) demonstrated that signaling through the TCR in adaptively tolerant T cells is impaired. In the current experiments, we explored in detail the response of these anergic T cells to TCR stimulation by antigen and APCs. Although initial conjugate formation appeared to be normal, we found that immunological synapse formation was greatly reduced. This led to the discovery that ITK and GADS molecules were down-regulated in these cells and that this, coupled with our previous finding of impairment in LAT phosphorylation, resulted in a failure to recruit VAV proteins into the T cell/APC interface. This in turn impaired actin filaments from congregating at the synapse and prevented the MTOC from orienting toward this site.
Materials and Methods
Mice and Adoptive transfer experiments
All the mice used in this study were bred at the National Institute of Allergy and Infectious Diseases contract facility at Taconic Farms (Germantown, NY). They were on the B10.A (H-2a) background. For adoptive transfer experiments, the donor T cells were from B10.A, TCR-5C.C7 transgenic (Tg), Rag2−/− mice that specifically recognize the pigeon cytochrome c (PCC) peptide 81–104 bound to I-Ek (6). The recipient mice (RO) were transgenic for PCC expression under the control of an MHC class I promoter and an Ig enhancer (6). They were also CD3ε−/−. Three million lymph node (LN) T cells from TCR-5C.C7 Tg mice were injected iv. into each recipient mouse. Mice were sacrificed from 4 to 6 weeks after transfer to provide the adaptively tolerant T cells. All of the animals were maintained in a specific pathogen-free environment, and all experiments were approved by the NIH Animal Care and Use Committee.
Abs and reagents
MCC peptide (aa 88–103 of moth cytochrome c) was synthesized through the National Institute of Allergy and Infectious Diseases Peptide Facility (National Institute of Health, Bethesda, MD). The PI-3K inhibitor LY294002 was purchased from Sigma-Aldrich. The Src-family kinase inhibitor PP2 and a control analogue PP3 that only inhibits the EGFR kinase were purchased from Calbiochem. The following Abs were used for flow cytometry: anti-CD4-PE-Cy5.5 (Caltag Laboratories) and anti-Vβ3-PE, anti-MHCII (I-Ek)-PE, anti-CD44-FITC, anti-CD62L-FITC, and anti-LFA-1-FITC (BD Pharmingen). Abs used for immunofluorescence were from Upstate (anti-LAT, anti-PLCγ1, and anti-phosphotyrosine (4G10)-biotin), Santa Cruz Biotechnology (anti-VAV (sc132) and anti-PKCθ (sc212)), BD Pharmingen (anti-TCRβ-biotin and anti-ZAP70-FITC), BioLegend (Alexa-Fluor 488-anti-mouse CD11a (LFA-1)), Invitrogen (Alexa-Fluor 488-phalloidin), NeoMarkers (anti-tubulin), or Jackson Immunoresearch Laboratories (donkey anti-mouse IgG-FITC and donkey anti-rabbit IgG-FITC). Cholera toxin B (CTx) was purchased from Sigma-Aldrich. Alexa-Fluor 488-Streptavidin was purchased from Molecular Probes. Abs used for TCR stimulation were from BD Pharmingen (anti-TCRβ-biotin, anti-CD4- biotin, anti-CD3-biotin, anti-CD28-biotin, anti-LFA-1, and anti-CD80). Abs used for immunoprecipitation and Western blot were from Upstate Biotechnology (anti-LAT, anti-phosphotyrosine 191 LAT, anti-VAV, anti-GADS, and anti-SLP76), Cell Signaling Technology (anti-phospho-AKTmAb, and anti-phospho-GSK3α/β Ab), Santa Cruz Biotechnology (anti-ZAP70 (sc574), anti-CDC42 (sc87), anti-VAV (sc132), and-RAP1 (sc65)), BD Pharmingen (anti-RAC1, anti-ITK, anti-ZAP70, and anti-phosphotyrosine 319 ZAP70), Sigma-Aldrich (anti-actin), or Bio-Rad (anti-mouse IgG-HRP and anti-rabbit IgG-HRP). Streptavidin was purchased from Southern Biotechnology.
Cell preparation
The CD4+ T cell population was purified (>90%) from lymph node and spleen cells by negative selection as previously described (51). P13.9 cells, used as APCs, are fibroblasts that had been transfected with MHC class II I-Ek, CD80, and ICAM1 and were kindly provided by Dr. R. N. Germain (NIAID, National Institutes of Health). P13.9 cells in log phase were pulsed with various concentrations of MCC peptide for 2 h in fresh medium at 37 °C.
In vitro pre-activated cells
In vitro pre-activated TCR-5C.C7 transgenic cells were made by stimulating naïve LN and splenic T cells with 1 μM moth cytochrome c (MCC) and a 10-fold excess of irradiated (3000 rad) B10.A, CD3ε−/− splenic APCs in EHAA/RPMI 1640 medium (6). After 72 h, the activated T cells were expanded with 10 U/ml of rIL-2 (BioSource). The cells were then rested in fresh medium without IL-2 and used after 10days.
Flow cytometry
Cell suspensions were stained with anti-Vβ3 TCR-PE and anti-CD4- PE Cy5.5. For analysis of CD62L, CD44 or LFA-1 expression, T cells were stained with anti-CD62L-FITC, anti-CD44-FITC or anti-LFA-1-FITC, respectively. Immunofluorescence analysis was performed on a FACSort cytometer (BD Biosciences), and data files were analyzed using CellQuest software (BD Biosciences).
Conjugate formation
P13.9 cells (4 × 105/well) or B10.A CD3ε−/− spleen cells (4 × 105/well) were pulsed with the indicated concentrations of MCC 88–103 peptide for 2 h in 96-well round-bottom plates. Two hundred thousand purified CD4+ T cells were mixed with the APCs at a 1:2 ratio and centrifuged briefly to promote conjugate formation. Samples were incubated at 37°C for the indicated periods of time. Cells were vigorously pipetted to disrupt nonspecific conjugates and then fixed with2% paraformaldehyde for 10 min at room temperature. The cells were then stained with anti-CD4-PE-Cy5.5 and anti-I-Ek-PE and analyzed by flow cytometry. Conjugates were identified as the percentage of CD4+ cells that were also MHC class II+ (I-Ek).
Immunocytochemistry
P13.9 APCs were pulsed with MCC for 2 h. Purified naïve, tolerant, and pre-activated T cells (2 × 105) were mixed with the APCs (4 × 105) at a 1:2 ratio. The cell mixtures were incubated at 37°C for 5 or 20min and then gently resuspended and spread (50μl/slide) onto pre-warmed poly-L-lysine-coated slides (Sigma-Aldrich). Slides were incubated for another 15 min at 37°C to promote cell attachment. For lipid raft staining, CD4+ T cells were stained with FITC-CTx before incubation with the APCs. Cells were fixed with 4% paraformaldehyde in PBS for 10 min and then permeabilized with 0.2% Triton X-100 for 5 min. Cells were blocked overnight with 10% horse serum/ 1% BSA/PBS and stained with anti-TCRβ-biotin or anti-phosphotyrosine (4G10)-biotin followed by Alexa-Fluor 488-Streptavidin. For LFA-1, cells were stained with Alexa-Fluor 488-anti-LFA-1. For ZAP70, cells were stained with anti-ZAP70-FITC. For LAT, PLCγ1, VAV, or PKCθ, cells were stained with rabbit anti-LAT, mouse anti-PLCγ1, rabbit anti-VAV, or rabbit anti-PKCθ, followed by donkey anti-mouse IgG-FITC or donkey anti-rabbit IgG-FITC. All images were taken using a Leica SP1 laser scanning confocal microscope. Twenty five to 50 conjugates were scored per condition. Data were analyzed by a two-way ANOVA and a Bonferroni’s multiple comparison test was used to determine statistical significance, which was accepted at a value of p<0.05 (GraphPad PRISM software). A Chi-square test was used to analyze the significance of the data on actin polymerization, MTOC polarization, and VAV translocation. p<0.025 was considered significant.
RAP1, RAC1 and CDC42 activation assays
Purified T cells (1 × 107 or 2.5 × 107) were rested for 2 hr in complete medium (51) followed by activation with anti-TCRβ-biotin plus anti-CD4-biotin or anti-CD3-biotin plus anti-CD28-biotin and cross-linked by streptavidin for the indicated times. For analysis of RAP1, cells were lysed in 25 mM Tris (pH 7.5), 250 mM NaCl, 0.5% Nonidet P-40, 1.25 mM MgCl2, 10% glycerol, and a mixture of protease inhibitors (Roche) and centrifuged for 5 min at 12,000 rpm and 4°C. Clarified lysates were incubated with the GST-RAL GDS-RAP binding domain (RBD) (Upstate Biotechnology) at 4°C for 1 h. After the incubation, beads were washed three times with ice-cold 1X lysis buffer and the GST-RAL GDS-RBD-bound proteins were eluted with 2X sample buffer. Samples were analyzed by SDS-PAGE. After electrophoresis, the proteins were transferred onto nitrocellulose membranes for immunoblotting. Blots were probed with rabbit anti-RAP1 followed by HRP-conjugated anti-rabbit IgG. The immunoblots were developed by ECL (Amersham Biosciences). In addition, 10%of whole cell lysates from each sample were saved prior to the assay for use as a loading control. For analysis of RAC1and CDC42 activation, cells were lysed in 25 mM HEPES (pH 7.5), 150 mMNaCl, 1% Nonidet P-40, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1mM sodium orthovanadate, and a mixture of protease inhibitors. Clarified lysates were incubated with the GST-PAK-1 p21-binding domain (PBD) (Upstate Biotechnology)at 4°C for 1 h. The GST-PAK-1 PBD-bound proteins were then analyzed as described above. Blots were probed with mouse anti-RAC1 or rabbit anti-CDC42 followed by HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG, respectively. A portion of the lysate was saved prior to the assay as described above.
