Abstract
We sought to delineate further the immunological significance of T lymphocytes infiltrating the valve leaflets in calcific aortic stenosis (CAS) and determine whether there were associated alterations in circulating T cells. Using clonotypic TCR β-chain length and sequence analysis we confirmed the repertoire of tricuspid CAS valves contains numerous expanded T cell clones with varying degrees of additional polyclonality, which was greatest in cases with severe calcification. We now report a similar proportion of clonal expansions in the much younger bicuspid valve CAS cases. Peripheral blood flow cytometry revealed elevations in HLA-DR+ activated CD8 cells and in the CD8+CD28nullCD57+ memory-effector subset that were significantly greater in both bicuspid and tricuspid CAS cases with more severe valve calcification. Lesser increases of CD4+CD28null T cells were identified, principally in cases with concurrent atherosclerotic disease. Upon immunostaining the CD8 T cells in all valves were mainly CD28null, and CD8 T cell percentages were greatest in valves with oligoclonal repertoires. T cell clones identified by their clonotypic sequence as expanded in the valve were also found expanded in the circulating blood CD28nullCD8+ T cells and to a lesser degree in the CD8+CD28+ subset, directly supporting the relationship between immunologic events in the blood and the valve. The results suggest that an ongoing systemic adaptive immune response is occurring in cases with bicuspid and tricuspid CAS, involving circulating CD8 T cell activation, clonal expansion and differentiation to a memory-effector phenotype, with trafficking of T cells in expanded clones between blood and the valve.
Keywords: Human, calcific aortic stenosis, bicuspid aortic valve, T cell receptors, activated T cells, memory-effector T cells, clonal expansions, cell trafficking
Introduction
Calcific aortic stenosis (CAS) is characterized by progressive remodeling of the valve matrix characterized by the formation of calcific nodules. The thickened and less compliant valve leaflets narrow the valve aperture, resulting in left ventricular hypertrophy and ultimately heart failure.(1) CAS is often relentlessly progressive and valve replacement is currently the only treatment. It has been classically envisioned as a degenerative response of the aortic valve to hemodynamic stress as reflected in its designation as senile aortic stenosis.(2) CAS is relatively frequent, developing in 2-3% of the population by the age of 75. However, nearly 10% of those with the uncommon and hemodynamically less adequate anatomic variant bicuspid valve develop CAS two or more decades earlier than those with tricuspid valves.
The normal valve leaflet is avascular and without evidence of infiltrating mononuclear cells. The more recent identification of a prominent mononuclear cell infiltrate in the vast majority of both bicuspid and tricuspid CAS valves introduced the concept of inflammation to this disease.(3-7) The infiltrate predominantly consisted of variably sized aggregates of activated T cells and macrophages adjacent to regions of heterotopic calcification and neoangiogenesis. However the questions of the immunologic nature of the T cell infiltrate in the valve and whether it was part of a broader systemic immune response remained unanswered. In particular it was not known whether the infiltrate entirely consisted of numerous polyclonal T cells that may be entering the valve secondarily in response to calcification and injury, or whether the infiltrate contained expanded T cell clones, which would suggest the possibility that T cells might play a more important pathogenic role in aspects of CAS through their activation, differentiation, clonal expansion, and mediator release in an adaptive immune response.
This question was addressed in an initial study of the TCR β-chain length and sequence analysis in the repertoire of the T cells infiltrating tricuspid CAS valves where on average over half of the infiltrating αβT cell repertoire was shown to consist of expanded clones.(8) Additionally, the large majority of expanded clones found in either blood or the valve exhibited clonotypes unique to that compartment and were not shared, emphasizing that the clonal expansion in the tricuspid valve was immunologically specific, although whether β-chain sequence analysis would show that clonal expansions were similarly present in bicuspid valve CAS was not determined. In contrast, normal valves did not have identifiable TCR β-chain transcripts.(8)
These finding of clonal expansions in the valves of CAS cases along with evidence suggesting involvement of CD8 T cells raised the major question of whether the T cells in the circulation of those developing CAS would exhibit features of an ongoing systemic immune response and furthermore whether the response would be greater in the CD8 subset.(8) The alteration in T cell phenotype during an immune response, especially evident in the CD8 T cells usually includes the transient expression of activation molecules, such as HLA-DR(9), and the development of memory-effector cells particularly in sustained responses, defined by the loss of co-stimulatory CD28 molecules and acquisition of structures including natural killer receptors, e.g. CD57.(10–13)
Accordingly, we hypothesized first, that the entrance of T cells into the valve might be a reflection of a major systemic immune response that would be characterized by T cell activation and differentiation to memory-effector phenotype in the blood, second, that the intensity of these systemic immune events would be related to the severity of the changes of CAS, and third, that clones shared between blood and the valve, reflecting lymphocyte trafficking, would be found in the memory-effector CD8+CD28null T cell subset.
However, tricuspid valve CAS is a disease of the elderly, and in normal chronologic aging the prevalence of CD28nullCD57+ T cells increases in the CD8+ and, to a much lesser extent, in the CD4+ compartment(14) along with large clonal expansions in this subset.(15–17) Therefore to address the potentially confounding effect of age-related alterations, we specifically sought to also study the much younger individuals who develop CAS in bicuspid aortic valves, and hypothesized that similar alterations would be found in the valve and peripheral blood of these younger cases of CAS. The primary object of the present study was to investigate these four hypotheses.
Materials and Methods
Study population
Cardiac valves and venous blood samples were obtained from 18 tricuspid and 9 bicuspid CAS cases with advanced CAS and/or ascending aortic disease at the time of valve replacement surgery. The T or B suffix of the CAS case identifier (ID) refers to the bicuspid or tricuspid valve structure. All of the study subjects participated voluntarily and gave informed consent. The study followed institutional guidelines and was approved by the Columbia University Institutional Review Board.
Pathologic grading of calcification
The severity of aortic valve pathology in CAS was quantified after Warren et al.(18) Atherosclerosis, was defined either as the requirement for a revascularization procedure, or as asymptomatic aortic or coronary artery atherosclerosis directly observed during surgery. Those with extensive histories of multiple (≥2) atherosclerotic events (peripheral vascular, coronary, carotid, etc.) are designated “V” (vasculopathic, n=6). In one case the event requiring revascularization was 3 months prior to aortic valve replacement, while in the others it was remote and ranged up to 30 years prior to valve surgery. Stable mild coronary artery disease for ≥ one year prior to valve replacement surgery, or those without clinical evidence of the presence or consequence of atherosclerotic disease, but were found to have coronary artery or aortic atherosclerotic involvement during surgery were designated as stable atherosclerotic disease cases, “S”, n=8. One additional case designated had polymyalgia rheumatica and an ascending thoracic aortic aneurysm. This case was not considered to have atherosclerotic disease.
Flow cytometry and immunohistochemistry
Peripheral blood mononuclear cells were stained with a combination of antibodies, including fluorescein (FITC)- or allophycocyanin (APC)-conjugated anti-CD3, phycoerythrin (PE)- or APC-conjugated anti-CD8, PE-conjugated anti- CD28 or anti-CD57 monoclonal antibodies, or FITC-conjugated anti-HLA-DR antibodies (all from BD Biosciences). Additionally, CD56, CD16, CD158e1 (KIR3DL1, NKB1) and CD69 were used. For the isolation of purified T cells, CD4+ T cells were first separated from blood mononuclear cells by positive selection using magnetic beads (Dynal). In a second step, the CD4-negative fraction was stained with anti-CD8-APC and anti-CD28-PE for cell sorting (FACSAria, BD).
