Abstract
Background
Excessive alcohol intake causes an increase in intestinal permeability that induces translocation of gut-derived lipopolysaccharide (LPS) to the portal vein. Increased LPS in the portal vein stimulates Kupffer cells through Toll-like receptor (TLR) 4 in the liver. Activated TLR4 signaling in Kupffer cells induces various inflammatory mediators including TNF-α, IL-1β and reactive oxygen species, resulting in liver injury. Hepatic stellate cells (HSCs) also express TLR4. This study investigates whether TLR4 on bone marrow (BM)-derived cells, including Kupffer cells, or non-BM-derived endogenous liver cells, including HSCs, contributes to the progression of alcohol-induced steatohepatitis and fibrogenesis in mice.
Method
TLR4 BM chimera (wild type (WT) mice with TLR4-/- BM or TLR4-/- mice with WT BM) were generated by the combination of liposomal clodronate injection with whole body irradiation and BM transplantation (BMT), followed by treatment with intragastric alcohol feeding.
Results
WT mice transplanted with WT BM exhibited liver injury, steatosis, inflammation and a fibrogenic response. Conversely, TLR4-/- mice with TLR4-/- BM displayed less steatosis, liver injury and inflammation. Notably, steatosis, macrophage infiltration and ALT levels in both TLR4 chimeric mice showed intermediate levels between WT mice transplanted with WT BM and TLR4-/- mice transplanted with TLR4-/- BM. Hepatic mRNA expression of fibrogenic markers (collagen α1(I), TIMP1, TGF-β1) and inflammatory cytokines (IL-1β, IL-6) were markedly increased in WT mice with WT BM, but there was less of an increase in both TLR4-chimeric mice and in TLR4-/- mice transplanted with TLR4-/- BM.
Conclusion
TLR4 signaling in both BM-derived and non-BM-derived liver cells is required for liver steatosis, inflammation, and a fibrogenic response after chronic alcohol treatment.
Keywords: innate immunity, LPS, alcoholic steatohepatitis, alcoholic liver fibrosis, Tsukamoto-French
Introduction
Chronic alcohol abuse is a major cause of cirrhosis and liver failure in adult patients in the United States (Diehl, 1997). Alcohol-induced liver cirrhosis is characterized by hepatocyte steatosis, ballooning, apoptosis, inflammatory cell infiltration, excessive deposition of extra cellular matrix (ECM) proteins and the appearance of regenerative nodules. These pathological changes produce hepatocellular dysfunction, increased intrahepatic resistance to blood flow and hepatocyte dysplasia, resulting in liver failure, portal hypertension and hepatocellular carcinoma (HCC), respectively (Adachi and Brenner, 2005; Bataller and Brenner, 2005; Diehl, 1997; Ramaiah et al., 2004). Excessive intake of ethanol induces changes in the composition of enteric microflora, the overgrowth of Gram-negative bacteria, and disrupts the intestinal epithelial barrier. These changes result in increased intestinal permeability, and translocation of bacterial lipopolysaccharide (LPS) to the portal vein. The LPS delivered into the liver activates Toll-like receptor (TLR) 4 signaling in Kupffer cells to produce inflammatory mediators, resulting in liver injury (Enomoto et al., 2000; Thakur et al., 2007; Thurman, 1998; Uesugi et al., 2001; Uesugi et al., 2002; Yan et al., 2010). In patients with alcoholic liver cirrhosis, LPS levels in the portal vein are elevated (Szabo and Bala). A continuous intragastric alcohol-feeding model in rodents demonstrates comparable histological findings with human alcoholic liver disease (Tsukamoto et al., 2008; Xiong et al., 2008). Increased plasma LPS levels in this rodent model correlate with the grade of histology of liver injury (Thurman, 1998; Tsukamoto et al., 2008; Xiong et al., 2008).
Mice with inactive mutant TLR4 or with inactivation of Kupffer cells are protected from alcohol-induced liver injury (Enomoto et al., 2000; Hritz et al., 2008; Uesugi et al., 2001). However, the direct relationship between TLR4 and Kupffer cells in alcohol-induced liver injury is unknown. Hepatic stellate cells (HSCs) are the major source of ECM protein, including collagen type I, III and IV in the fibrotic liver (Bataller and Brenner, 2005; Seki et al., 2007). Our previous study demonstrated that TLR4 on HSCs, but not on Kupffer cells or hepatocytes, is crucial for various murine models of liver fibrosis (Bataller and Brenner, 2005; Seki et al., 2007). In HSCs, TLR4 signaling enhances TGF-β signaling by down-regulating Bambi, a transmembrane inhibitor of TGF-β (Seki et al., 2007), which is required for liver fibrosis (Hellerbrand et al., 1999). Currently, the role of TLR4 on HSCs during alcohol-induced steatohepatitis and fibrogenesis is unknown. TLR4 signaling also induces various chemokines (CCL2/ MCP-1, CCL3/ MIP-1α, CCL4/ MIP-1β and CCL5/ RANTES) in HSCs that recruit inflammatory cells, including Kupffer cells/macrophages, to the injured site (Seki et al., 2007). We and others have demonstrated that Kupffer cells are important for HSC activation in vitro and in vivo (Nieto, 2006; Seki et al., 2007). Indeed, Kupffer cells are the major producer of fibrogenic cytokine TGF-β (Seki et al., 2007).
On the basis of these observations, we hypothesize that TLR4 signaling in both Kupffer cells and HSCs is required for alcohol-induced liver injury, inflammation, steatosis, and fibrogenesis. To test this hypothesis in vivo, we have generated TLR4 bone marrow (BM) chimeric mice by the combination of Kupffer cell depletion, lethal irradiation and BM transplantation (BMT), which fully reconstitutes endogenous Kupffer cells with BM-derived cells without affecting endogenous liver cells, including HSCs, hepatocytes and endothelial cells (Seki et al., 2007). These TLR4-chimeric mice contain TLR4+/+ BM-derived cells, including Kupffer cells, and TLR4-/- endogenous liver cells, including HSCs, and vice versa. This study investigates the distinct roles of BM-derived cells and endogenous liver cells in TLR4-mediated experimental alcohol-induced liver pathogenesis.