Immunoprecipitation and western blot analysis
T cells were stimulated with anti-TCRβ biotin plus anti-CD4 biotin or anti-CD3 biotin plus anti-CD28 biotin followed by cross-linking with streptavidin at 37°C as described above. Alternatively, T cells were also activated with Ag-pulsed P13.9 cells following the procedure described earlier. T cells were then washed in ice-cold PBS and resuspended in lysis buffer containing 1% Nonidet P-40, 10 mM Tris (pH 7.5),150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM sodium orthovanadate, 10 mM NaF, 1 mM DTT, 1 mM PMSF, and a mixture of protease inhibitors (Roche). After incubating on ice for 30 min, lysates were spun at 12,000 rpm in the cold for 10 min. Lysates were then immunoprecipitated with protein (A/G)-Sepharose conjugated with rabbit polyclonal anti-LAT, anti-VAV1, or anti-ZAP70 Abs or sheep polyclonal anti-SLP76 Ab. The precipitates were washed three times with lysis buffer, resuspended in 2X SDS sample buffer, and boiled for 5 min. Samples were then run on SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked with a buffer containing 5% skim milk for 1 h at room temperature. Membranes were incubated with primary Ab for 4 h and then with a secondary Ab conjugated to HRP. The immunoblots were developed by the ECL technique (Amersham Biosciences). Band intensities were measured by Gel-Pro 32 software (Media Cybernetics), and arbitrary intensity units are shown.
Real-time quantitative PCR analysis
Total RNA from sorted cells were purified by using TRIzol plus an RNA purification kit (Invitrogen) and then transcribed by using Superscript II reverse-transcriptase and oligo (dT)12–18 primers (Invitrogen). RNA samples were treated with RNase-free DNase I (Invitrogen) before reverse transcription to eliminate contaminating genomic DNA. The expression of the mRNA for Itk, Gads, Rlk, Tec and cyclophilinA were determined by real time PCR using the primers below. The expression of individual genes was normalized to the expression of cyclophilinA. Gene-specfic primer sets used in the real-time PCR assays were: Itk, 5′-AAGGTGTCCGACTTTGGGAT-3′ and 5′-GACCACACATCTGACTTGCT-3′; Gads, 5′-GACACTGCGGCCTTGGAAGT-3′ and 5′-CAGCTCGTCCTCTTCCAGAG-3′; Rlk, 5′-GACTTCGGCATGGCGAGGTA-3′ and 5′-ACTCCGAACGACCAGACATC-3′; Tec, 5′-TATCTGATTTTGGAATGGCC-3′ and 5′-AAACGACCAGACGTCTGACT-3′; cyclophilinA, 5′-AAAGCATACAGGTCCTGG CA-3′and 5′-TCATGCCTTCTTTCACCTTC-3′.
Results
Adaptively tolerant CD4+ T cells can form conjugates with splenic APC
Naïve PCC-specific TCR-transgenic CD4+ T cells express high levels of CD62L and low to intermediate levels of CD44 (Fig. 1A). Ten days following pre-activation with Ag and splenic APCs, 55% of the T cells expressed high levels of CD44 and 67% expressed low levels of CD62L. Adaptively tolerant T cells, representing naïve TCR transgenic cells injected into CD3ε−/− hosts expressing the antigen and isolated several weeks later (see Materials and Methods), had a phenotype more like that of the T cells pre-activated in vitro. Two thirds of them expressed high levels of CD44 and 95% expressed low levels of CD62L (Fig. 1A). Despite their activated phenotype, however, the tolerant T cells failed to produce much IL-2 when stimulated with agonist peptide and splenic APCs (see reference 6).
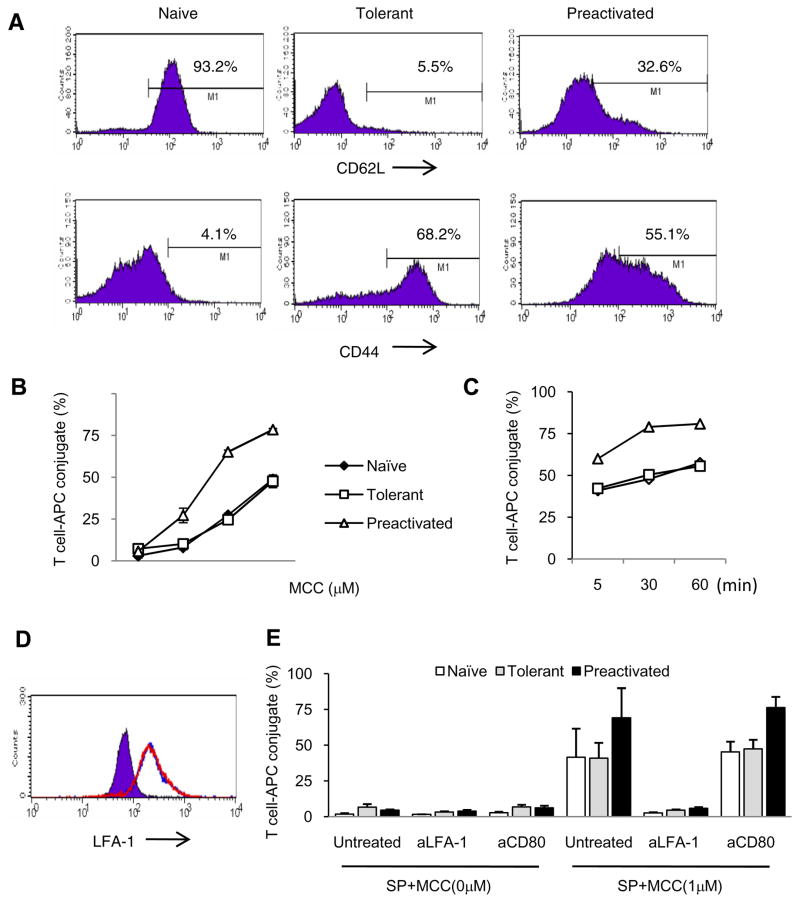
Figure 1. Phenotype and conjugate formation of adaptively tolerant T cells with spleen cells.
A, Purified naïve, adaptively tolerant, or pre-activated 5C.C7 T cells were stained with anti-CD4, anti-Vβ3 and anti-CD62L or anti-CD44 mAb. Expression of CD62L and CD44 on Vβ3+CD4+ T cells is shown. Percentages of CD62Lhigh and CD44high T cells are indicated.
B and C, Splenocytes from B10. A CD3ε−/− mice were pre-pulsed with 0, 0.01, 0.1, or 1μM MCC(88–103) peptide and mixed with naïve (◆), tolerant (□), or pre-activated (Δ) T cells for 30 min (B) or pulsed with 1μM MCC(88–103) peptide and mixed for 5, 30, or 60 min (C). The cells were harvested, fixed and stained with anti-CD4 and anti-MHC class II. The percentages of MHC class II+ cells within the CD4+ T cell population were determined. The data shown in B are the averages from 2 experiments. The data in C are all from one experiment.
D, The expression levels of LFA1 on the surface of CD4+ and Vβ3+ cells from purified naïve (purple), adaptively tolerant (blue line), or pre-activated T cells (red line) were determined by FACS.
E, The splenocytes from B10.A CD3ε−/− mice were pre-pulsed in the presence or absence of 1μM MCC (88–103) peptide and mixed with purified naïve, adaptively tolerant, or pre-activated T cells for 30 min in the presence or absence of anti-LFA1 mAb (1μg / 2 × 105 cells) or anti-CD80 mAb (2μg / 2 × 105 cells). The percentages of CD4+ and MHC class II+ conjugates were determined. The results shown are the means ± SD for three experiments.
Efficient TCR signaling for normal T cell activation requires conjugate formation with an APC. The hyporesponsive state of adaptively tolerant T cells could thus be due to inefficient conjugate formation. To investigate whether tolerant T cells could form conjugates with APCs, we purified the CD4+ transgenic T cells and mixed them with spleen cells from CD3ε −/− mice, with or without the antigen MCC peptide 88–103. Following fixation and flow cytometry analysis, very few conjugates were detected in the absence of antigen, no matter which cell population was examined: naïve, pre-activated, or tolerant T cells (Fig. 1B). Within 5 minutes, however, a significant number of conjugates were detected (Fig. 1C). Tolerant cells formed as many conjugates as naïve T cells, but less than that achieved with the pre-activated T cells. The maximum numbers were achieved around 30 to 60 minutes when 1uM MCC peptide was used (Fig. 1C).
TCR-mediated recognition of peptide/MHC complexes on APCs triggers inside-out signaling, leading to integrin activation and integrin-mediated formation of T cell/APC conjugates. Among a number of molecules, the β2-integrin LFA1 plays an important role in this conjugate formation. Therefore, LFA1 expression on the surface of these T cells was analyzed by FACS. Tolerant and pre-activated T cells expressed 3 fold higher levels of LFA1 than naïve T cells (Fig. 1D). The addition of anti-LFA1 mAb to the cultures nearly completely blocked all conjugate formation in the presence of antigen by naïve, tolerant and pre-activated T cells (Fig. 1E). In contrast, anti-CD80 had no effect. The pretreatment with anti-CD80 also had no effect when the T cells were stimulated with lower concentrations of the peptide (Supplemental Fig. 1). These results show that LFA1/ICAM interactions are the essential component for conjugate formation by all the T cell populations under these circumstances.