For double immunostaining, the paraffin sections were stained with the first primary antibody from either rabbit anti-human CD3 or CD4 (Cell Marque Inc, CA), or goat anti-human CD28 (R&D Systems, MN) or monoclonal mouse anti-human CD8 or CD20 (DAKO). This was followed by addition of a species-specific secondary biotinylated antibody and Streptavidin conjugated with Alexa Fluo 555 (Vector Laboratories). After blocking, second stage staining was similarly performed followed by fluorescein-conjugated avidin, and DAPI counterstaining. The three fluorescent images were taken at different wavelengths and merged. Four to six images were used to count the proportion of CD8+CD3+ T cells in the samples.
T cell repertoire β-chain length distribution analysis
Total RNA from homogenized valve tissue or separated T cell subsets was extracted and reverse-transcribed as described.(19) The third complementarity determining region (CDR3) region within the TCR β-chain and its flanking sequences were amplified in 24 separate polymerase chain reactions (PCR), using forward primers specific for each TCR β-chain variable region (Vβ) family.(8) The International Immunogenetics Information System (IMGT) and Arden nomenclature for variable region families are used. (8, 20, 21) Each of the PCR products was examined in TCR β-chain length distribution (spectratype) analysis, performed as described(8) using an ABI 3100 capillary sequencer. The output files containing peak size in base pairs, height and peak area was used to create histograms of peak area versus size and then converted to peak area versus CDR3 length plots. For each Vβ family, a reference repertoire was compiled from the averaged peripheral CD4+ T cell β-chain length distribution of 15 individuals.(22) The Hamming Distance (HD), a measure of the difference between a histogram describing the TCR β-chain length distribution of a polyclonal reference repertoire and the test repertoire length distribution, was calculated for each Vβ family as the difference in normalized peak area between the test population and the reference (range: 0-100, with 0 indicating the test repertoire diversity was maximal and equivalent to the polyclonal reference of CD4 T cells from healthy individuals, and 100 indicating that no β-chain product was shared with the reference repertoire) as described.(23) If a BV product was not detected in the sample for a particular family, that result was excluded when the HD for each T cell subset was averaged across all BV families tested, yielding a mean Hamming distance (mHD). The proportion of the repertoire occupied by a given BV family was approximated by summing the peak intensities for all spectratype peaks across all BV families and determining the proportion attributable to a given BV family.
T cell receptor β-chain sequence analysis
To define the degree of clonal expansion in the circulating T cell subsets and identify clonal trafficking between blood and the valve, limited cloning and nucleotide sequencing of selected amplified β-chain CDR3 regions were performed as described.(8) These samples were selected on the basis of the initial spectratype analysis, and usually included combinations of samples that shared oligoclonal expansions at the same CDR3 length. Typically 48 clones were sequenced for each BV family for each valve or peripheral blood subset. CDR3 sequences were edited and aligned (Geneious) and analyzed using IMGT/V-QUEST criteria.(20) Representative sequences from the valve leaflets and blood subsets comprise Genbank accession numbers JF731129-JF731232. (http://www.ncbi.nlm.nih.gov/genbank) In the instance of an expanded clone in a given site with multiple identical sequences only one representative sequence was entered into Genbank and the clone size noted. To describe repertoire diversity in each Vβ family analyzed, the Simpson’s diversity index (SDI) as a measure of the repertoire sequence diversity was calculated as described.(24) The SDI ranges from 0 to 1, with 1 representing maximal diversity (a polyclonal repertoire consisting of unexpanded T cells) and 0 representing maximal contraction (a single monoclonal expansion). The clonotype frequency in a given BV family was directed calculated from the number of sequences in the given clone divided by the total number of sequences obtained for that BV family in the particular sample. The proportional representation of each BV family in the repertoire was approximated by summing the peak intensities for all peaks across all BV families and determining the fraction accounted for by a specific BV family. This latter calculation is potentially influenced by differences in PCR efficiencies, and is considered an approximation. The percentage across the entire repertoire in the sample occupied by the expanded clone in a sample across the entire repertoire was reported as the product of the clonotype frequency in a given BV family times the proportional representation of that BV sample.
Statistical Analysis
The Wilcoxon rank sum test was used for comparisons of valve calcification score, chronologic age, aortic valve subtype and immunophenotype. Spearman’s or non-parametric correlation (2-tailed) was used to assess the relationship between the relative proportion of T cell subsets and the grade of valve calcification. A t-test was used to compare the means of independent samples. A two-tailed p-value < 0.05 was considered significant. SPSS 17 software was used for all analyses (SPSS, Chicago, Il).
Results
Peripheral blood T lymphocytes express activation markers in CAS
The proportion of circulating CD3+ T cells expressing HLA-DR in CAS was considerably increased in the peripheral T cell compartment, range 4.7 to 32.9%, mean 16.1% and 16.3% in tricuspid and bicuspid CAS, respectively, compared to the expected frequency of ≤ 5% in healthy controls. (Table I) HLA-DR expression on T cells was more strikingly increased within the CD8 subset, mean 24.4% and 28.4% in tricuspid and bicuspid disease, respectively, range 5.6 to 42.8% (table I). CD69 expression also correlated with HLA-DR expression on T cells (data not shown). A greater proportion of activated HLA-DR+ T cells was observed in those with a calcification score ≥ 5 (p = 0.03), correlation, ρ = 0.530, p = 0.024. Interestingly the percentage of the CD8+CD57+ T cell subset expressing HLA-DR ranged up to 49.9% and was greater than that found on CD8 T cells in 11 of 14 CAS cases, indicating the CD8 T cells that have differentiated to a memory-effector phenotype continue to be strongly activated, table I. This increase in HLA-DR and CD69 expression was independent of the atherosclerosis status of the cases. Additionally, the expression of HLA-DR on CD4 T cells was increased over the reference samples in 10 of 18 cases, mean 6.9 %, but was considerably less marked than that observed on CD8 T cells, Table I.
Table I.