Materials and Methods
Animals and generation of TLR4-chimeric mice
Specific pathogen-free wild-type (WT) C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) and C57BL/6 background TLR4-/- mice generated by Dr. Shizuo Akira (Osaka University, Suita, Japan) were used (Hoshino et al., 1999). BMT was performed as previously described (Seki et al., 2007). Because only 30% of Kupffer cells are reconstituted by donor-derived BM cells six months after BMT by the standard protocol, mice received liposomal clodronate injection (200 μl) before irradiation to deplete Kupffer cells and accelerate tissue macrophage turnover in order to obtain fully-reconstituted BM-derived cells (Kennedy and Abkowitz, 1997; Seki et al., 2009a; Seki et al., 2009b; Van Rooijen and Sanders, 1996). 1×107 BM cells from the tibias and femurs of donor mice were injected into the tail vein of lethally irradiated (11Gy) recipient mice. The TLR4-chimeric mice were generated by WT mice transplanted with TLR4-/- BM and vice versa. They were subjected to intragastric ethanol feeding 12 weeks after BMT. All animal experiments were approved by the University of Southern California, Los Angeles, and the University of California, San Diego, Institutional Animal Care and Use Committees.
Intragastric alcohol feeding mouse model
The intragastric feeding model of continuous ethanol infusion in mice was performed in the Animal Core facility of the Southern California Research Center for Alcoholic Liver and Pancreatic Disease and Cirrhosis (Tsukamoto et al., 1995; Tsukamoto et al., 2008; Tsukamoto et al., 2001; Xiong et al., 2008). Mice were implanted with a long-term gastrostomy catheter under anesthesia. After one week of infusion of a control liquid diet, ethanol infusion was initiated at a dose of 18g/kg/day which was increased until it reached 29.4g/kg/day on day 28. At the initial ethanol dose, total caloric intake derived from a diet and ethanol was set at 533 Cal/kg and the caloric percentages of ethanol, dietary carbohydrate (dextrose), protein (lactalbumin hydrolysate) and fat (corn oil) are 24.3%, 15.7%, 25%, and 35%, respectively. The ethanol dose at the end of 4 weeks accounted for 34.4% of calories. The isocaloric high fat diet without ethanol was used as a control diet.
Histological analysis
For histochemical analysis, liver specimens were fixed in 10% buffered formalin, followed by H&E, and immunohistochemistry. Immunohistochemistry were performed by using monoclonal antibody against α-smooth muscle actin (α-SMA) (DakoCytomation, Carpinteria, CA) with an M.O.M. kit (Vector Laboratories, Burlingame, CA), and rat anti mouse F4/80 (eBioscience, San Diego, CA)(Miura et al.). For immunofluorescent staining, frozen sections were incubated with antibody to GFP (Abcam, Cambridge, MA), F4/80, and desmin (Neomarkers, Fremont, CA), followed by imaging with fluorescent microscopy (Seki et al., 2007). For Oil red O staining, frozen sections were stained as described previously (Miura et al.). A liver pathological score was determined by H&E staining as described previously (Nanji et al., 1989; Uesugi et al., 2001): steatosis (the number of hepatocytes that accumulated lipid droplet): <25%=1+;<50%=2+;<75%=3+;>75%=4+; inflammation and necrosis: 1 focus per low-power field=1+; 2 or more=2+. One point was given for each grade of severity of histological change, and a total score was calculated in each animal.
Lipid isolation and measurement
Hepatic triglyceride contents were measured using the TRIGLYCERIDE REAGENT SET (Pointe Scientific, Cantom, MI).
Measurement of serum ethanol concentration
Serum ethanol levels were measured using the Ethanol Assay Kit (Biovision, Mountain View, CA).
Quantitative real-time polymerase chain reaction
Total RNA was prepared from cells or frozen liver tissues using TRIzol regent (Invitrogen, Carlsbad, CA) and cleaned by an RNeasy kit, followed by DNase treatment (Qiagen, Valencia, CA). RNA was reverse transcribed using a high-capacity complementary DNA reverse-transcription kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed using an ABI PRISM 7000 Sequence Detector (Applied Biosystems, Foster City, CA) (Seki et al., 2009a). PCR primer sequences are listed in supplementary table 1. The expression of respective genes was normalized to 18S RNA as an internal control.
Flow cytometry
Liver mononuclear cells were prepared as previously described (Karlmark et al., 2009). Briefly, the liver was perfused and homogenated. The cells were resuspended in 36% Percoll and were centrifuged at 700g for 10 minutes. After Fc receptor blockade, cells were stained with anti F4/80 (eBioscience, San Diego, CA). Samples were analyzed on a FACSCanto flow cytometer (BD bioscience, San Jose, CA) and Flow-Jo software (Tree Star, Ashland, OR).
Statistical Analysis
All data were expressed as mean ± standard error of the mean. Data between groups were analyzed by Student's t-tests. Differences between multiple groups were compared using one-way ANOVA (GraphPad Prism 4.02; GraphPad Software, La Jolla, CA); P-values less than 0.05 were considered statistically significant.