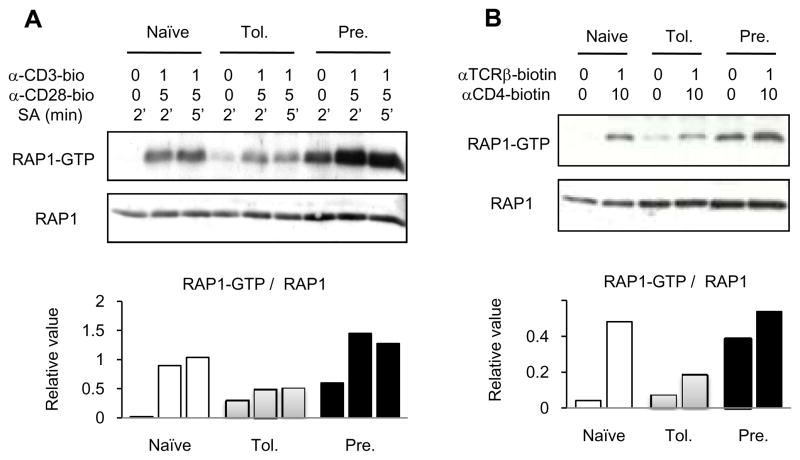
The small GTPase, RAP1, is a signaling molecule that plays a critical role in mediating inside-out signaling to activate β2 integrins. Because the tolerant T cells showed relatively stable LFA1-dependent conjugates with splenic APCs, we thought that the signaling pathways leading to RAP1 activation would be intact in tolerant T cells. To test this, we examined TCR-stimulated RAP1 activation with a pull-down assay using GST-RAL-RBD, which binds specifically to the RAP1-GTP active form. Naïve, resting T cells had very low levels of RAP1-GTP, but antibody activation with anti-CD3 and anti-CD28 (Fig. 2A) or anti-TCR and anti-CD4 (Fig. 2B), induced a substantial increase within 1 to 2 min. Pre-activated, resting T cells, in contrast, already had increased levels of RAP1-GTP, although mAb stimulation elevated this level further. The adaptively tolerant T cells also showed a significant level of RAP1-GTP in the resting state, but less than that seen in the pre-activated, resting T cells. The increase in RAP1-GTP following antibody stimulation, however, was very modest, resulting in levels that were below either the naïve or pre-activated, stimulated T cell populations. This may explain the failure to achieve as high a number of conjugates under maximal activation conditions (Fig. 1B and C) using the adaptively tolerant cells, despite their increased level of LFA1 expression. Nonetheless, we conclude that the adaptively tolerant T cells are capable of forming conjugates to a significant degree.
Figure 2. RAP1 activation in adaptively tolerant T cells.
A and B, Purified naïve, adaptively tolerant, or pre-activated T cells were unstimulated or stimulated for 2 or 5 min with anti-CD3-biotin mAb (1μg/ml) and anti-CD28-biotin mAb (5μg/ml) (A) or for 1 min with anti-TCRβ-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10 μg/ml) (B), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer, and active GTP-bound RAP1 was detected with a pull-down assay using GST-fusion RAL GDS-RBD. Bound RAP1 (upper gels) and total RAP1 (lower gels) were detected by Western blotting with anti-RAP1 Ab. The density of each band was determined using GelPro software. The relative values normalized to the total level of RAP1 expression are shown in the lower figures. The data show one representative experiment from two that were performed.
Adaptively tolerant CD4+ T cells form conjugates with P13.9 APCs
Our initial plan was to next look at the T cell/APC interface for formation of an immunological synapse in tolerant cells. However, we could not obtain very good fluorescent signals with the control naïve or pre-activated T cells when we used splenic APCs. To circumvent this problem, we switched to a fibroblast cell line called P13.9 as the APC. This cell line can present the MCC agonist peptide because P13.9 has been transfected with the MHC class II molecule, I-Ek, as well as the co-stimulatory molecule CD80, and the cell adhesion molecule ICAM1. As shown in Fig. 3A, P13.9 could stimulate naïve and pre-activated T cells to produce IL-2 in a peptide dose-dependent manner, peaking at 1uM peptide (Fig. 3A). In contrast, the amount of IL-2 produced by the tolerant T cell population was 10% of this maximal amount. These results are similar to our previous experiments carried out with splenic APC (6) and thus allowed us to use P13.9 as the APC for our conjugate and synapse formation experiments.
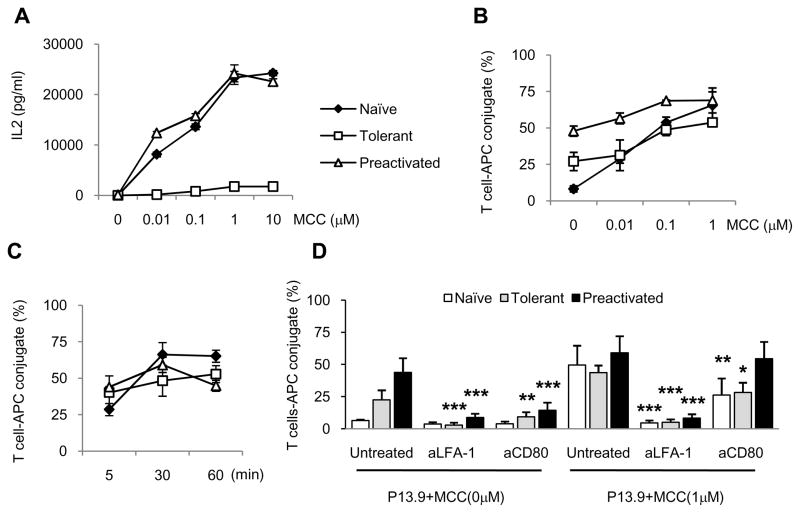
Figure 3. IL2 production and conjugate formation of adaptively tolerant T cells with the P13.9 cell line as APCs.
A, Purified naïve (◆), adaptively tolerant (□), or pre-activated (Δ) T cells were stimulated with various doses of MCC peptide in the presence of irradiated APCs. The P13.9 cell line (MHC-II+, CD80+, ICAM1+) was used as the APC. The supernatant was collected after 38 h of culture, and IL-2 was assayed by ELISA. The data in A are all from one experiment of 2 performed.
B and C, The P13.9 cells were pre-pulsed with the indicated concentrations of MCC(88–103) peptide and the cells mixed with naïve (◆), tolerant (□), or pre-activated (Δ) T cells (B) for 30 min or pulsed with 1μM MCC(88–103) peptide and mixed for 5, 30, or 60 min (C). The percentages of MHC class II+ cells within the CD4+ T cell population were determined. The data shown in B and C are the averages from 2 experiments.
D, The P13.9 cells were pre-pulsed in the presence or absence of 1μM MCC (88–103) peptide and mixed with purified naïve, adaptively tolerant, or pre-activated T cells for 30 min in the presence or absence of anti-LFA1 mAb (1μg / 2 × 105 cells) or anti-CD80 mAb (2μg / 2 × 105 cells). The percentages of CD4+ and MHC class II+ conjugates were determined. The results shown are the means ± SD for three experiments. Values of p were calculated using a two-way ANOVA and a Bonferroni post hoc test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. (the asterisk indicates a significant difference from untreated P13.9 with or without MCC).
When P13.9 cells were used in the conjugate assay, the patterns observed with naïve T cells were similar to those observed with splenic APC. Less than 10% of naïve T cells formed conjugates in the absence of antigen (Fig. 3B and D). Thirty minutes after stimulation with 1μM MCC peptide, however, greater than 50% of these cells were involved in conjugates. The number of conjugates formed depended on the dose of the antigen (Fig. 3B), and their formation was detected as early as 5min after mixing the cells (Fig. 3C). Maximal conjugate formation was achieved with 1μM peptide after 30 minutes of incubation (Fig. 3C). In contrast, many pre-activated T cells (48% ± 4) were able to form conjugates in the absence of antigen. Addition of MCC peptide increased the number of conjugates by about 1.5 fold and this was Ag dose dependent (Fig. 3B). The maximum number formed was achieved at 30 min with a dose of 1μM peptide (Fig. 3C). The number of conjugates formed by the adaptively tolerant T cells in the absence of antigen was also quite significant (27% ± 6), but it was intermediate between that of the naïve and pre-activated T cell populations. In the presence of 1μM peptide for 30 min this increased to 54% ± 3 (Fig. 3B and D). Thus, similar to the results with splenic APC, the tolerant T cells can form conjugates with P13.9 APCs, but only half of these required antigen stimulation to initiate the process.
The significant number of conjugates observed in the absence of antigen with P13.9 APCs appears to be at least partially due to the high expression of CD80 on these cells. Such conjugates could be significantly inhibited with anti-CD80 mAb (Fig. 3D). A similar antibody-mediated inhibition was seen with anti- LFA1 (Fig. 3D), suggesting that both molecules are required. The anti-CD80 inhibition was less prominent after peptide stimulation, but still significant for the naïve and tolerant T cells. LFA1 in contrast was nearly completely inhibitory under all conditions (Fig. 3D). The reason that antigen-independent conjugates are seen only with the pre-activated and tolerant T cells could be because they express 3 fold higher levels of surface LFA1 (Fig. 1D). Imaging of the T cell/APC interface at 5 or 20 min after formation of the conjugates showed an enrichment of LFA1 at the interface (compared to the rest of the plasma membrane) for all three T cell populations (Supplemental Fig. 2). The ratio of the mean signal intensity at the interface relative to the intensity over the rest of the T cell membrane was not significantly different for the preactivated and adaptively tolerant T cells, which express equally high levels of LFA1. In contrast, the naïve cells showed a somewhat larger ratio, because the total signal in these cells was lower as a consequence of their decreased level of LFA1 expression in the membrane.