Among Circulating T Cells in CAS, the CD8 Lineage Exhibits the Greatest Differentiation to an Activated and Memory-Effector Phenotype
| ID* | Age | CAS score* | Athero- sclerosis ** | CD8 (%) | CD3 (%) | CD4 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA- DR+ | CD28null | CD57+ | CD57+ HLA- DR+ | HLA- DR+ | CD57+ | CD57+ HLA- DR+ | CD28null | HLA- DR+ | CD57+ | ||||
| T55 | 83 | 2 | 0 | 8.6 | 36.6 | 31.0 | 21.2 | 5.7 | 10.1 | 24.0 | 4 | 2.4 | 3.3 |
| T11 | 64 | 2 | 0 | 67.0 | 3.0 | ||||||||
| T43 | 92 | 2 | S | 60.0 | |||||||||
| T19 | 79 | 3 | V | 54.0 | 9.3 | ||||||||
| T35 | 75 | 3 | V | 17.0 | 63.5 | 48.8 | 21.3 | 11.6 | 29.1 | 17.1 | 2.6 | 2.6 | 3.4 |
| T16 | 72 | 3 | S | 68.0 | 6.9 | ||||||||
| T34 | 68 | 3 | 0 | 25.1 | 50.5 | 41.3 | 36.9 | 12.1 | 11.8 | 38.7 | 0.5 | 8.0 | 1.4 |
| T37 | 82 | 4 | 0 | 85.2 | |||||||||
| T10 | 66 | 4 | S | 90.0 | 19.3 | ||||||||
| T51 | 86 | 5 | PMR | 41.3 | 84.8 | 53.3 | 40.4 | 32.9 | 46.4 | 47.3 | 50.5 | 18.3 | 44.3 |
| T57 | 79 | 5 | V | 15.4 | 73.0 | 46.2 | 21.5 | 8.2 | 12.8 | 22.1 | 3.0 | 2.5 | 0.7 |
| T53 | 84 | 5 | S | 15.1 | 65.3 | 41.4 | 13.9 | 4.7 | 4.8 | 13.4 | 5.1 | 3.3 | 0.9 |
| T54 | 88 | 5 | S | 38.0 | 81.2 | 57.3 | 48.2 | 30.2 | 41.6 | 46.2 | 38.5 | 10.1 | 30.2 |
| T52 | 81 | 6 | 0 | 42.8 | 66.6 | 37.6 | 49.9 | 20.4 | 14.8 | 52.7 | 7.1 | 6.7 | 6.0 |
| T58 | 60 | 6 | 0 | 29.6 | 69.0 | 49.9 | 37.0 | 19.6 | 23.6 | 39.9 | 8.0 | 5.2 | 4.8 |
| T56 | 84 | 7 | S | 10.8 | 78.3 | 52.8 | 8.7 | 10.6 | 33.2 | 11.3 | 25.5 | 0.0 | 15.4 |
| T13 | 77 | 8 | V | 96.0 | 27.7 | ||||||||
| T60 | 80 | 8 | 0 | 27.4 | 83.6 | 70.3 | 26.4 | 17.3 | 41.3 | 26.9 | 7.7 | 3.8 | 6.4 |
| T61 | 90 | 8 | V | 26.8 | 83.9 | 33.8 | 34.6 | 20.3 | 23.2 | 35.4 | 20.3 | 7.4 | 14.8 |
| B38 | 31 | 1 | 0 | 70.7 | 60.0 | 31.0 | 10.0 | 13.0 | |||||
| B48 | 53 | 2 | 0 | 5.6 | 20.0 | 3.9 | 12.9 | 4.7 | 2.1 | 21.3 | 2.9 | 2.7 | 0.1 |
| B50 | 75 | 4 | 0 | 23.6 | 74.4 | 68.0 | 26.3 | 13.4 | 35.0 | 24.5 | 3.3 | 8.6 | 16.0 |
| B75 | 44 | 4 | 0 | 26.0 | 58.2 | 32.2 | 31.3 | 12.6 | 17.9 | 28.9 | 13.6 | 3.9 | 6.6 |
| B14 | 49 | 6 | 0 | 80.0 | 7.0 | ||||||||
| B08 | 90 | 6 | 0 | 60.4 | 15.0 | ||||||||
| B72 | 54 | 6 | V | 21.3 | 76.0 | 46.0 | 14.2 | 16.3 | 28.0 | 15.1 | 16.0 | 10.4 | 18.0 |
| B46 | 66 | 7 | S | 20.7 | 85.5 | 81.0 | 20.7 | 19.4 | 50.0 | 20.7 | 45.0 | 12.9 | 32.0 |
| B63 | 39 | 8 | S | 39.7 | 67.4 | 48.0 | 46.8 | 31.1 | 32.8 | 48.7 | 32.5 | 15.3 | 24.8 |
ID case number prefix T or B refers to tricuspid or bicuspid valve. CAS score=Measure of severity of pathologic changes.
Atherosclerosis or associated comorbidity: 0=not detectable, S=Clinically stable atherosclerosis present as temporally remote need for a single revascularization procedure, or asymptomatic coronary artery or aortic atherosclerosis found at time of surgery, V=vasculopthic with history of multiple occlusions requiring revascularization procedures, PMR=polymyalgia rheumatic and dissecting ascending aortic aneurysm.
Peripheral blood of CAS cases contains an expanded population of CD28null memory-effector T lymphocytes
The proportion of circulating CD8+ T cells that extinguished expression of CD28, indicating differentiation to the memory-effector phenotype, was substantially increased in tricuspid CAS, range 36.6 to 96%, mean 69.7% of CD8 T cells, and in bicuspid CAS, range 20 to 85.5%, mean 65.8%, Table I. Among tricuspid cases the percentage of CD8+CD28null T cells correlated with valve calcification severity (ρ = 0.666, p = 0.003). Although their mean age was 79±8 years, the correlation between CD8+CD28null T cells and calcification remained significant even when controlling for chronologic age (ρ = 0.694, p = 0.002). In the much younger bicuspid CAS patients, mean age 56± 18 years, the proportion of CD8+CD28null T cells was more than double the level expected in normal age-matched individuals.(13) For all valve types a greater proportion of CD8+CD28null T cells was seen in cases where the valve calcification score was ≥ 4 (p = 0.0006), and the correlation with calcification score wasρ = 0.590, p = 0.001. Further phenotyping, to verify the CD8 T cells had additional characteristics of the effector memory subset(25), showed the frequency of CD8+ CD57+ T cells was elevated, Table I, mean 47% and 48.4% in tricuspid and bicuspid CAS. We also measured the per cell expression of CD56, CD16 and CD158e1 (KIR3DL1, NKB1) on CD4 and CD8 T cell subsets and these structures were also principally expressed on CD28null T cells, (data not shown).
In peripheral blood, the percentage of CD4+CD28null T cells was more moderately elevated, mean = 14.8%, range 2.6 to 50.5% (Table I). This subset was increased among cases with extensive valvular calcification, CAS score ≥4 (p = 0.007). There was a significant difference in mean frequency of the CD4+CD28 T cell subset between the atherosclerotic positive (19.36%) and negative (6.94%) subsets, p= 0.007396, while all other cell populations in the table did not differ significantly between the two atherosclerotic disease subsets. However there was no difference between cases with stable isolated atherosclerotic disease requiring a revascularization procedure in the past and extensive ongoing vasculopathic disease. The mean percentage of CD4+CD57+ T cells was 12.7%.