Results
TLR4 on both BM-derived cells and endogenous liver cells contributes to alcohol-induced liver injury and steatosis
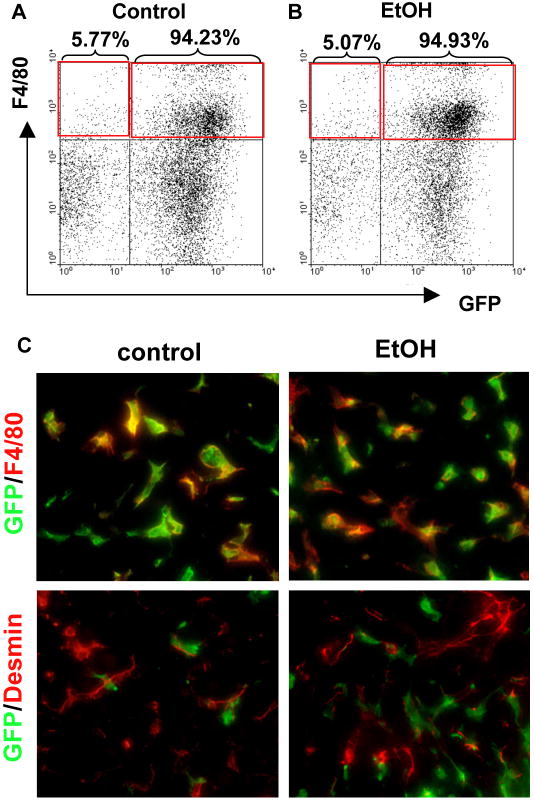
As we previously demonstrated (Seki et al., 2009b; Seki et al., 2007), endogenous Kupffer cells were efficiently reconstituted by BM-derived cells in the BM-chimeric mice generated by our protocol. The mice transplanted with BM isolated from β-actin promoter-driven GFP transgenic (GFP-Tg) mice were treated with intragastric continuous feeding of control- or ethanol containing diet for 4 weeks. FACS analysis confirmed that GFP-expressed F4/80-positive Kupffer cells/macrophages were efficiently substituted in control- and ethanol-fed GFP-Tg BM-transplanted mice (Fig. 1A, B). The livers were stained with either the macrophage marker F4/80 or the HSC marker Desmin. Thus, Kupffer cells were reconstituted as GFP-positive Kupffer cells, and all HSCs and hepatocytes were GFP-negative (Fig. 1C).
Figure 1. Bone marrow (BM) transplantation sufficiently replaced Kupffer cells with BM-derived cells in the livers of alcohol-fed mice.
(A-C) Bone marrow (BM) transplantation was performed to generate the chimeric mice; (A-B) BM from β-actin promoter-driven GFP Tg mice was transplanted into WT mice treated with liposomal clodronate and whole body irradiation. Liver mononuclear cells were isolated from control and ethanol diet-fed mice. F4/80 positive GFP-expressing hepatic non-parenchymal cells were determined by FACS analysis. (C) Liver sections from control and ethanol diet-fed mice were stained with anti-F4/80 (upper, red color) and desmin (lower, red color) antibodies as the markers for HSCs and Kupffer cells, respectively. Green fluorescence signals represent endogenous GFP expression as BM-derived cells.
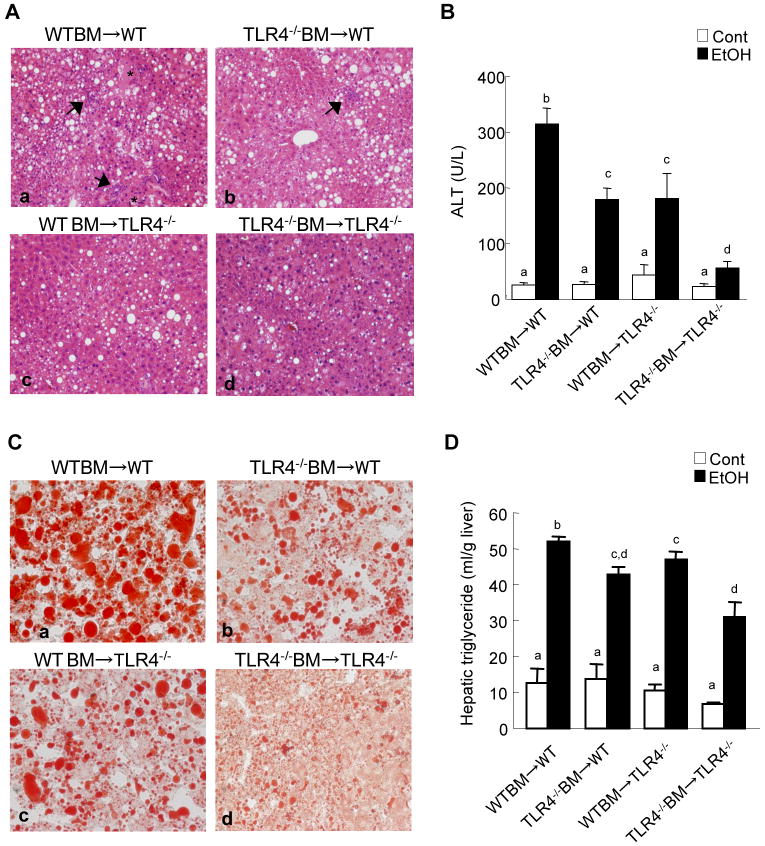
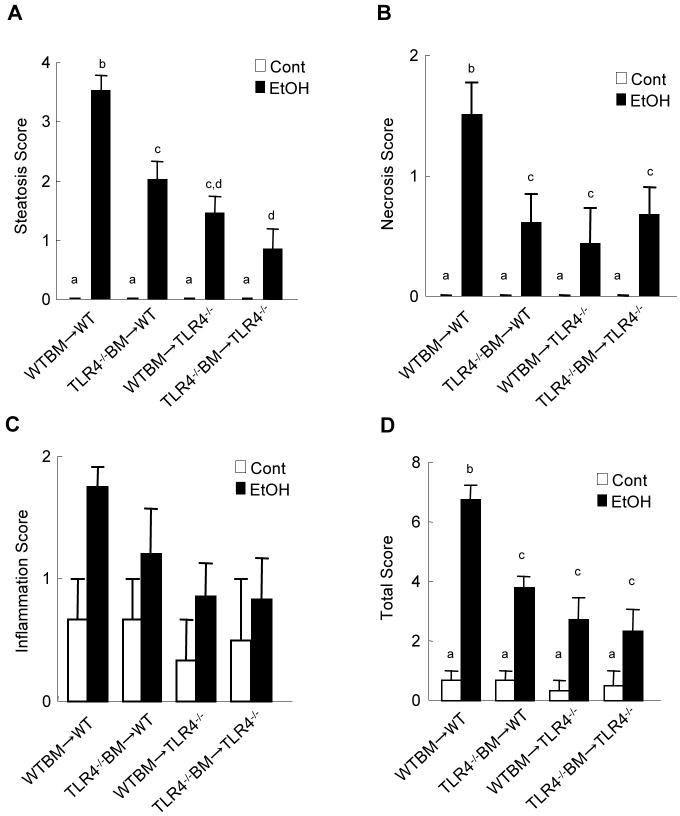
TLR4-inactivated mice have a marked reduction of liver injury, steatosis and inflammation after chronic ethanol treatment (Hritz et al., 2008; Uesugi et al., 2001). To investigate the specific roles of TLR4 expressed on BM-derived cells, including Kupffer cells, compared with endogenous liver cells, including HSCs, in alcohol-induced steatohepatitis, TLR4-chimeric mice (WT mice transplanted with WT BM; WT mice transplanted with TLR4-/- BM; TLR4-/- mice transplanted with WT BM; and TLR4-/- mice transplanted with TLR4-/- BM) were generated. These mice were then treated with intragastric alcohol feeding for 4 weeks. There were no significant differences in mouse body weight of each group of BM chimeric mice before and after intragastric ethanol feeding (Supplementary table 2). There were also no significant differences in serum ethanol concentrations among