Translocation of signaling molecules into the contact site is impaired in adaptively tolerant T cells
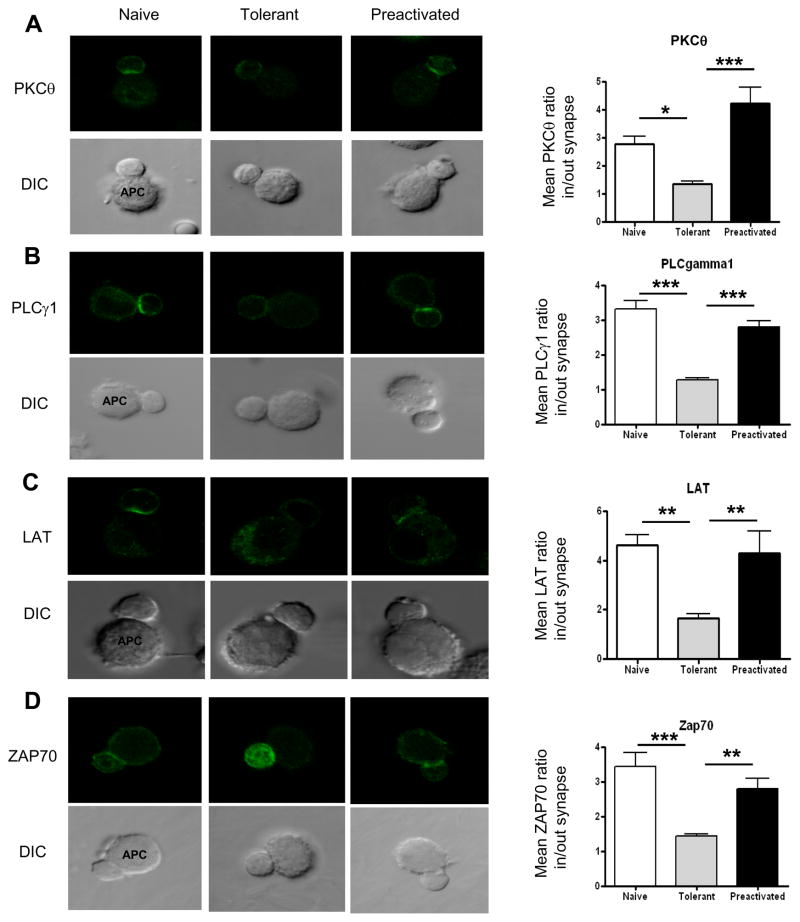
Immunological synapse formation is required for full activation of T cells leading to consequences such as proliferation and IL-2 production. We investigated whether adaptively tolerant T cells could accumulate signaling molecules in their contact site with the APC, which is the hallmark of this process. Purified T cells were initially incubated with MCC peptide-pulsed P13.9 APC and confocal microscopy was used to see the localization of ZAP70, LAT, PLCγ1 and PKC-θ (protein kinase C-theta) at the T cell/APC interface. T cells were mixed with the APCs for 5 min because the extent of conjugate formation was similar at this time point in all 3 populations examined, naïve, pre-activated, and tolerant T cells (Fig. 3C). For these 4 proteins involved in early TCR signal transduction, both naïve and pre-activated T cells showed a polarized pattern of localization at the T cell/APC interface as measured by the ratio of the inside to outside distribution of Ab staining at the synapse (Fig. 4, A-D). In contrast, no polarization was observed for these proteins in the tolerant T cells, although the enhanced expression of ZAP70 in the tolerant T cells (reference 6 and Fig. 6A) made this particular analysis more difficult. Nonetheless, the difference from the naïve or pre-activated T cells was statistically significant for the means of 25 measurements in each of 2 experiments. We conclude that the translocation of ZAP70, LAT, PLCγ-1 and PKC-θ into the T cell-APC contact area after Ag stimulation is impaired in the tolerant T cells.
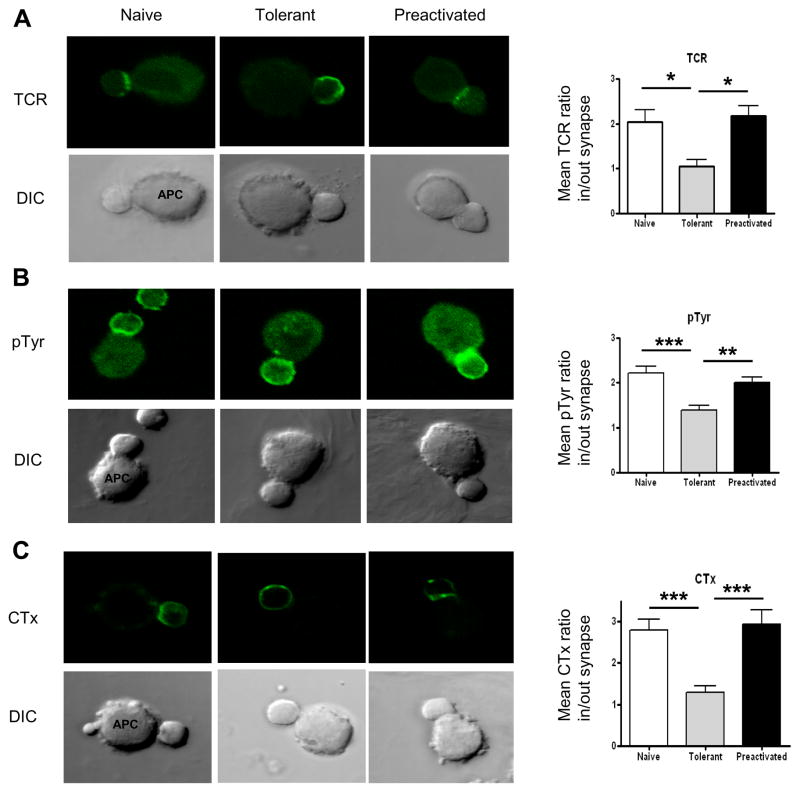
Figure 4. Translocation of signaling molecules to the T cell/APC contact region is greatly impaired in adaptively tolerant T cells.
A, B, C, and D, Purified naïve, adaptively tolerant or pre-activated T cells were stimulated for 5 min with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). Cells were fixed, permeabilized, and stained for PKCθ, PLCγ1, LAT, or ZAP70. Representative images for T cell/APC conjugates and the localization of PLCγ1, LAT, or ZAP70 are shown in the left panels. The localization of PLCγ1, LAT, or ZAP70 was measured with ImageJ software (http://rsb.info.nih.gov/ij/). The means ± SEM of the inside to outside distribution of staining at the synapse for A) PKCθ, B) PLCγ1, C) LAT or D) ZAP70 are shown in the right panels. Twenty five conjugates were counted in each of 2 experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
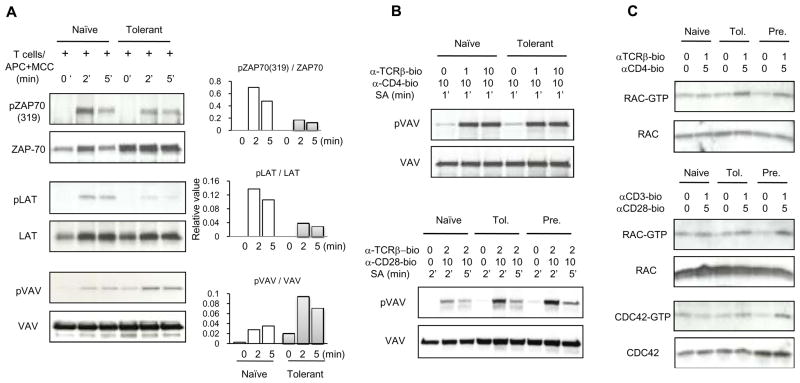
Figure 6. Activation of ZAP70, LAT, VAV1, RAC1 and CDC42 in adaptively tolerant T cells.
A, Purified naïve and adaptively tolerant T cells were stimulated for 2 min or 5 min with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-ZAP70, anti-LAT or anti-VAV1Abs. Western blots were performed and probed with an anti-phospho-ZAP70 mAb specific for the tyrosine phosphorylation site Y319, or an anti-phosphotyrosine mAb, and then reprobed with an anti-ZAP70, anti-LAT or anti-VAV1 mAb. The density of each band was determined using GelPro software. The relative values normalized to the total level of ZAP70, LAT or VAV1 expression are shown in the right panels.
B, Purified naïve, adaptively tolerant or pre-activated T cells were stimulated for 1 min with anti-TCRβ-biotin mAb (0, 1, or 10μg/ml) and anti-CD4-biotin mAb (10μg/ml) or for 2 or 5 min with anti-TCRβ-biotin mAb (2μg/ml) and anti-CD28-biotin mAb (10μg/ml), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-VAV1, and run on SDS-PAGE. Western blots were performed and probed with an anti-phosphotyrosine mAb and then reprobed with anti-VAV mAb. The density of each band was determined using GelPro software.