T cell repertoire in CAS valves
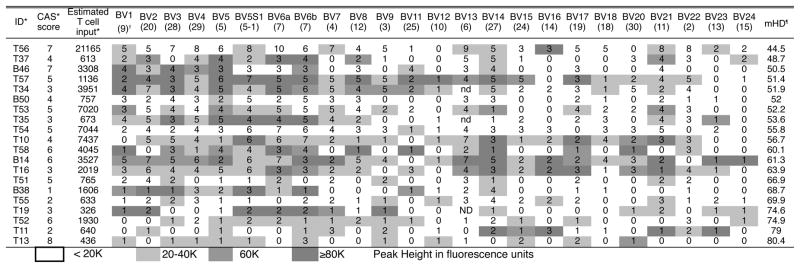
Figure 1 summarizes the repertoire characteristics of the infiltrating T cells for each BV family in 16 tricuspid CAS valves in terms of the number of peaks found on the β-chain length distribution and their peak area in fluorescence intensity units, illustrating that despite considerable heterogeneity, the majority of the valve infiltrating T cells in most valves and in most BV families consist of oligoclonal expansions, as shown by entries containing one, two, or three peaks. The average quantity of the BV family was classified as <20,000, 20-40,000, 40-80,000, and >80,000 according to the total fluorescence intensity units of summed for all peaks found in the BV family of that sample. The mean HD (mHD) across all expressed BV families was 60, range 45–80, illustrating the marked difference of the tricuspid valve repertoire from a polyclonal reference repertoire (p < 0.001) and emphasizing the overall oligoclonal character of the T cell infiltrate in CAS valves. We found the same pattern of extensive oligoclonality and variable polyclonality in bicuspid valves and their repertoire characteristics were indistinguishable from those of the tricuspid valves, Figure 1, mHD 57 (range 51–69). Additionally, a median of 13 BV families across all samples had no detectable or very low expression (<20K fluorescent units), consistent with highly selective entrance of clones into the valve.
Figure 1. Predominance of Oligoclonal Expansions across the BV Families among T cells Infiltrating Valve Leaflets in Tricuspid and Bicuspid CAS as determined by TCR β-chain length distribution analysis.
Each box contains the number of peaks for that BV family found on spectratyping and their peak height intensity, as shown by the shading intensity *ID case number prefix T or B refers to tricuspid or bicuspid valve. *CAS score=Measures severity of pathologic changes. Estimated T cell input based on equivalence of the quantity of RNA per reaction obtained from the valves, to the amount of RNA obtained from a given number of circulating T cells. †BV family, TRBV designation is in parentheses. ¶mHD=Mean Hamming Distance, which measures the average of the distance of the observed TCR β-chain length distribution from a polyclonal reference distribution.
Concerning heterogeneity among CAS samples in the extent of multi or polyclonality versus oligoclonality, seven CAS cases had at least one BV family with 6 or more peaks and 13 valves had fewer than 6 peaks in all BV families, Figure 1. The figure includes quantitation of the estimated T cell input determined by real time PCR, and is represented in terms of the RNA in the given number of peripheral blood T cells, although because of variability in per cell mRNA in tissue this does not represent the actual number of T cells in the tissue sample. No polyclonal BV families were found in any of the cases with CAS calcification scores of 1 or 2. Increasing valve polyclonality as measure by lower mHD correlated with estimated T cell input rank order, reflecting recovery of greater numbers of T cells from the polyclonal valves, (ρ = − 0.525, p = 0.039).
In 8 valves selected to represent the observed range of calcification severity, the presence of individual clonal expansions in the TCR repertoire was confirmed with higher resolution β-chain nucleotide sequencing (Supplemental table I). Some clones were considerably expanded, and when adjusted for the proportion of the BV family representation, were calculated to occupy a considerable fraction of the repertoire of valve-infiltrating T cells. For example clone JF731133 was calculated to occupy 10.94% of the repertoire of valve-infiltrating T cells (Supplemental table I). The sequence data summarized in table II using Simpson’s diversity index (SDI) of 0.8, further emphasizes the departure of the valve TCR repertoire from a polyclonal distribution. Although there was no correlation between β-chain length polyclonality, as measured by mHD, and CAS score, the degree of TCR repertoire polyclonality in the valve as measured using βchain nucleotide sequencing data (Table II) was directly and significantly correlated with the severity of calcification (ρ = 0.887, p = 0.003).
Table II.
Oligoclonality in the T Cell Repertoire of CAS Valves and in Peripheral Blood Phenotypic Subsets, Determined by β-Chain Nucleotide Sequencing and by β-Chain Length Distribution Analysis
| ID† | CAS Score† | Valve
|
Peripheral Blood
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repertoire Complexity | CD8* | CD8+ CD28null |
CD8+ CD28+ |
CD4+ |
||||||||
| % CD8 T Cells | Repertoire Complexity | % CD8 T Cells | Repertoire Complexity | Repertoire Complexity | ||||||||
| SDI‡ | mHD‡ | % | SDI | mHD | SDI | mHD | SDI | mHD | ||||
| T55 | 2 | 0.27 | 69.9 | 99 | 38 | 0.57 | 64 | 72 | 0.67 | 48.2 | 0.99 | 20.2 |
| T19 | 3 | 0.68 | 74.6 | - | 54 | - | - | 46 | - | - | - | - |
| T10 | 4 | 0.84 | 56.7 | 61 | 90 | - | - | 10 | - | - | - | - |
| T54 | 5 | 0.91 | 55.8 | 50 | 81 | 0.05 | 62.8 | 19 | 0.89 | 54.6 | 0.99 | 20.3 |
| T57 | 5 | 0.82 | 51.4 | 44 | 72 | 0.75 | 60.9 | 28 | 0.79 | 35.6 | 0.9 | 11.2 |
| T56 | 7 | 0.98 | 44.5 | 15 | 79 | 0.52 | 67.4 | 21 | 0.96 | 49.8 | 0.99 | 38.1 |
| B38 | 1 | 0.76 | 68.7 | 83 | 60 | 0.89 | 50.6 | 40 | 0.99 | 29.8 | 1 | 10.9 |
| B14 | 6 | 0.93 | 61.3 | 70 | 74 | - | - | 26 | - | - | - | - |
| B46 | 7 | - | 50.5 | - | 72 | 0.85 | 61.1 | 28 | 0.78 | 40 | 0.94 | 16.3 |
|
| ||||||||||||
| Mean | 4.4 | 0.8 | 59.3 | 68.9 | 0.6 | 61.1 | 32.2 | 0.8 | 43.0 | 1.0 | 19.5 | |
CD8 refers to percentage of CD3+ T cells in the valve that are CD8+ by double immunostaining. (data not shown for 7 additional samples)
ID case number, prefix T or B refers to tricuspid or bicuspid valve. CAS score=Measure of severity of pathologic changes defined in the methods.
Simpson’s Diversity Index, an index of repertoire diversity determined from β-chain sequencing, 1.0=polyclonal. mHD=Mean Hamming Distance, the average of the distance between the observed TCR β-chain length distribution and a polyclonal reference, 0=polyclonal.