the groups at the end of the experiments (Supplementary table 3). Then, we assessed hepatocyte injury and steatosis by serum ALT levels, H-E staining and Oil-red O staining (Fig. 2). The WT mice transplanted with WT BM had necrosis, large lipid droplets in hepatocytes, and a significant increase of hepatic triglyceride and serum ALT levels (Fig. 2A-D, 3A). As expected, TLR4-/- mice transplanted with TLR4-/- BM exhibited few necrotic areas and had significantly less steatosis, hepatic triglyceride and serum ALT levels (Fig. 2A-D, 3A). Notably, after alcohol feeding, hepatic steatosis and triglyceride levels in TLR4-/- mice with WT BM and WT mice with TLR4-/- BM were significantly decreased compared to those in WT mice transplanted with WT BM (Fig.2A, C, D, 3A). Although ALT levels in the chimeric mice were intermediate between the complete WT mice and the complete TLR4-/- mice (Fig.2B), the hepatic necrotic score in both TLR4 chimeric mice was similar to that in the complete TLR4-/- mice (Fig. 3B). Infiltration of inflammatory mononuclear cells was observed in WT mice with WT BM (Fig. 2A, 3C). In contrast, a reduction of mononuclear cell infiltration was seen in TLR4-/- mice with WT BM and complete TLR4-/- mice (Fig. 2A, 3C). The total pathology score in the complete WT mice was significantly greater than that of both types of TLR4 chimeric mice and of the complete TLR4-/- mice (Fig. 3D).
Figure 2. Alcohol-induced liver inflammation and steatosis in mice with TLR4 deficiency in BM-derived cells or endogenous liver cells.
(A-C) Bone marrow (BM) transplantation was performed to generate the TLR4 chimeric mice; (a) WT mice with WT BM (n=9); (b) TLR4-/- mice with WT BM (n=6); (c) WT mice with TLR4-/- BM (n=7); (d) TLR4-/- mice with TLR4-/- BM (n=6). TLR4-chimeric mice were treated with intragastric feeding of alcohol for 4 weeks. The livers were harvested and stained for H-E (A) and oil red O (C). The asterisk indicates necrotic area and the black arrow represents inflammatory cell infiltration (original magnifications are ×100 in A, ×200 in C.). Serum ALT levels were measured (B). Values with different letters are significantly different, p<0.05.
Figure 3. Alcohol-induced steatohepatistis through TLR4 on BM-derived cells and endogenous liver cells.
(A-D) The TLR4 chimeric mice [(a) WT mice with WT BM (n=9); (b) TLR4-/- mice with WT BM (n=6); (c) WT mice with TLR4-/- BM (n=7); (d) TLR4-/- mice with TLR4-/- BM (n=6)] were treated with intragastric feeding of alcohol for 4 weeks. Pathological changes were scored as described in Materials and Methods. (A) necrosis, (B) steatosis, (C) inflammation, and (D) total score are shown. Values with different letters are significantly different, p<0.05.