C, Purified naïve, adaptively tolerant, or pre-activated T cells were stimulated for 2 min with anti-TCRβ-biotin mAb (0 or 1μg/ml) and anti-CD4-biotin mAb (0 or 5μg/ml) or for 2 min with anti-CD3-biotin mAb (0 or 1μg/ml) and anti-CD28-biotin mAb (0 or 5μg/ml), followed by cross-linking with streptavidin. Samples were lysed and active GTP-bound RAC1 or active GTP-bound CDC42 were detected with a pull-down assay using the GST-fusion protein PAK1-PBD. Bound RAC (upper panel) and total RAC (lower panel) were detected by Western blotting with anti-RAC Ab. Bound CDC42 (upper panel) and total CDC42 (lower panel) were detected by Western blotting with anti-CDC42 Ab.
TCR clustering in the immunological synapse is another polarization event that accompanies T cell activation by Ag and APC. As shown in Fig 5A this was observed for naïve and pre-activated T cells, but not for the tolerant T cells. TCR engagement also leads to the phosphorylation of many proteins on tyrosine residues. This can be detected with an anti-phosphotyrosine mAb. In naïve and pre-activated T cells the distribution of these phosphorylated proteins was preferentially polarized over the T cell/APC synapse. In contrast, for the tolerant T cells, no significant polarization was seen. These differences from the naïve and pre-activated T cells were significant. Finally, lipid raft polarization to the T cell/APC contact site was measured using cholera toxin (CTx). CTx specifically binds to the glycosphingolipid GM1, which is enriched in membrane lipid rafts. As shown in Fig 5C, GM1 accumulated in the T cell/APC interface after Ag-induced activation of both naïve and pre-activated T cells, but not at the interfaces of tolerant T cells. This difference was also statistically significant (p< 0.001). Overall these observations demonstrate that the formation of an immunological synapse following Ag-induced activation is greatly impaired in the adaptively tolerant T cells.
Figure 5. Translocation of the TCR, tyrosine-phosphorylated proteins, and lipid rafts to the T cell/APC contact site is impaired in adaptively tolerant T cells.
A and B, Purified naïve, adaptively tolerant or pre-activated T cells were stimulated for 5 min with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). Cells were fixed, permeabilized, and stained for TCRs or tyrosine phosphorylated molecules. Representative images for Tcell/APC conjugates and the localization of TCRs or tyrosine phosphorylated molecules are shown in the left panels. The ratios of the mean number of phosphorylated molecules or TCRs in/out of the synapse were measured with ImageJ software and are shown in the right panels. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
C, Purified naïve, adaptively tolerant, or pre-activated T cells were stained with FITC- CTx and then incubated with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). After 5 min, the cells were fixed. In the left panels, representative images for Tcell/APC conjugates and localization of the lipid rafts are shown. The mean CTx ratios in/out of the synapse were measured with ImageJ software and are shown in the right panel. Thirty conjugates were counted in each of 2 experiments. ***, p < 0.001.
Activation of ZAP70 and LAT, but not activation of VAV1, RAC1, and CDC42 is impaired in adaptively tolerant T cells
Next, we analyzed the biochemical basis for the defective translocation of signaling molecules to the T cell/APC interface of tolerant T cells. ZAP70 activity is required for the recruitment of signaling molecules such as PKC-θ and PLCγ1 to the synapse (52). To examine its activation, we stimulated naïve and tolerant CD4+ T cells with Ag and P13.9 APCs for 2 or 5min. As we reported previously with splenic APCs (6), ZAP70 phosphorylation was decreased in tolerant T cells compared to naïve T cells, when normalized to the total amount of ZAP70 immunoprecipitated (Fig. 6A). Part of this effect, however, was caused by an increase in the level of ZAP70 in the tolerant T cells only. Tyrosine phosphorylation of the scaffolding molecule LAT was also decreased in the tolerant T cells (Fig. 6A) This dramatic effect, however, was not impacted by any change in the level of the protein. Thus, a major block in TCR signaling in tolerant T cells responding to pMHC is at the level of ZAP70 phosphorylation of LAT. VAV, a guanine nucleotide exchange factor for the Rho family GTPases RAC1 and CDC42, is a critical regulator of immunological synapse formation. We investigated its tyrosine phosphorylation in anti-VAV1 immunoprecipitates following antigen/APC stimulation. Interestingly, VAV1 phosphorylation was not decreased in the tolerant T cells compared to naïve T cells (Fig. 6A). In fact its phosphorylation was not reduced in either cell type out to 20 min. (Supplemental Fig. 3B). We also stimulated these cells with anti-TCRβ and anti-CD4 and obtained similar results (Fig. 6B top). When the cells were stimulated with anti-TCRβ and anti-CD28, the tolerant T cells actually showed a slightly increased VAV phosphorylation compared to that of naïve T cells, although it was similar to the amount seen in stimulated pre-activated T cells (Fig. 6B bottom).
We also investigated activation of the downstream GTPases RAC1 and CDC42 by a pull- down assay using GST-PAK1-RBD, which specifically binds to RAC-GTP and CDC42- GTP. When the cells were stimulated with anti-TCRβ and anti-CD4 or anti-CD3 and anti-CD28, tolerant T cells showed an intact activation of RAC1 and CDC42 (Fig. 6C), equivalent to that seen in pre-activated T cells.
F-Actin enrichment and MTOC polarization at the immune synapse are impaired in adaptively tolerant T cells
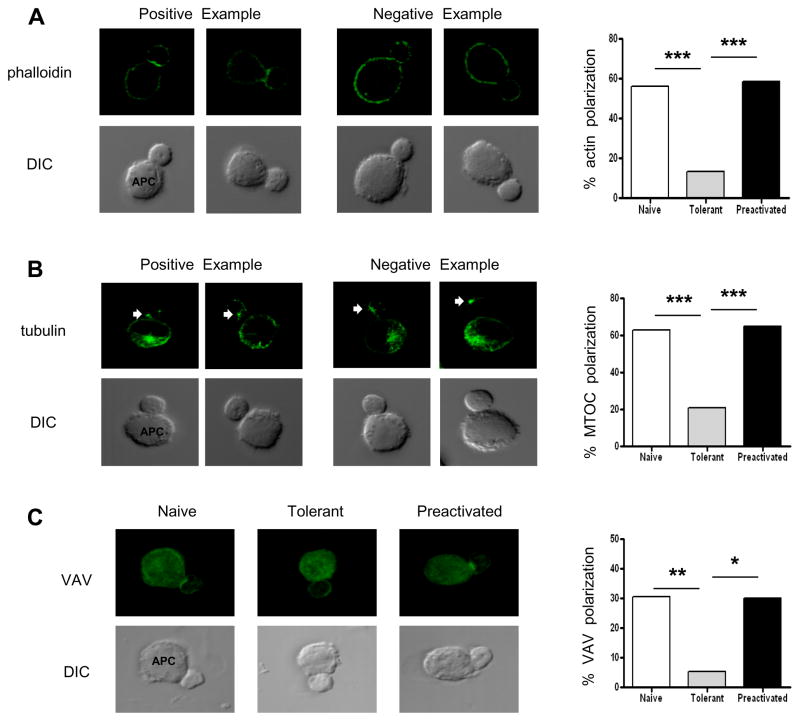
Because tolerant T cells could efficiently form conjugates with APCs and VAV1 signaling seemed to be intact, we wondered whether the enhanced actin polymerization that normally occurs at the immunological synapse would also take place. However, tolerant T cells did not accumulate augmented amounts of F-actin (phalloidin staining) at the synapse 20 minutes following pMHC presentation by P13.9 APCs (Fig. 7A). In contrast, naïve and pre-activated T cells do show this enhancement. Moreover, labeling with anti-tubulin revealed that the tolerant T cells do not polarize their MTOC toward the bound APC as efficiently as either naïve or pre-activated T cells (Fig. 7B). A Chi-square statistical analysis of two experiments showed that the tolerant T cells have impairments in both of these antigen-induced responses.
Figure 7. Actin polymerization at the immune synapse, MTOC polarization toward the synapse, and translocation of VAV to the contact site in adaptively tolerant T cells.
A and B, Purified T cells were conjugated for 20 min with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). Cells were fixed, permeabilized, and stained with Alexa488- phalloidin to visualize F-actin (A) or anti-tubulin to visualize the MTOC (B). Representative images are shown in the left panels for Tcell/APC conjugates revealing the enhancement of F-actin at the immune synapse or the MTOC localized to the proximal third of the T cell facing the synapse. The quantitation of the number of cells in which the F-actin localized to the synapse or of conjugates showing the MTOC localized to the proximal third of the T cell are summarized in the right panels for 2 experiments of 25 and 50 conjugates examined. ***, p < 0.0001 by Chi square analysis.
C, Purified naïve, adaptively tolerant or pre-activated T cells were stimulated for 20 min with the P13.9 cell line pre-pulsed with 1μM MCC(88–103). Cells were fixed, permeabilized, and stained for VAV. Representative images for Tcell/APC conjugates and the localization of VAV are shown in the left panels. The quantitation of the number of cells in which VAV localized to the synapse is summarized in the right panel for 2 experiments of 25 and 30 conjugates examined. *, p < 0.001; **, p < 0.0005 by Chi square analysis.
VAV synapse localization is impaired in adaptively tolerant T cells
In TCR-induced T cell activation, VAV plays a major role in the initiation of actin polymerization during the formation of the immunological synapse. Since VAV1 phosphorylation was intact in the tolerant T cells, but the enhancement of F-actin at the synapse did not occur, we investigated whether instead VAV1 concentration into the synapse was what was impaired in these cells. Although the background level of staining with anti-VAV1 in P13.9 cells was somewhat high, a careful examination of the interface showed that the tolerant T cells were indeed defective in VAV1 accumulation in the synapse (Fig. 7C). This correlated well with the lack of enhanced actin polymerization (Fig. 7A). Thus, in the adaptively tolerant T cells, improper VAV1 localization seems to contribute to the impaired immunological synapse formation in spite of VAV1’s intact phosphorylation.