Immunohistologic staining of valves
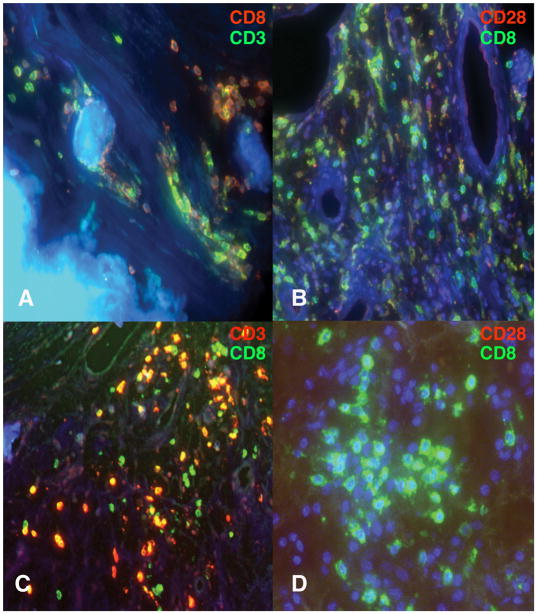
Immunostaining of the valves was performed to define the phenotype and distribution of infiltrating T cells in relationship to the repertoire analyses. Valves with multi or polyclonal T cell infiltration usually exhibited many regions of very abundant infiltration by CD8 and CD4 T cells, Figure 2, panels A and B. This abundance on staining was consistent with estimated T cell recovery from the valve during RNA extraction, Figure 1. Sometimes infiltration by CD20 staining B cells was also found in these valves, not illustrated. In contrast more oligoclonal samples, Figure 2, panels C and D had more sparse regions of T cell infiltration that predominantly stained for CD8. Among the 14 cases available for immunostaining the percentage of CD3 T cells that expressed CD8 directly correlated with the degree of repertoire oligoclonality as measured by mHD (ρ = 0.679, p = 0.011, table II). In all valves, the majority of CD8 T cells either lacked detectable expression of CD28 or staining was very dim, as shown in Figure 2, frames B and D. These CD28null or CD28dimCD8+ T cells were often found in proximity to sites of calcification (not illustrated) or neovascularization, Figure 2, panel C. Reciprocally, in valves with greater proportions of polyclonal T cells, with higher percentages of CD4+CD3+ T cells, more were CD28brtCD8−.
Figure 2. Representative examples of CAS valve immunostaining.
Frame A, from valve T50 with a repertoire containing more polyclonal T cells, stained for CD3 (Green) and CD8 (Red) shows a mixture of infiltrating CD8 and CD3+CD8−, presumably CD4 T cells, in a region of calcification. Some CD8 T cells are closely applied to the calcified nodule. Frame B stained for CD8 (Green) and CD28 (Red) shows a major predominance of CD8+CD28null T cells in a region of neovascularization in valve T10, which also contains more polyclonal T cells. Some T cells appear to be CD8dimCD28dim on the merged image. A few CD8−CD28+ T cells are present. Frame C shows neovascularization in oligoclonal valve T13 containing a predominant infiltrate of CD8+CD3+ T cells. Frame D stained for CD8 (Green) and CD28 (Red) shows an aggregate of CD8+CD28null T cells in valve B38 also infiltrated with an oligoclonal T cell repertoire.
Clonal expansions in circulating T cell subsets
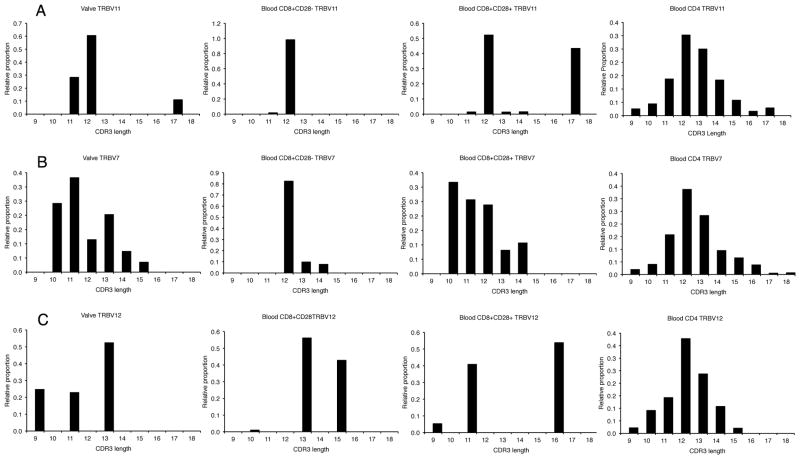
Three examples of the spectratypes of the circulating T cell subsets and those of the paired valve are illustrated in Figure 3. Comparison of Figure 3, panel B with figure 4 shows that in general the overall results of the low resolution spectratype method parallel the distribution of clone β-chain sequences. A marked degree of oligoclonality in the circulating CD28null CD8+ memory-effector subset was seen and it was usually more restricted than the heart valve. A summary analysis of the clonal composition of the TCR repertoires in two subsets of circulating CD8 T cells also showed that the CD8+CD28null repertoire has the greatest degree of oligoclonality, mHD 61.1, Table II. This oligoclonality was also illustrated by sequencing in representative case T54, Figure 4 and table III and Supplemental table I. In contrast, the CD4+ repertoire was uniformly highly polyclonal (Figure3, Figure 4, bottom right panel) mHD of 19.5 and SDI of 1.0 (Table II). Figure 4 shows that the CASSLDT sequence (JF731330) accounts for virtually all of the TRBV7 memory-effector repertoire (97.4%), and that this clonotype accounts for 2.47% of this individual’s entire CD8 T cell repertoire, as shown in the supplementary table. Interestingly, the CD8+CD28+ T cell subset in CAS also contained a variable number of clones that have undergone limited proliferation, mHD of 43 and SDI of 0.8 (Table II, Figure 3 and figure 4), e.g. JF731135, JF731137, JF731139, and JF731222-JF731229. Table III illustrates three examples of the clonotype frequency of selected clones in the CD8+CD28null and the CD8+CD28+ T cell subset, showing that the clonal expansions in these instances occupied up to 5.41% of the total subset repertoire in the case of the CD8+CD28+ T cell subset and 1.51% of the CD8+CD28null repertoire.
Figure 3. Comparison of β-chain length distribution histograms (spectratypes) of the T cell repertoire in CAS valves and associated peripheral blood subsets.
Each section contains four histograms depicting the T cell repertoire composition in the valve and circulating lymphocyte CD8+CD28null, CD8−CD28+, and CD4 subsets. Section A from sample T55 illustrates an oligoclonal pattern in the valve and the two CD8 subsets, with a major expansion seen at CDR3 length of 12 in the first three distributions. The subsequent sequence-based repertoire analysis of this sample in Table III illustrates that this peak consists of a single shared CD8 T cell clonotype. The CD4 T cell repertoire was polyclonal. Section B from sample T54 illustrates a slightly more multiclonal valve sample. The CD8+CD28null repertoire was highly oligoclonal, while the CD8−CD28+repertoire was more multiclonal, but clearly different from the polyclonal CD4 repertoire. Subsequent sequence analysis in Figure 4 shows an expanded clone at CDR3 length 12 in the CD8+CD28null subset that was shared with the valve and that the major CDR3 length 10 expansion in the CD8−CD28+subset was shared with the valve. Section C shows a markedly oligoconal pattern in the valve of T54 and in the CD8 subsets of the TRBV 12 family repertoire. Sequence analysis reveals that the major expansion at CDR3 length of 13 present in the valve and in the CD8+CD28null subset was due to a highly expanded shared clonotype, see JF731131 and JF731132 in the supplementary table.