Both BM-derived cells and endogenous liver cells require TLR4 for liver inflammation after chronic ethanol treatment
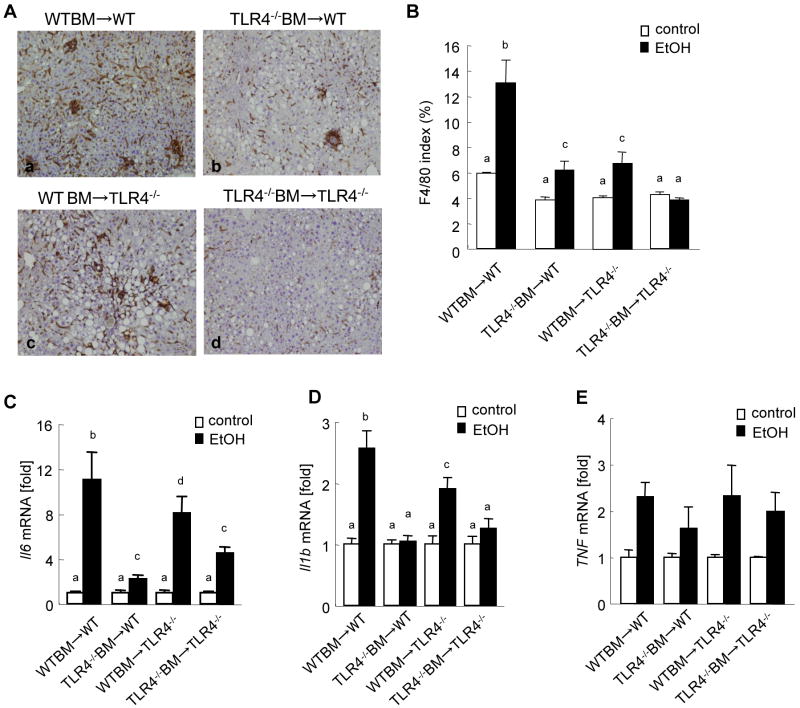
We further examined liver inflammation by assessing macrophage infiltration and expression of inflammatory cytokines in the liver. Kupffer cell/macrophage accumulation was significantly increased in WT mice with WT BM (Fig. 4A, B). In contrast, TLR4-/- mice with TLR4-/- BM had no increase of Kupffer cells in the liver after chronic ethanol feeding (Fig. 4A, B). The accumulation of Kupffer cells/macrophages in WT mice with TLR4-/- BM and TLR4-/- mice with WT BM was intermediate between the complete WT mice and the complete TLR4-/- mice (Fig. 4A, B). Hepatic mRNA levels of IL-6 and IL-1β were markedly increased in the complete WT mice (Fig. 4C, D). Interestingly, the levels of IL-6 and IL-1β in the chimeric mice with WT BM were significantly higher than those in both the chimeric mice with TLR4-/- BM and the complete TLR4-/- mice, suggesting a more important role of TLR4 on BM cells in IL-6 and IL-1β production than that of TLR4 on endogenous cells (Fig. 4C, D). No significant differences were seen in TNFα expression among the groups of chimeric mice after ethanol treatment (Fig. 4E).
Figure 4. TLR4 on either BM-derived cells or endogenous liver cells participates in liver inflammation in mice fed ethanol diet.
(A-D) The TLR4 chimeric mice [(a) WT mice with WT BM (n=9); (b) TLR4-/- mice with WT BM (n=6); (c) WT mice with TLR4-/- BM (n=7); (d) TLR4-/- mice with TLR4-/- BM (n=6)] were treated with intragastric feeding of alcohol for 4 weeks. Immunohistochemistry against F4/80 (A) are shown (original magnifications are ×100). An F4/80 index was calculated by measuring positive area using Image J software in five high power fields (B). Hepatic mRNA levels of IL-6 and IL-1β were measure by qPCR and are expressed as fold change compared with corresponding pair-fed mice (C, D). Values with different letters are significantly different, p<0.05.
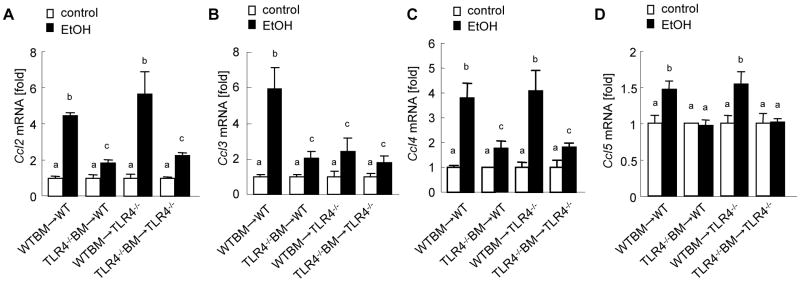
TLR4-mediated chemokine production in alcohol-induced steatohepatitis
CC-chemokines including CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β and CCL5/RANTES are important for chemotaxis of inflammatory cells, including Kupffer cells/macrophages, and non-inflammatory endogenous cells, including HSCs, in chronic liver inflammation and fibrosis (Schwabe et al., 2003; Seki et al., 2009a; Seki et al., 2009b). Both Kupffer cells/macrophages and HSCs produce CC-chemokines through TLR4 (Paik et al., 2003; Seki and Brenner, 2008; Seki et al., 2007). To examine whether TLR4 signaling induces CC-chemokine production in alcohol-induced steatohepatitis, hepatic mRNA expression of CC-chemokines was measured in TLR4-chimeric mice after chronic alcohol feeding. Hepatic mRNA levels of CCL2, CCL3, CCL4 and CCL5 were significantly elevated in the complete WT mice after ethanol treatment (Fig. 5A-D). In contrast, the expression of these CC-chemokines was significantly suppressed in the total TLR4-/- mice in comparison to the complete WT mice (Fig. 5A-D). Of note, the TLR4 chimeric mice with WT BM, but not the mice with TLR4-/- BM, showed sufficient levels of CCL2, CCL4 and CCL5 (Fig. 5A, C, D). Hepatic CCL3 mRNA levels were significantly decreased in both TLR4-chimeric mice and total TLR4-/- mice compared with those in the complete WT mice (Fig. 5B).
Figure 5. TLR4 induces CC-chemokines in BM-derived cells and endogenous liver cells in alcoholic liver disease.