ITK and GADS are down-regulated in adaptively tolerant T cells
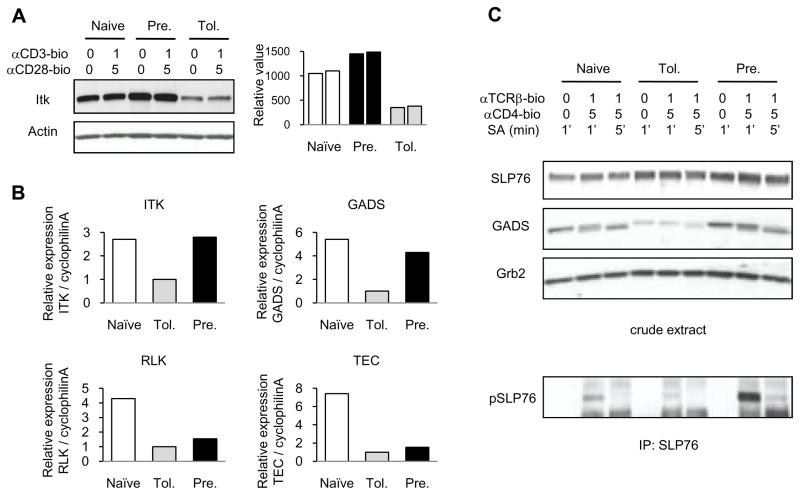
ITK has been reported to be critical for recruiting VAV into the immunological synapse (53). ITK-deficient mice show impaired immune synapse formation, although VAV1 activation is not impaired. Interestingly, ITK protein levels in adaptively tolerant T cells were down-regulated about 3 fold (Fig. 8A). Real-time quantitative PCR analysis showed that these T cells also had significantly lower levels (3 fold) of mRNA encoding ITK (Fig. 8B). We also examined the other TEC kinases (RLK and TEC) by real-time quantitative PCR analysis. The levels of mRNA encoding RLK and TEC were substantially down-regulated in both adaptively tolerant T cells and pre-activated T cells (Fig. 8B). Thus, ITK is the only family member that is uniquely down-regulated in adaptively tolerant T cells.
Figure 8. GADS and ITK expression and SLP76 activation in adaptively tolerant T cells.
A, Purified naïve, adaptively tolerant, or pre-activated T cells were stimulated for 2 min with anti-CD3-biotin mAb (0 or 1μg/ml) and anti-CD28-biotin mAb (0 or 5μg/ml) followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and run on SDS-PAGE. Western blots were performed and probed with an anti-ITK mAb and then reprobed with an anti-actin mAb. The relative values normalized to the total level of actin expression are shown in the right panel.
B, Real-time quantitative RT-PCR analyses of expression of genes encoding Itk, Gads, Rlk and Tec is presented relative to the expression of cyclophilin A in naïve, adaptively tolerant, or pre-activated T cells.
C, Purified naïve, adaptively tolerant, or pre-activated T cells were stimulated for 1 or 5 min with anti-TCRβ-biotin mAb (0 or 1μg/ml) and anti-CD4-biotin mAb (0 or 5μg/ml), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and the crude lysate run on SDS-PAGE and immunoblotted with anti-SLP76 Ab, anti-GADS Ab or anti-Grb2 mAb. The lysate was also immunoprecipitated with anti-SLP-76 and run on SDS-PAGE. Western blots were performed and probed with an anti-phosphotyrosine mAb (lowest panel). This experiment was performed 3 times.
ITK is normally recruited to the TCR-signaling complex by SLP76 following its phosphorylation by activated ZAP70. SLP76 in turn is brought to the complex by GADS, which recognizes ZAP70-phosphorylated tyrosines on LAT. In adaptively tolerant T cells, we found that SLP76 levels were if anything slightly elevated, but that GADS levels were down about 2–3 fold (Fig. 8C). The decrease in GADS was also observed at the mRNA level (Fig. 8B). This combined with the inhibition of LAT phosphorylation greatly reduced the phosphorylation of SLP76 in adaptively tolerant T cells, presumably by preventing SLP76 recruitment to the TCR signaling complex (Fig. 8C). Thus, defective ITK and GADS expression, in addition to the impaired LAT phosphorylation by ZAP70, appear to be the major impediments to VAV1 recruitment to the T cell/APC interface in adaptively tolerant T cells. These defects would also contribute to the impaired phosphorylation of PLCγ1 we noted previously in adaptively tolerant T cells (6).
Discussion
The development of an immune synapse between T cells and APCs is a key step in the events leading to full T cell activation. When T cells interact with APCs, their F-actin and signaling molecules are enriched in the specialized junction between the T lymphocyte and the APC, which consists of a central cluster of T cell receptors surrounded by a ring of adhesion molecules. Actin polymerization at the immune synapse stabilizes conjugate formation and facilitates T cell activation (54). Ise et al. (55) have shown that orally tolerant T cells can form conjugates with APCs, but that they are defective in immunological synapse formation. Their results are consistent with ours in that orally tolerant T cells could not translocate TCR, PKC-θ, or lipid rafts to the T cell/APC contact site. Heissmeyer et al. (34) also demonstrated that immunological synapse formation by in vitro-anergized T cells induced with ionomycin treatment was unstable. Their observations showed by live cell imaging that the synapses formed normally at early time points after incubation on lipid bilayers, but that at later time points these broke down. Such an analysis remains to be carried out in our adaptive tolerance model.
Integrin-mediated adhesion is essential for the formation of stable contacts between T cells and APCs. RAP1 in T cells is a critical activator of these integrins and plays an essential role in LFA1-mediated interaction with ICAMs on the APCs (17). Adaptively tolerant T cells showed only modest activation of RAP1 upon TCR stimulation, although the basal activity of RAP1 was increased. The almost normal conjugate formation that we observed may be due to the combination of this modest activation of RAP1 and the increased expression of LFA1 (3 fold) observed on the adaptively tolerant T cells. In other models, VAV1 has been shown to be required for integrin-mediated adhesion of T cells to peptide-loaded APCs (56). The guanine nucleotide exchange factor (GEF) activity of VAV1 was required for conjugate formation and to a lesser extent for integrin activation (57). However, whether VAV recruitment into the synapse is required for the integrin-mediated adhesion of the T cells to peptide-loaded APCs was unclear in these studies. Our data would suggest that VAV activation without enrichment into the cSMAC is sufficient for conjugate formation.
Nonetheless, VAV normally does play an important role in immune synapse formation, TCR capping and lipid raft clustering into the immune synapse (56, 58). Interestingly, tyrosine phosphorylation of VAV was not impaired in adaptively tolerant T cells and in fact was sometimes enhanced following stimulation with Ag-APC or anti-TCR/CD28. In addition, this led to normal activation of RAC1 and CDC42. Because Cbl-b deficient T cells also show enhanced tyrosine phosphorylation of VAV (59), Cbl-b levels were investigated in adaptively tolerant T cells. However, similar to other models of anergy (60), cbl-b was found to be upregulated instead of down-regulated (S. Choi and R. H. Schwartz, unpublished observations). VAV1 can be tyrosine phosphorylated by Lck in vitro (44). Fyn also plays a major role in controlling VAV1 phosphorylation after stimulation through the TCR and CD28 (45). Our studies show that VAV phosphorylation requires Src family kinase activity in both normal and adaptively tolerant T cells as we could completely inhibit the tyrosine phosphorylation with the SRC-family kinase inhibitor PP2 (Supplemental Fig. 3A and Supplemental Fig. 4A and B). We have shown previously that LCK activity is only marginally decreased in adaptively tolerant T cells, and that FYN activity is dramatically increased (6). This enhanced SRC-family kinase activity might be responsible for the increase in VAV1 phosphorylation that we sometimes observed. In other cells this phosphorylation event has been reported to require phosphotidylinositol 3-kinase (PI3-K)-generated lipid products (44) in order to recruit VAV to the plasma membrane via its pleckstrin homology domain. Furthermore, VAV tyrosine phosphorylation upon costimulation of the T cell with anti-CD3/CD28 has been reported to be PI3-K dependent (61). In our hands, however, pretreatment with PI3-K inhibitors did not reduce VAV tyrosine phosphorylation following stimulation of naïve transgenic CD4+ T cells with either anti-TCR/CD4 or anti-CD3/CD28 (Supplemental Fig. 4A) or with MCC peptide-prepulsed APC (Supplemental Fig. 3A), even though the downstream effector of PI3-K, AKT, and the downstream effector of AKT, GSK3α/β, were both normally phosphorylated upon anti-CD3/CD28 stimulation, in adaptively tolerant T cells (Supplemental Fig. 4C). Thus, we think that the major pathway for VAV tyrosine phosphorylation is through SRC-family kinases and that VAV must get to the plasma membrane by some other mechanism.