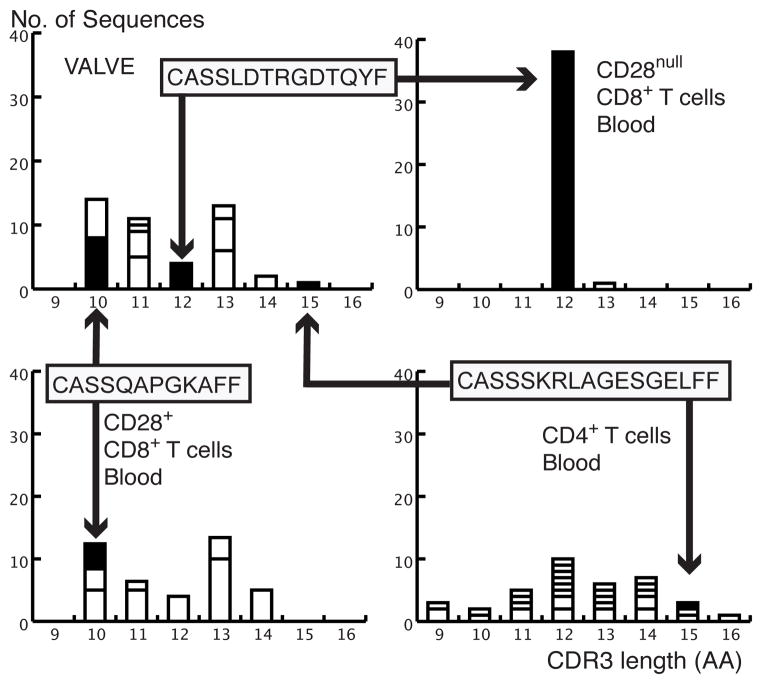
Figure 4. The clonal composition in the valve and in three peripheral blood CD8+ and CD4+ subsets for case T54 as determined by TCR β-chain nucleotide sequencing for the BV6 (TRBV7) family.
The CD8+CD28null subset was the most oligoclonal, while the CD8+CD28+ subset contains 9 clones of which 8 are moderately expanded. 22 of the 29 clones in the CD4 subset are single sequence indicating a highly polyclonal population. Solid rectangles containing the clonotypic CDR3 sequences denote clonotypes shared between the circulation subsets and the valve, with the arrows reflecting clonal lymphocyte trafficking between the sites. One clone with the sequence CASSLDTRGDTQYF was shared between the valve and the CD8+CD28null subset, where it was highly expanded (Accession numbers JF731129 and JF731130). A second clone with the sequence CASSQAPGKAFF was shared between the valve and the CD8+CD28+ subset, and was proportionally more expanded in the valve than in the CD8+CD28+ subset (Accession numbers JF731136 and JF731137). A third clone with the sequence CASSSKRLAGESGELFF was shared between the valve and the CD4 T cell subset and was present as a single sequence clone in both sites (Accession numbers JF731140 and JF731141).
Table III.
Two Examples of Shared T Cell Clones Identified in the Aortic Valve and in Peripheral Blood Phenotypic Subsets with Identical β-Chain Sequences, Indicating Clonal trafficking
| GenBank Accession number | Case | Sample Source | CDR3 length | CDR3 Region Nucleotide Sequence, Junction Analysis and Translation* | Number sequences in clone† | Clonotype frequency in BV family (%) | Clonotype frequency in repertoire (%) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JF731133 | T55 | Tricuspid Valve | 12 | TGTGCCAGCAGCTTAGctttGGCtttCAATGAGCAGTTCTTC | 18 | 85.7 | 10.94 | ||||||||||||||||||
| C | A | S | S | L | A | L | A | F | N | E | Q | F | F | ||||||||||||
| JF731134 | T55 | Circulating CD8+CD28neg T cells | 12 | TGTGCCAGCAGCTTAGctttGGCtttCAATGAGCAGTTCTTC | 3 | 17.7 | 1.51 | ||||||||||||||||||
| C | A | S | S | L | A | L | A | F | N | E | Q | F | F | ||||||||||||
| JF731135 | T55 | Circulating CD8+CD28+ T cells | 12 | TGTGCCAGCAGCTTAGctttGGCtttCAATGAGCAGTTCTTC | 4 | 20 | 1.97 | ||||||||||||||||||
| C | A | S | S | L | A | L | A | F | N | E | Q | F | F | ||||||||||||
| JF731138 | T55 | Tricuspid Valve | 17 | TGTGCCAGCAGCTTAaatGACAGGGGagtaggacttagCTCCTACGAGCAGTACTTC | 2 | 9.5 | 1.21 | ||||||||||||||||||
| C | A | S | S | L | N | D | R | G | V | G | L | S | S | Y | E | Q | Y | F | |||||||
| JF731139 | T55 | Circulating CD8+CD28+ T cells | 17 | TGTGCCAGCAGCTTAaatGACAGGGGagtaggacttagCTCCTACGAGCAGTACTTC | 11 | 55 | 5.41 | ||||||||||||||||||
| C | A | S | S | L | N | D | R | G | V | G | L | S | S | Y | E | Q | Y | F | |||||||
Upper case denotes germ line residues encoded respectively by the TRBV element, TRBD element (underlined) and TRBJ element. Lower case denotes N or P non-germline encoded nucleotides.
The number of identical TCRB sequences found in the sample that comprises the expanded clone, only one sequence of which has been entered in Genbank.
Identification of the same expanded clones in both valve tissue and peripheral blood
Of 33 expanded clones in the peripheral blood found by sequence analysis 27 clonotypes were not present in the valve, and 13 of 19 clones expanded in the valve were not detected in blood, indicating that T cell entrance into the valve was selective and expansion may occur after entrance of a progenitor T cell (Supplement Table 1, and data not shown).
Figure 3 panel A depicts the spectratype result showing the striking predominance of an expansion at CDR3 length 12 in the first three frames. Table III illustrates that in sequence analysis a clone, JF731133, was present in the valve in case T55 with the CDR3 sequence CASSLALAFNEQFF and length 12 and that this accounted for 85.7% of the TRBV 11 repertoire. Moreover the sequence of this clone was identical, including regions encoded by N and P diversity nucleotides, to the sequences of clones present both in the circulating CD28+ CD8+ subset, JF731134, and the CD28null CD8+ memory-effector subset, JF731135, which respectively account for 17.7% and 20% of the two CD8 subset repertoires, table III. The JF731133 clone is substantially expanded in the valve, accounting for 10.94% of the entire repertoire of T cells infiltrating the valve, table III. The presence of the same clonotype in these three sites indicates clonal traffic between the circulation and the valve, and suggests the CASSLALAFNEQFF clone is both proliferating and in the process of extinguishing CD28 expression in the blood. Table III also illustrates the sequence evidence showing a second expanded clone was shared between the valve leaflets, JF731138, and the CD28+ CD8+ subset, JF731139, which has not yet extinguished CD28 expression. The potential sharing of this clone was also evident by spectratype analysis, with an expansion at CDR3 length 17, Figure 3 panel A.
Figure 4 illustrates additional examples of the clonal trafficking between the valve and the T cell subsets as determined by nucleotide sequencing. For case T54, one expanded valve clone, JF731129, with the clonotype CASSLDTRGDTQYF, which accounts for 8.9% of the TRBV7 family in the valve was also the highly expanded dominant clone in the circulating memory-effector CD8+CD28null subset, JF731130, as described above. The expanded clone in the valve at CDR3 length=10, JF731136, which accounts for about 1.7% of the entire T cell repertoire in the valve, was shared with an expanded clone in the naïve CD28+CD8+ subset, JF731137, that has not yet extinguished the expression of CD28. In 5 similar instances, the valve clonotype was traced to a CD8+ precursor, e.g. JF731131 and JF731132, Table III, Supplement Table I, and data not illustrated. We identified only one single sequence clone from the valve, JF731140, from atherosclerotic case T55 that was identical to a single sequence clone in the CD4+ compartment, JF731141, (Supplement Table I, Figure 4).