(A-D) The TLR4-chimeric mice [WT mice with WT BM (n=9); TLR4-/- mice with WT BM (n=6); WT mice with TLR4-/- BM (n=7); TLR4-/- mice with TLR4-/- BM (n=6)] were treated with intragastric ethanol feeding for 4 weeks. Hepatic mRNA levels of CCL2 (A), CCL3 (B), CCL4 (C) and CCL5 (D) were measure by qPCR and are expressed as fold change compared with corresponding pair-fed mice. Values with different letters are significantly different, p<0.05.
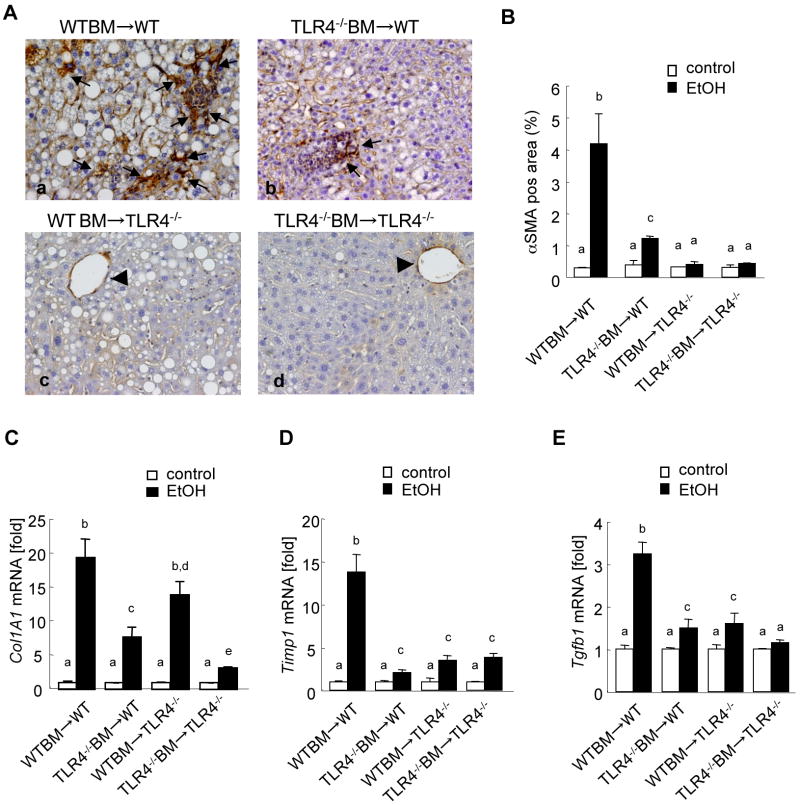
Alcohol mediates fibrogenesis through TLR4 signaling in the liver
Although collagen deposition is not prominent in the intragastric continuous ethanol feeding model, we found strong αSMA expression in the liver, demonstrating that HSCs are activated to myofibroblasts (Fig. 6Aa). αSMA expression was markedly increased in the livers from alcohol fed WT mice transplanted with WT BM (Fig. 6Aa, B). Some αSMA positive cells accumulated in WT mice with TLR4-/- BM (Fig. 6Ab, B). In contrast, TLR4-/- mice with WT BM and total TLR4-/- mice had αSMA expression only in the vessels, but had no myofibroblasts (Fig. 6Ac, d, B). These results suggest that TLR4 signaling in endogenous liver cells is more important for activating myofibroblasts than that in BM-derived cells. Since fibrogenic gene expression is a more sensitive marker than collagen protein production and deposition, we measured hepatic fibrogenic gene expression (collagen α1(I), TIMP1, and TGF-β1) in the intragastric ethanol feeding model. Hepatic mRNA levels of collagen α1(I), TIMP1 and TGF-β1 were significantly increased in the complete WT mice after ethanol treatment in comparison to those in the pair-fed WT mice (Fig. 6C-E). In contrast, hepatic mRNA levels of collagen α1(I), TIMP-1 and TGF-β1 were significantly lower in the complete TLR4-/- mice than those in the complete WT mice (Fig. 6C-E). The chimeric mice with TLR4-/- BM cells, but not the mice with WT BM cells, exhibited significantly less collagen α1(I) expression than the complete WT mice (Fig. 6C). Both types of TLR4-chimeric mice showed similar levels of TIMP-1 and TGF-β1 mRNA as the complete TLR4-/- mice (Fig. 6D, E).
Figure 6. Both Kupffer cells and HSCs contribute to TLR4-mediated fibrogenic responses in continuous intragastric ethanol feeding.
(A-E) The TLR4 chimeric mice [(a) WT mice with WT BM (n=9); (b) TLR4-/- mice with WT BM (n=6); (c) WT mice with TLR4-/- BM (n=7); (d) TLR4-/- mice with TLR4-/- BM (n=6)] were subjected to intragastric feeding of alcohol for 4 weeks. Immunohistochemistry for αSMA (A) are shown. The black arrow represents αSMA expression in myofibroblasts and arrowheads indicate αSMA expression in vessels (original magnifications are ×200). αSMA expression was quantified by counting five randomly chosen high power fields (B). Hepatic mRNA levels of Collagen α1(I), TIMP1 and TGF-β1 were measure by qPCR and are expressed as fold change compared with corresponding pair-fed mice (C-E). Values with different letters are significantly different, p<0.05.
Discussion
The liver is a major target of alcohol-induced tissue injury. Chronic alcohol abuse is a major cause of cirrhosis and liver failure in adult patients in the United States (Szabo and Bala, 2010). Alcohol itself, as well as its metabolite acetaldehyde is a hepatotoxin. In addition, intestine-derived LPS is a major contributor to alcoholic liver injury. Excessive intake of ethanol induces changes in intestinal microflora and injures the tight junctions of intestinal epithelial cells, which cause increased intestinal permeability. This allows for translocation of intestine-derived LPS and other bacterial products to the liver through the portal vein (Szabo and Bala, 2010). This suggests that alcohol-induced steatohepatitis is tightly associated with LPS-TLR4 signaling. Indeed, alcohol-induced liver injury is significantly ameliorated in mice mutated in the TLR4 gene (Hritz et al., 2008; Uesugi et al., 2001). Downstream of TLR4, adaptor proteins MyD88 and TRIF are activated for transmitting their signals. The TRIF-dependent pathway is more important than the MyD88-dependent pathway in alcohol-induced liver injury after the Lieber-DeCarli diet feeding (Hritz et al., 2008; Zhao et al., 2008). The TRIF-dependent pathway induces IRF3 activation and TNF-α production after chronic ethanol treatment (Zhao et al., 2008).