Despite an intact activation pathway for VAV, RAC1, and CDC42 in adaptively tolerant T cells, it was not sufficient to allow VAV1 to be recruited to the immunological synapse (Fig. 7C and D). What then is missing? Surprisingly, adaptively tolerant T cells showed decreased expression of ITK at both the mRNA and protein levels. ITK plays an important role in actin polymerization and in VAV recruitment to the immune synapse (62). It also plays a key role in regulating TCR-mediated polarization of integrins and signaling molecules to the site of TCR stimulation, as well as the up-regulation of integrin adhesion (63). Like our results, T cells from ITK−/− mice show normal tyrosine phosphorylation of VAV (62, 64). Similarly, loss of ITK expression by siRNA knock down did not alter the pattern of VAV tyrosine phosphorylation, but instead disrupted the interaction of VAV with SLP76 (53). SLP76 is present in normal amounts in adaptively tolerant T cells; however, interestingly, GADs levels were significantly diminished along with ITK. GADs is a linker protein required to bring SLP76 to the TCR activation complex in the plasma membrane by binding to tyrosine phosphorylation sites on LAT via an SH2 domain interaction (65). LAT phosphorylation, however, is greatly impaired in adaptively tolerant T cells (Fig. 6A), reducing the number of active binding sites for the limited amount of GADs in the cell to find. This combination of negative effects leads to an impairment in SLP76 recruitment to the TCR activation complex. As a consequence, SLP76 cannot be phosphorylated by ZAP70, and this impairs its ability to recruit the limited amount of ITK in the cell to the TCR activation complex. Without ITK mobilization, PLCγ1 is not optimally phosphorylated, leading to an impairment in PIP2 hydrolysis and a failure to generate adequate amounts of the second messengers IP3 and diacylglycerol (DAG) (66). In addition, the curtailment of ITK binding limits the recruitment of VAV into the immunological synapse.
As a consequence of these signaling defects, we found that enhancement of actin polymerization at and MTOC polarization toward the T cell/APC contact area were greatly impaired in adaptively tolerant T cells. The actin cytoskeleton is critical for T cell signaling and normal T cell-APC conjugate formation (67). VAV1 regulates this actin polymerization (68) and thus its reduced presence in the synapse would impair F-actin accumulation in this part of the cell. MTOC polarization is controlled by ZAP70 kinase activity (52). Recently, Quann et al. (69) have shown that this polarization is driven by localized accumulation of DAG. Our previous experiments (6) showed an impairment in ZAP70 kinase activity and a profound defect in PLCγ1 phosphorylation in adaptively tolerant T cells. This PLCγ1 defect may result in a limited production of DAG, which could cause the impairment of MTOC polarization seen in these cells.
In this paper, we have shown that adaptively tolerant T cells retain the ability to form conjugates with APC, but are defective in translocation of TCR signaling molecules into the contact site. The tolerant T cells showed both increased LFA1 expression levels and increased basal RAP1 activity compared to naïve T cells, even though they could only modestly activate RAP1 upon TCR stimulation. These alterations were adequate for initial conjugate formation. In addition, Ag-induced activation of VAV1 was not impaired, only its accumulation into the immunological synapse. ITK has been previously reported to facilitate this recruitment process and interestingly ITK levels were down-regulated in adaptively tolerant T cells. Also, ITK is normally recruited to the TCR-signaling complex by SLP76 following its phosphorylation by activated ZAP70. SLP76 is brought to the complex by GADs, which recognizes ZAP70-phosphorylated tyrosines on LAT. In adaptively tolerant T cells we found that SLP76 levels were normal, but that SLP76 phosphorylation and GADs levels were down-regulated. Thus, defective ITK and GADs expression, in addition to impaired tyrosine phosphorylation of LAT by the ZAP70 kinase, appear to be the major impediments to VAV1 recruitment for enabling formation of a stable immunological synapse.
Supplementary Material
Supplemental Figure 1. The effect of CD80 on the T cell-primary APC conjugation with low dose concentration of the peptide.
The splenocytes from B10. A CD3ε−/− mice were pre-pulsed in the presence or absence of 0.05μM MCC (88–103) peptide and mixed with purified naïve, adaptively tolerant, or pre-activated T cells for 30 min in the presence or absence of anti-LFA1 mAb (1μg / 2 × 105 cells) or anti-CD80 mAb (2μg / 2 × 105 cells). The percentages of CD4+ and MHC class II+ conjugates were determined. The bars represent the means ± SD for triplicate determinations.
Supplemental Figure 2. Enrichment of LFA-1 at the T cell/APC contact site.
Purified naïve, adaptively tolerant and preactivated T cells were stimulated for 20 min with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). Cells were fixed, permeabilized, and stained for LFA-1. Representative images for T cell/APC conjugates and the localization of LFA-1 molecules are shown in the left panels. The mean LFA-1 ratios in/out of the synapse were measured with ImageJ software and are shown in the right panel. Thirty conjugates were counted in total from 2 experiments. ***, p < 0.001.
Supplemental Figure 3. The effect on VAV1 phosphorylation of a PI3K inhibitor or a SRC-family kinase inhibitor, and the kinetics of VAV1 phosphorylation following stimulation with APC+Ag.
A, The P13.9 cells were pre-pulsed with 1μM MCC(88–103) peptide. Purified naïve and adaptively tolerant T cells were pretreated for 30 min with DMSO (D), 100μM of LY294002 (L), 100μM of PP3 (3) or 100μM of PP2 (2). Pretreated T cells were stimulated for 2 min with the pre-pulsed P13.9 cell line. Samples were lysed with 1% NP40 buffer and immunoprecipitated with an anti-VAV1 Ab. Western blotting was performed and probed with an anti-phosphotyrosine mAb, and then reprobed with an anti-VAV1 mAb. The crude lysate was used for immunoblotting with an anti-phospho-LAT Ab specific for the tyrosine phosphorylation site Y191, and then reprobed with an anti-LAT Ab.
B, Purified naïve and adaptively tolerant T cells were stimulated for 5 min, 10 min or 20 min with the P13.9 cell line pre-pulsed with 1μM MCC(88–103) peptide. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-VAV1 or anti-ZAP70 Abs. Western blots were performed and probed with anti-phosphotyrosine mAb, and then reprobed with an anti-VAV1mAb or an anti-ZAP70 mAb.
Supplemental Figure 4. The effect on VAV1 phosphorylation of a PI3K inhibitor or a SRC-family kinase inhibitor in the presence of an intact activation of the AKT pathway in adaptively tolerant T cells.
A, Purified naïve T cells were pretreated for 30 min with DMSO (D), 10μM of LY294002 (L), 10μM of PP3 (3) or 10μM of PP2 (2). Pretreated T cells were stimulated for 2 min with anti-TCR-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10μg/ml) or for 2 min with anti-CD3-biotin mAb (1μg/ml) and anti-CD28-biotin mAb (5μg/ml), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-VAV1, and run on SDS-PAGE. Western blots were performed and probed with an anti-phosphotyrosine mAb and then reprobed with anti-VAV mAb. The crude lysate was used for immunoblotting with an anti-phospho-ZAP70mAb specific for the tyrosine phosphorylation site Y319, and then reprobed with an anti-ZAP70, or an anti-phospho-AKTmAb.
B, Purified naïve T cells were pretreated for 30 min with 50μM of PP3 (3) or PP2 (2). Pretreated T cells were stimulated for 2 min with anti-TCR-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10μg/ml) followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and immunoprecipitated with anti-VAV1. Western blots were performed and probed with an anti-phosphotyrosine mAb and then reprobed with anti-VAV mAb. The crude lysate was used for immunoblotting with anti-phospho-LAT Ab specific for the tyrosine phosphorylation site Y191, and then reprobed with an anti-LAT Ab.
C, Purified naïve or adaptively tolerant T cells were stimulated for 2, 5 or 20 min with anti-CD3-biotin mAb (1μg/ml) and anti-CD28-biotin mAb (5μg/ml), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and run on SDS-PAGE. Western blots were performed and probed with an anti-phospho-AKT mAb, anti-phospho-GSK3α/β Ab or anti actin mAb.
Acknowledgments
We thank members of the NIAID Biological Imaging facility for technical help in these experiments and Jeff Skinner for statistical analysis. We are grateful to Drs. Nevil Singh, Rajat Varma, and Pascal Chappert for critical reading of this manuscript and helpful suggestions and discussion. In addition, we thank Dr. Irena Stefanova for providing the P13.9 cell line and Dr. Pam Schwartzberg for helpful comments on these experiments.