Discussion
In this study we sought to evaluate the immunological significance of T lymphocytes infiltrating the valve leaflets in CAS. The central findings included, first that clonal expansions in cases with bicuspid or tricuspid CAS valves were associated wih parallel changes in the circulating lymphocyte population including, marked T cell activation, denoted by HLA-DR expression, and extensive clonal expansion with differentiation to to memory-effector status, reflected by extinguishing expression of CD28 and acquisition of NK markers. These changes were most strikingly evident in the CD8 T cell subset and were highly correlated with the severity of valve calcification and stenosis. Second, on immunostaining of the valve, the infiltrating CD8 T cells were mainly CD28null orCD28dim. Third, we extended the initial finding in tricuspid CAS(8) by identifying similar clonal expansions in bicuspid valves by β-chain sequencing and obtaining evidence indicating that polyclonal CD4 T cells often also infiltrated valves with higher degrees of calcification. Lastly the clonal expansions in the valve were directly linked to the activation and differentiation events in the peripheral blood by demonstrating trafficking of members of the same T cell clone between the peripheral circulation and the valve. Taken together these data suggest the interpretation that a systemic adaptive immune response is occurring in individuals developing CAS, and that this systemic response is coupled to the lymphocytic infiltration of the valves, where it could be driven by the recognition of peptide antigens expressed in the valve.
Several findings point to an important role of CD8 T cells and in particular the CD8+CD28null T cells in CAS. They include identification of this subset in the valve on immunostaining, as well as the correlation of the degree of its expansion in blood with the severity of valve calcification. Activation and differentiation to memory-effector status among circulating lymphocytes also strongly predominated in the CD8 T cell subset. Moreover, HLA- DR expression was particularly increased on the CD8+CD57+ T cell subset, indicating their continued activation. Furthermore 8 instances of sharing of the same T cell clones expanded both in the valve and in the peripheral blood memory-effector CD8+CD28null T cells shown in figure 4 and table III directly link clonal entrance and expansion of T cells within the valve to the activated and memory-effector T cells in the circulation. The quantitative characteristics of the expansion of the CD8 T cell clones involved in trafficking between the circulating T cell population and the heart valve included an example of where a shared clone accounted for 10.94% of all of the T cells infiltrating the valve. Additionally most of these cells had an approximate individual clone frequency in blood of 1 to 2% or more of all T cells in the CD8 memory-effector subset, suggesting that they account for a considerable proportion of the circulating T cells exhibiting a memory-effector phenotype. Together with the earlier findings where 23 of 24 clones identified both among the circulating lymphocytes and in the valve were CD8 lineage T cells(8) these findings also suggest the peptides driving this aspect of the immune response in CAS likely have a cytoplasmic origin.
Evidence was also obtained that CD4 T cells might also be involved in aspects of CAS. Maturation to memory-effector subsets of the circulating CD4 T cells was found, albeit at a much lower and more variable level. There were two features to the elevation in CD4+CD28null T cells: first, the intensity of this response correlated with the severity of valve disease; and second, of great interest, the primary association of elevations in this CD4+CD28null T cell subset appeared to be with clinically significant atherosclerotic disease (Table I). The relationship between this CD4 T cell subset and unstable atherosclerotic disease and with rheumatoid arthritis is well recognized(26, 27), although none of these conditions were noted in the present CAS cases. Subsetting atherosclerotic disease into extensive vasculopathic versus stable did not account for the elevation in the CD4+CD28null T cell subset, which appeared to be with demonstrable atherosclerotic disease, per se. One instance of a shared, but not detectably expanded, clone between the CD4 blood subset was found (Figure 4), JF731140 and JF731141 in a case that also had atherosclerotic disease. It is possible this instance of a shared T cell clone reflects the contribution of the co-morbid atherosclerotic process(28, 29). To the extent that CD4+CD28null T cells reflect an atherosclerotic process, in the subset of about half of the cases with atherosclerosis, it appears this process is linked to enhancing CAS severity. However importantly, as emphasized by Wu et al.(8), the overall oligoclonal nature and CD8 lineage of valve-infiltrating T cells that predominate in CAS contrast sharply with the findings reported in atherosclerosis(28, 29), emphasizing the difference of the immunopathologic events that characterize CAS.
While oligoclonal expansions were identified in all valves, some valves with higher degrees of calcification also exhibited an additional component of polyclonal T cell infiltration. They had a lower proportion of CD3+CD8+ T cells and increased CD28brt T cells, frequently together with infiltrating CD20+ B lymphocytes. We interpret this to indicate that in valves with a multi or polyclonal TCR repertoire, more of the infiltrating T cells are naïve, clonally unexpanded CD4 lineage cells, which likely do not participate in the process via cognitive recognition by their clonotypic TCR but may reflect a response to chemokines released in later stages of immune-mediated tissue injury. Reciprocally, these findings also suggests that the T cell infiltration initially consists of a relatively few dominant CD8 T cell clonal expansions.
In normal chronologic aging the prevalence of CD28nullCD57+ T cells increases in the CD8+ and, to a much lesser extent, in the CD4+ compartment(14) along with large clonal expansions in this subset.(15-17) These expansions been attributed to the sustained proliferation of CD8+ T cells involved in maintaining viral latency, as well as the involvement of CD4 and CD8 T cells in diverse chronic inflammatory conditions. It is well recognized that responses to persistent viral infection such as CMV also contribute to the expansion of the CD8+CD28null T cell population.(30, 31) five of a subset of 11 cases in table I examined for the presence of CMV infection by PCR were positive, a level consistent with the age and ethnicity of these cases.(32) There was no correlation between CMV positive or negative status and the size of the CD8+CD28null population, or the mean CAS score, data not illustrated. Effros and colleagues advanced the concept that CD8+CD28null T cells are immunosenescent and that their expansion modulates both immune and non-immune functions and contributes to various age-related pathologies. Intriguingly, the present findings in tricuspid CAS are generally consistent with the association noted by Effros et al., especially given the advanced age of the tricuspid CAS cases, and in this respect tricuspid valve CAS can be added to conditions characterized by expansions of memory-effector T cells. However our interpretation of mechanism differs from that proposed by Effros et al. in that CAS does not appear to be a secondary consequence of immunosenescence. Our data are more consistent with the possibility that a specific immune response involving the valve drives the activation of CD8 T cells and their maturation to CD28null phenotype. This interpretation is supported by: a) The high expression of HLA-DR and CD69 on CD8+CD28null T cells in CAS that distinguishes this condition from the CD8+CD28null T cell populations in aging; b) The extent of the expansion of the CD28nullCD8+subset was directly and significantly correlated with the CAS severity score (p=0.003), but was independent of chronologic age; and c) Perhaps most consequentially, the complete resemblance of the findings in blood and in the valve between the much younger bicuspid valve CAS cases and the older tricuspid valve cases. The counter possibility could be raised that those with bicuspid aortic valves preferentially develop age inappropriate immunosenescence to account for this finding, but then one would have to propose an explanation for the age inappropriate immunosenescence. Accordingly, while multiple conditions contribute to the expansion of the expansion of the CD28nullCD8+subset, a major proportion of the elevation in these CAS cases appears to be contributed by the immune processes operating in CAS. However, the study was limited by its overall design and the lack of a large control population of age-matched individuals with varying co-morbidities to provide a better measure of the specificity of the findings for CAS.