A high level of TLR4 is expressed on Kupffer cells in the liver. Inactivation of Kupffer cells with gadolinium chrolide or liposomal clodronate efficiently suppressed alcoholic-induced liver injury (Adachi et al., 1994; Koop et al., 1997). As reported previously, both TLR4 and Kupffer cells are critical for the development of experimental alcohol-induced steatohepatitis (Hritz et al., 2008; Uesugi et al., 2001) (Adachi et al., 1994; Koop et al., 1997). However, these studies on TLR4 and Kupffer cells were two independent studies, and no study has clearly investigated the direct link between TLR4 and Kupffer cells in experimental alcohol-induced steatohepatitis. We have shown that TLR4 expressed on HSCs is crucial for liver inflammation and fibrosis in cholestatic liver disease (Seki et al., 2007). Current rodent models of alcohol-induced steatohepatitis are not amenable to studying liver fibrosis (Pradere et al., 2010). Our current study attempts to determine the responsible cell types expressing TLR4 in alcohol-induced liver injury, steatosis, inflammation and fibrogenesis. To examine specific roles of TLR4 on different liver cell types, we generated TLR4-BM chimeric mice, which were subjected to continuous intragastric ethanol feeding (Seki et al., 2007; Tsukamoto et al., 2008; Xiong et al., 2008). Overall, our results suggest that both BM-derived cells and non-BM-originated endogenous liver cells contribute to TLR4-mediated alcohol-induced liver pathophysiology, including hepatocyte injury, steatosis, inflammation and fibrogenesis.
Among BM-derived cells, Kupffer cells/macrophages highly express TLR4 and produce inflammatory cytokines in response to LPS (Crispe, 2009; Seki and Brenner, 2008). Non-BM-originated endogenous liver cells include HSCs and hepatocytes (Crispe, 2009; Seki and Brenner, 2008). Our previous study demonstrated that TLR4 signaling is more important in HSCs rather than in hepatocytes in mouse models of liver fibrosis (Seki et al., 2007). The present study demonstrated that WT mice transplanted with WT BM exhibited the most severe pathology with respect to hepatocyte damage, inflammation, steatosis and fibrogenesis among the groups of TLR4 chimeic mice. In contrast, TLR4-/- mice transplanted with TLR4-/- BM had a significant reduction of liver pathology compared to the complete WT mice. To determine the relative roles of TLR4 signaling in BM-derived cells and endogenous liver cells, alcohol-treated TLR4 chimeric mice were analyzed. Our data demonstrated that both TLR4 chimeric mice had intermediate levels between the complete WT mice and the complete TLR4-/- mice in serum ALT, hepatocyte steatosis and Kupffer cell infiltration after chronic alcohol treatment (Fig. 2-4). The complete WT mice had substantial necrotic areas, while similar low number of necrosis areas were seen in both TLR4-chimeric mice and the complete TLR4-/- mice (Fig. 3A). However, ALT levels in both chimeric mice were still higher than those in the total TLR4-/- mice. This might be due to other types of hepatocyte injury such as severe steatosis. Our results suggest that TLR4 signaling in both BM-derived cells, including Kupffer cells, and endogenous liver cells, including HSCs, contributes to hepatocyte damage, liver steatosis and Kupffer cell/macrophage infiltration after chronic alcohol intake. Although both Kupffer cells and HSCs produce inflammatory cytokines and CC-chemokines via TLR4 signaling (Pradere et al., 2010), our results suggest TLR4 signaling in BM-derived cells are more important for the production of IL-6, IL-1β and CC-chemokines CCL2, CCL4 and CCL5 than that in non-BM cells after ethanol treatment (Fig. 4, 5). In contrast, CCL3 production requires TLR4 on both BM-derived cells and endogenous liver cells (Fig. 4B). Our study did not identify a specific role of TLR4 signaling in alcohol-induced TNFα production in the liver.
Although cirrhosis is the major complication in patients with alcoholic liver disease, alcohol-induced liver fibrosis is difficult to study in current rodent models of chronic alcohol intake (Pradere et al., 2010). Even the murine intragastric continuous ethanol feeding model produces only minimal fibrillar collagen deposition. Our data demonstrated that the intermediate fibrosis biomarkers αSMA protein expression and mRNA expression of collagen α1(I), TIMP-1 and TGF-β1 are significantly upregulated in these livers. This indicated that many HSCs are activated, and fibrogenic responses are induced in the intragastric continuous ethanol feeding model. The complete TLR4-/- mice and both TLR4-chimeric mice had significant reductions of TIMP-1 and TGF-β1 mRNA expression after ethanol treatment (Fig. 6), suggesting that both BM- and non BM-derived cells are crucial for TGF-β1 and TIMP-1 production. On the other hand, collagen α1(I) expression is more dependent on BM-derived cells than non-BM cells (Fig. 6). This suggests that cytokines released from Kupffer cells in a TLR4-dependent manner induce activation of myofibroblasts. Our study also demonstrated that both TLR4-/- mice transplanted with WT BM and TLR4-/- mice transplanted with TLR4-/- BM showed no activated myofibroblasts, while WT mice transplanted with TLR4-/- BM cells had a low number of myofibroblasts (Fig. 6). Consistent with our previous study, this result suggests that HSCs require TLR4 signaling for their activation, although cytokines, including TGF-β secreted by Kupffer cells through TLR4, are major contributors for HSC activation (Seki et al., 2007). Taken together, our results suggest that both BM-derived cells, including Kupffer cells, and endogenous liver cells, including HSCs, require TLR4 for alcohol-induced fibrogenic response.