Abbreviations used in this paper
- MCC
moth cytochrome c
- SMAC
supramolecular activation cluster
- CTx
cholera toxin
- MTOC
microtubule organizing center
- PKCθ
protein kinase C theta
- LAT
linker for activation of T cells
- ITK
interleukin-2-inducible T-cell kinase
- GADS
growth-factor- receptor-bound protein-2-related adaptor downstream of SHC
- PLCγ1
phospholipase C γ1
- SLP76
SRC homology-2-domain- containing leukocyte protein of 76 kDa
Footnotes
This work was supported by the National Institutes of Health Intramural Research Program.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983;157:1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–309. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 4.Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Seminars in Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 6.Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol. 2006;176:2279–2291. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–2039. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- 9.Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zell T, Kivens WJ, Kellermann SA, Shimizu Y. Regulation of integrin function by T cell activation: points of convergence and divergence. Immunol Res. 1999;20:127–145. doi: 10.1007/BF02786469. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naïve T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 12.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 14.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nature Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 15.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 16.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–258. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 18.Ardouin L, Bracke M, Mathiot A, Pagakis SN, Norton T, Hogg N, Tybulewicz VL. VAV1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur J Immunol. 2003;33:790–797. doi: 10.1002/eji.200323858. [DOI] [PubMed] [Google Scholar]
- 19.Katagiri K, Shimonaka M, Kinashi T. RAP1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-γ1. J Biol Chem. 2004;279:11875–11881. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, Billadeau DD, Shimizu Y. Protein kinase D1 and the 1 integrin cytoplasmic domain control β1 integrin function via regulation of RAP1 activation. Immunity. 2005;23:213–226. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 22.Lafuente EM, van Puijenbroek AAFL, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertier FB, Boussiotis VA. RIAM, an Ena/VASP and profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase MST1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nature Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaudhlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu QH, Pribila JT, Freedman BD, et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths EK, Krawczyk C, Kong YY, Raab M, Hyduk SJ, Bouchard D, Chan VS, Kozieradzki I, Oliveira-Dos-Santos AJ, Wakeham A, et al. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293:2260–2263. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Moon EY, Azouz A, Wu X, Smith A, Schneider H, Hogg N, Rudd CE. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat Immunol. 2003;4:366–374. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 28.Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of RAP1. Mol Cell Biol. 2006;26:7130–7144. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 30.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 31.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 32.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 33.Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK, Shaw AS. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 34.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 35.Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 36.Tybulewicz VL. VAV-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nature Rev Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 38.Costello PS, Walters AE, Mee PJ, Turner M, Reynolds LF, Prisco A, Sarner N, Zamoyska R, Tybulewicz VL. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc Natl Acad Sci USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, Billadeau DD, Burkhardt JK. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, Heldebrant MP, Labno CM, McKean DJ, McNiven MA, Burkhardt JK, Billadeau DD. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 41.Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. SLP-76 coordinates NCK-dependent Wiskott–Aldrich syndrome protein recruitment with VAV-1/CDC42-dependent Wiskott–Aldrich syndrome protein activation at the T cell–APC contact site. J Immunol. 2003;171:1360–1368. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 42.Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM. Role for the ABI/WAVE protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 43.Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Kristina UM, Falck JR, White MA, Broek D. Role od substrates and products of PI3-kinase in regulating activation of rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 45.Michel F, Grimaud L, Tuosto L, Acuto O. Fyn and ZAP-70 are required for Vav phosphorylation in T cells stimulated by antigen-presenting cells. J Biol Chem. 1998;273:31932–31938. doi: 10.1074/jbc.273.48.31932. [DOI] [PubMed] [Google Scholar]
- 46.Kim HH, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J Biol Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- 47.Turner M, Billadeau DD. Vav proteins as signal integrators for multi- subunit immune-recognition receptors. Nature Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 48.Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–2766. [PubMed] [Google Scholar]
- 49.Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 50.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inobe M, Schwartz RH. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J Immunol. 2004;173:7239–7248. doi: 10.4049/jimmunol.173.12.7239. [DOI] [PubMed] [Google Scholar]
- 52.Blanchard N, Di Bartolo V, Hivroz C. In the immune synapse, Zap-70 controls T cell polarization and recruitment of signaling proteins but not formation of the synaptic pattern. Immunity. 2002;17:389–399. doi: 10.1016/s1074-7613(02)00421-1. [DOI] [PubMed] [Google Scholar]
- 53.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. Kinase-independent functions for Itk in TCR-induced regulation of vav and the actin cytoskeleton. J Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 54.Fuller CL, V, Braciale L, Samelson LE. All roads lead to actin: the intimate relationship between TCR signaling and the cytoskeleton. Immunol Rev. 2003;191:220–236. doi: 10.1034/j.1600-065x.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- 55.Ise W, Nakamura K, Shimizu N, Goto H, Fujimoto K, Kaminogawa S, Hachimura S. Orally tolerized T cells can form conjugates with APCs but are defective in immunological synapse formation. J Immunol. 2005;175:829–838. doi: 10.4049/jimmunol.175.2.829. [DOI] [PubMed] [Google Scholar]
- 56.Krawczyk C, Oliveira-dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- 57.Saveliev A, Vanes L, Ksionda O, Rapley J, Smerdon SJ, Rittinger K, Tybulewicz VLJ. Function of the nucleotide exchange activity of Vav1 in T cell development and activation. Sci Signal. 2009;2:ra83. doi: 10.1126/scisignal.2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villalba M, Bi K, Rodriguez F, Tanaka Y, Schoenberger S, Altman A. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol. 2001;155:331–338. doi: 10.1083/jcb.200107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of the T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 60.Macián F, García-Cózar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 61.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by cbl-b- mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 62.Labno CM, Lewis CM, You D, Leung DW, Takesono A, Kamberos N, Seth A, Finkelstein LD, Rosen MK, Schwartzberg PL, Burkhardt JK. Itk functions to control actin polymerization at the immune synapse through localized activation of cdc42 and WASP. Curr Biol. 2003;13:1619–1624. doi: 10.1016/j.cub.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finkelstein LD, Shimizu Y, Schwartzberg PL. Tec kinase regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J Immunol. 2005;175:5923–5930. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- 64.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Law CL, Ewings MK, Chaudhary PM, Solow SA, Yun TJ, Marshall AJ, Hood L, Clark EA. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J Exp Med. 1999;189:1243–53. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 67.Cannon JL, Burkhardt JK. The regulation of actin remodeling during T- cell-APC conjugate formation. Immunol Rev. 2002;186:90–99. doi: 10.1034/j.1600-065x.2002.18609.x. [DOI] [PubMed] [Google Scholar]
- 68.Fischer KD, Kong YY, Nishina H, Tedford K, Marengère LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 69.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The effect of CD80 on the T cell-primary APC conjugation with low dose concentration of the peptide.
The splenocytes from B10. A CD3ε−/− mice were pre-pulsed in the presence or absence of 0.05μM MCC (88–103) peptide and mixed with purified naïve, adaptively tolerant, or pre-activated T cells for 30 min in the presence or absence of anti-LFA1 mAb (1μg / 2 × 105 cells) or anti-CD80 mAb (2μg / 2 × 105 cells). The percentages of CD4+ and MHC class II+ conjugates were determined. The bars represent the means ± SD for triplicate determinations.
Supplemental Figure 2. Enrichment of LFA-1 at the T cell/APC contact site.
Purified naïve, adaptively tolerant and preactivated T cells were stimulated for 20 min with the P13.9 cell line pre-pulsed with 1μM MCC (88–103). Cells were fixed, permeabilized, and stained for LFA-1. Representative images for T cell/APC conjugates and the localization of LFA-1 molecules are shown in the left panels. The mean LFA-1 ratios in/out of the synapse were measured with ImageJ software and are shown in the right panel. Thirty conjugates were counted in total from 2 experiments. ***, p < 0.001.
Supplemental Figure 3. The effect on VAV1 phosphorylation of a PI3K inhibitor or a SRC-family kinase inhibitor, and the kinetics of VAV1 phosphorylation following stimulation with APC+Ag.
A, The P13.9 cells were pre-pulsed with 1μM MCC(88–103) peptide. Purified naïve and adaptively tolerant T cells were pretreated for 30 min with DMSO (D), 100μM of LY294002 (L), 100μM of PP3 (3) or 100μM of PP2 (2). Pretreated T cells were stimulated for 2 min with the pre-pulsed P13.9 cell line. Samples were lysed with 1% NP40 buffer and immunoprecipitated with an anti-VAV1 Ab. Western blotting was performed and probed with an anti-phosphotyrosine mAb, and then reprobed with an anti-VAV1 mAb. The crude lysate was used for immunoblotting with an anti-phospho-LAT Ab specific for the tyrosine phosphorylation site Y191, and then reprobed with an anti-LAT Ab.
B, Purified naïve and adaptively tolerant T cells were stimulated for 5 min, 10 min or 20 min with the P13.9 cell line pre-pulsed with 1μM MCC(88–103) peptide. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-VAV1 or anti-ZAP70 Abs. Western blots were performed and probed with anti-phosphotyrosine mAb, and then reprobed with an anti-VAV1mAb or an anti-ZAP70 mAb.
Supplemental Figure 4. The effect on VAV1 phosphorylation of a PI3K inhibitor or a SRC-family kinase inhibitor in the presence of an intact activation of the AKT pathway in adaptively tolerant T cells.
A, Purified naïve T cells were pretreated for 30 min with DMSO (D), 10μM of LY294002 (L), 10μM of PP3 (3) or 10μM of PP2 (2). Pretreated T cells were stimulated for 2 min with anti-TCR-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10μg/ml) or for 2 min with anti-CD3-biotin mAb (1μg/ml) and anti-CD28-biotin mAb (5μg/ml), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer, immunoprecipitated with anti-VAV1, and run on SDS-PAGE. Western blots were performed and probed with an anti-phosphotyrosine mAb and then reprobed with anti-VAV mAb. The crude lysate was used for immunoblotting with an anti-phospho-ZAP70mAb specific for the tyrosine phosphorylation site Y319, and then reprobed with an anti-ZAP70, or an anti-phospho-AKTmAb.
B, Purified naïve T cells were pretreated for 30 min with 50μM of PP3 (3) or PP2 (2). Pretreated T cells were stimulated for 2 min with anti-TCR-biotin mAb (1μg/ml) and anti-CD4-biotin mAb (10μg/ml) followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and immunoprecipitated with anti-VAV1. Western blots were performed and probed with an anti-phosphotyrosine mAb and then reprobed with anti-VAV mAb. The crude lysate was used for immunoblotting with anti-phospho-LAT Ab specific for the tyrosine phosphorylation site Y191, and then reprobed with an anti-LAT Ab.
C, Purified naïve or adaptively tolerant T cells were stimulated for 2, 5 or 20 min with anti-CD3-biotin mAb (1μg/ml) and anti-CD28-biotin mAb (5μg/ml), followed by cross-linking with streptavidin. Samples were lysed with 1% NP40 buffer and run on SDS-PAGE. Western blots were performed and probed with an anti-phospho-AKT mAb, anti-phospho-GSK3α/β Ab or anti actin mAb.