The questions arise of what immune recognition event, such as a response to intracellular pathogens or self-antigens, might be driving the immune events and what role does this immune response play in CAS. Because CAS occurs in the setting of different clinical situations of bicuspid and tricuspid aortic valves and co-morbidities including hypertension and atherosclerosis that have in common enhanced hemodynamic strain and diminished valvular compliance, we hypothesize that valve mesenchymal cells alter their transcriptional phenotype in response to enhanced strain, resulting in the cellular expression of stress-induced molecules. We envision peptides from these stress-induced molecules, to which the individual may not be fully tolerized, are expressed in the context of class I MHC, and an idiosyncratic CD8 T cell immune response is induced in a small subset of individuals. This response seen in the circulating T cells is analogous to a peptide-driven immune response and results in infiltration of the valve by expanding and activated T cell clones that recognize the stress neoantigen. We speculate these proliferating T cells release cytokines that alter the expression of valve mesenchymal cells and recruit additional lymphocytes into the valve, suggesting that at a minimum one consequence of this inflammation is a further and potentially reversible decrease in valvular compliance. Support for this possibility comes in part from preliminary data in 8 cases where significant production of γ interferon was found in the CD8+CD28null T cell subset, data not shown, and from the earlier reports identifying HLA-DR expression on valve mesenchymal cells.(33) It is possible that the activated memory-effector CD8 T cells directly injure target valve cells, driving the development of heterotopic calcification, with precedent for development of analogous heterotopic calcification in the setting of the autoimmune CD8+CD28null T cell response in diseases like psoriatic arthritis or ankylosing spondylitis. Further study of the immunologic specificity and features of these T cells may provide additional insight into the pathogenesis and the nature of the underlying immune recognition events of this increasingly common valvular disease and bear on whether the immune response might be a potential target for immunosuppressive therapy.
Supplementary Material
Acknowledgments
Sources of Funding
*Supported by a grant from the USPHS R01 HL084599 to R.J.W. and a generous gift of the Siegel Family (Advanced Traineeship in Rheumatology, to M.W.)
Abbreviations
- CAS
Calcific aortic stenosis
- IMGT
International Immunogenetics Information System
- AA
Amino Acid
- HD
Hamming Distance
- SDI
Simpson’s diversity index
References
- 1.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 2.Pomerance A. Pathogenesis of aortic stenosis and its relation to age. Br Heart J. 1972;34:569–574. doi: 10.1136/hrt.34.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 4.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 5.Wallby L, Janerot-Sjoberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88:348–351. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol. 2003;34:756–763. doi: 10.1016/s0046-8177(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 7.Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, et al. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol. 2004;43:1670–1676. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 8.Wu HD, Maurer M, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stuart AS, Winchester R. The Lymphocytic Infiltration In Calcific Aortic Stenosis Predominantly Consists of Clonally Expanded T Cells. Journal of Immunology. 2007;178:5329–5339. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
- 9.Yu DT, Winchester RJ, Fu SM, Gibofsky A, Ko HS, Kunkel HG. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. Journal of Experimental Medicine. 1980;151:91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speiser DE, Valmori D, Rimoldi D, Pittet MJ, Lienard D, Cerundolo V, MacDonald HR, Cerottini JC, Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990–1999. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 13.Hsu HC, Scott DK, Zhang P, Zhou J, Yang P, Wu Q, Schroeder HW, Jr, Gerald LB, Ravussin E, Jazwinski SM, et al. CD8 T-cell immune phenotype of successful aging. Mech Ageing Dev. 2006;127:231–239. doi: 10.1016/j.mad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schächter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 15.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandres E, Merino J, Vazquez B, Inoges S, Moreno C, Subira ML, Sanchez-Ibarrola A. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(-)CD57(+) subpopulation. Clin Immunol. 2000;96:230–235. doi: 10.1006/clim.2000.4894. [DOI] [PubMed] [Google Scholar]
- 17.Vallejo AN. Age-dependent alterations of the T cell repertoire and functional diversity of T cells of the aged. Immunol Res. 2006;36:221–228. doi: 10.1385/IR:36:1:221. [DOI] [PubMed] [Google Scholar]
- 18.Warren BA, Yong JL. Calcification of the aortic valve: its progression and grading. Pathology. 1997;29:360–368. doi: 10.1080/00313029700169315. [DOI] [PubMed] [Google Scholar]
- 19.Curran SA, FitzGerald OM, Costello PJ, Selby JM, Kane DJ, Bresnihan B, Winchester R. Nucleotide sequencing of psoriatic arthritis tissue before and during methotrexate administration reveals a complex inflammatory T cell infiltrate with very few clones exhibiting features that suggest they drive the inflammatory process by recognizing autoantigens. J Immunol. 2004;172:1935–1944. doi: 10.4049/jimmunol.172.3.1935. [DOI] [PubMed] [Google Scholar]
- 20.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- 22.Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debre P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nature Medicine. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 23.Costello PJ, Winchester RJ, Curran SA, Peterson KS, Kane DJ, Bresnihan B, FitzGerald OM. Psoriatic arthritis joint fluids are characterized by CD8 and CD4 T cell clonal expansions appear antigen driven. J Immunol. 2001;166:2878–2886. doi: 10.4049/jimmunol.166.4.2878. [DOI] [PubMed] [Google Scholar]
- 24.Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol Methods. 2007;321:182–195. doi: 10.1016/j.jim.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Werwitzke S, Tiede A, Drescher BE, Schmidt RE, Witte T. CD8beta/CD28 expression defines functionally distinct populations of peripheral blood T lymphocytes. Clin Exp Immunol. 2003;133:334–343. doi: 10.1046/j.1365-2249.2003.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. The Journal of clinical investigation. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 28.Stemme S, Rymo L, Hansson GK. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991;65:654–660. [PubMed] [Google Scholar]
- 29.Oksenberg JR, Stavri GT, Jeong MC, Garovoy N, Salisbury JR, Erusalimsky JD. Analysis of the T-cell receptor repertoire in human atherosclerosis. Cardiovasc Res. 1997;36:256–267. doi: 10.1016/s0008-6363(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 30.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, et al. The immune system in extreme longevity. Experimental gerontology. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. Journal of Immunology. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 32.Boppana SB, Fowler KB. Persistence in the population: epidemiology and transmisson. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge; 2007. [Google Scholar]
- 33.Olsson M, Rosenqvist M, Nilsson J. Expression of HLA-DR antigen and smooth muscle cell differentiation markers by valvular fibroblasts in degenerative aortic stenosis. J Am Coll Cardiol. 1994;24:1664–1671. doi: 10.1016/0735-1097(94)90172-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.