Although endogenous liver cells include not only HSCs, but also hepatocytes, the role of TLR4 signaling in hepatocytes is reported to be minor in the liver (Isogawa et al., 2005; Seki et al., 2007). However, in alcohol-induced streatohepatitis, the characteristics of hepatocytes might change and hepatocytes might acquire responsiveness to LPS due to excessive lipid accumulation in hepatocytes. A previous study demonstrated that alcohol-induced liver injury and oncogenesis are more evident in transgenic mice expressing the hepatitis C virus (HCV) non-structural protein NS5A than those in WT mice (Machida et al., 2009). HCV-NS5A transgenic mice, but not WT mice, showed enhanced TLR4 expression in hepatocytes. This suggests that TLR4 signaling in hepatocytes is strongly associated with HCV NS5A transgenic mice treated with alcohol, whereas TLR4 signaling in WT hepatocytes had a minor role during alcohol-induced steatohepatitis.
In summary, we demonstrated that TLR4 signaling in BM-derived cells, including Kupffer cells, and endogenous liver cells, including HSCs, contributes to the development of alcohol-induced steatohepatitis and fibrogenesis. We propose that the crosstalk between Kupffer cells and HSCs through TLR4 is critical for alcohol-mediated liver inflammation and fibrosis. The present study demonstrates that TLR4 expression on different liver cells is required for alcohol-mediated hepatic pathophysiology, and modulating TLR4 signaling on either Kupffer cells or HSCs can become a therapeutic target for the treatment of alcoholic liver disease.
Supplementary Material
Acknowledgments
Grant support: This study was supported by a Liver Scholar Award from the American Association for the Study of Liver Diseases/ American Liver Foundation (ES), a research grant from ABMRF (ES), NIH grant 1R01AA020172 (ES), 1R01DK085252 (ES), and a pilot project (ES) and the Animal Core from the Southern California Research Center for ALPD and Cirrhosis (P50 AA11999) funded by NIAAA/NIH (HT) and NIH grant 5R01GM041804 (DAB).
Abbreviations
- ALT
alanine aminotransferase
- BM
bone marrow
- BMT
bone marrow transplantation
- CCL
C-chemokine ligand
- CCR
CC-chemokine receptor
- ECM
extracellular matrix
- GFP
green fluorescent protein
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- IL
interleukin
- KO
knockout
- LPS
lipopolysaccharide
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory proteins
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- RANTES
regulated on activation normal T cell expressed and secreted
- SMA
smooth muscle actin
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinase
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- WT
wild-type
Footnotes
No conflicts of interest exist.
Contributor Information
Sayaka Inokuchi, Email: sinokuchi@ucsd.edu.
Hidekazu Tsukamoto, Email: htsukamo@usc.edu.
EekJoong Park, Email: ejpark@usc.edu.
Zhang-Xu Liu, Email: zxliu@usc.edu.
David A. Brenner, Email: dbrenner@ucsd.edu.
References
- Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis. 2005;23(3-4):255–63. doi: 10.1159/000090173. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–60. [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Alcoholic liver disease: natural history. Liver Transpl Surg. 1997;3(3):206–11. [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, Takei Y, Hirose M, Shimizu H, Miyazaki A, Brenner DA, Sato N, Thurman RG. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20–5. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30(1):77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–52. [PubMed] [Google Scholar]
- Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48(4):1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79(11):7269–72. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90(3):986–93. [PubMed] [Google Scholar]
- Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51(6):944–50. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, Seki E, Deshaies R, Miyake K, Lai MM. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106(5):1548–53. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1beta in Mice. Gastroenterology. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1989;13(1):15–9. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44(6):1487–501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37(5):1043–55. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like Receptor 4 and Hepatic Fibrogenesis. Semin Liver Dis. 2010;30(3):232–244. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah S, Rivera C, Arteel G. Early-phase alcoholic liver disease: an update on animal models, pathology, and pathogenesis. Int J Toxicol. 2004;23(4):217–31. doi: 10.1080/10915810490502069. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G949–58. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48(1):322–35. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009a;119(7):1858–70. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009b;50(1):185–97. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16(11):1321–9. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22 1:S53–6. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275(4 Pt 1):G605–11. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96(1):620–30. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Mkrtchyan H, Dynnyk A. Intragastric ethanol infusion model in rodents. Methods Mol Biol. 2008;447:33–48. doi: 10.1007/978-1-59745-242-7_3. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, Hill D, Schmidt J, Deaciuc I, Barve S, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC, Yokoyama H, Okamura Y, Nakamura Y, Ishii H, Chawla RK, Barve S, Joshi-Barve S, Watson W, Nelson W, Lin M, Ohata M, Motomura K, Enomoto N, Ikejima K, Kitamura T, Oide H, Hirose M, Bradford BU, Rivera CA, Kono H, Peter S, Yamashina S, Konno A, Ishikawa M, Shimizu H, Sato N, Thurman R. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):171S–181S. doi: 10.1097/00000374-200105051-00029. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168(6):2963–9. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23(5):1239–43. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- Xiong S, She H, Zhang AS, Wang J, Mkrtchyan H, Dynnyk A, Gordeuk VR, French SW, Enns CA, Tsukamoto H. Hepatic macrophage iron aggravates experimental alcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G512–21. doi: 10.1152/ajpgi.90327.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts D, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2010 doi: 10.1002/hep.24018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181(5):3049–56. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.