Abstract
Objective
There is no general consensus regarding the optimal follow-up strategy for patients with melanoma. We sought to determine the utility and cost effectiveness of radiologic restaging of patients with stage IIB-IIIC melanoma at the 3-year follow-up time point.
Methods
A retrospective review of 210 patients diagnosed with stage IIB–IIIC melanoma seen in the Cutaneous Oncology Program at Beth Israel Deaconess Medical Center (BIDMC) between January, 2001 and July, 2006 was conducted. 52 patients were asymptomatic and continuously disease free and underwent re-staging head CT/MRI and torso CT scans three years after completion of local-regional therapy or initiation of adjuvant treatment. True positive, false positive and normal scans were identified and the cost per diagnosis calculated.
Results
Fifty-five percent of patients followed at BIDMC recurred; 88% before 3 years (median time to recurrence 12 months, 95% CI: 10–16 months). The majority (69%) recurred with disease symptoms. Twenty five head CT scans, 27 head MRIs, and 52 torso CTs were performed. One false positive head CT and 5 abnormal torso CT scans (3 false positive, 2 true positive) were identified. The total cost per diagnosis was $312,990.
Conclusions
Extensive 3-year re-staging imaging appears to be of limited value for symptomatic and continuously disease free patients with stage IIB–IIIC melanoma. Furthermore, given the low risk of recurrence beyond 3 years, it is likely that subsequent routine imaging would have similarly low utility.
Keywords: melanoma, imaging, CT scan, cost-benefit analysis
Introduction
In 2010, there will be an estimated 68,130 new cases of melanoma and 8,700 deaths in the United States [1]. Patients with stage IIB to IIIC melanoma have a lifetime risk of melanoma related mortality of 30–60% [2]. Most recurrences are detected within the first two to three years after diagnosis of the primary and/or regional nodal disease [3]. No general consensus exists regarding the optimal follow-up strategy for patients at high risk of disease recurrence. However, it is generally accepted that the goal of surveillance approaches is to detect recurrent disease at a time when it is most treatable. Patients with high risk melanoma often undergo extensive staging studies including computed tomography (CT), positron emission tomography (PET), and/or magnetic resonance imaging (MRI) prior to surgical management and as part of routine follow-up. However, routine imaging has not been shown to be clinically useful in this setting [4–6]. Several retrospective studies evaluating the methods of detection of first disease recurrence have demonstrated that most recurrences are detected by the patient as either a new symptom or by the patient or physician as a new physical finding [3,7–9]. Furthermore, there appeared to be no difference in survival based on whether the recurrence was detected by the patient or the physician [3,7,10]. This was affirmed recently in a large retrospective study involving follow-up patients with stage III disease. This study showed that 47% of first recurrences were discovered by the patient or family member and further suggested that physical exam beyond 3 years for patients with stage IIIA, 2 years for patients with stage IIIB and 1 year for patients with stage IIIC would detect few recurrences, as ≤5% of patients had local/regional recurrences after these time points [11].
Currently, the National Comprehensive Cancer Network guidelines suggest that patients with high risk melanoma should undergo a history and physical every 3–6 months for 2 years, then every 3–12 months for 3 years and then annually as clinically indicated [12]. In addition, chest x-ray, CT and/or PET-CT scans as well as annual brain MRI are suggested to be considered in screening for recurrence. Routine laboratory testing and imaging is not recommended after 5 years[12]. Thus, despite the lack of supportive data, many patients with history of melanoma who are asymptomatic continue to undergo serial routine surveillance imaging aimed at detection of distant disease relapse.
The surveillance approach for patients with stage IIB-IIIC melanoma managed by Beth Israel Deaconess Medical Center Cutaneous Oncology Program (COP) involves visits every 6 weeks for the first 6 months post surgery, then every 3 months until year 3, then every 6 months until year 5, and annually, thereafter. Patients are subjected to a focused history, physical exam and have a complete blood count and serum lactate dehydrogenase measured at each visit. Surveillance chest x-rays are performed every other visit for the first 5 years and annually after year 5. CT scans or other imaging are not routinely performed and are only obtained in the setting of worrisome new symptoms, physical findings, or laboratory or chest x-ray abnormalities. In 2004, the COP began performing restaging CT scans of the torso and head imaging three years from completion of local-regional therapy or initiation of adjuvant treatment in patients with stage IIB–IIIC melanoma. The goal was to provide an additional measure of confidence of disease free status before moving from 3 to 6 month interval follow-up visits. Five and 1/2 years into this practice, we examined the COP patient database to determine the utility and cost effectiveness of this approach.
Methods
This study involved a retrospective review of patients diagnosed with stage IIB–IIIC melanoma between January, 2001-July, 2006 and followed within the COP at Beth Israel Deaconess Medical Center. Patients who underwent routine re-staging head CT/MRI and torso CT scans three years after completion of their local regional therapy or initiation of their adjuvant treatment were identified from the existing COP database of over 2000 patients with melanoma. Formal analysis was restricted to those patients who were over 18 years of age, free from disease recurrence and remained asymptomatic at the time of their 3 year follow-up imaging. Imaging occurred between 1/2004 and 7/2009. Baseline demographic and disease characteristics of all patients diagnosed with stage IIB–IIIC melanoma in this time period were documented. Information was collected regarding their disease outcome, treatment and follow-up imaging. The standard follow-up schedule involved visits every 6 weeks for the first 6 months post surgery, then every 3 months until year 3, then every 6 months until year 5, and annually, thereafter. Thus, most patients were seen at 3 month intervals during the 3 year time period and had chest x-rays every 6 months. With rare exception, torso CT or head CT/MRIs were performed only for patients with new symptoms, physical findings, laboratory or chest X-ray abnormalities. This research study was approved by the Dana Farber/Harvard Cancer Center Institutional Review Board.
Findings on the 3 year imaging studies were categorized as either: 1) true positive, 2) false positive, or 3) normal. Follow-up procedures arising from the positive findings were documented and the cost per true positive diagnosis of recurrent melanoma was determined using the standard cost for each imaging procedure during the follow-up imaging time period. The cost for head MRI was $5,195, for head CT $2,734 and torso CT was $7,592. Additional costs of follow-up imaging or biopsy procedures for patients with abnormal 3 year imaging were also estimated and added to the overall cost of surveillance. The medical records were reviewed to a median follow up of 23 months after 3-year imaging and patient outcomes were documented.
Statistical Considerations
Outcomes were determined by review of the raw data contained in the COP Database. Fisher’s exact tests and Wilcoxon two-sample tests were used to compare clinial features such as age, gender, location of tumor, Breslow thickness of tumor, Clark level, disease stage and interferon useage (yes or no) between the group of patients who underwent 3 years scans and those who did not undergo 3 years scans. A Kaplan-Meier curve was used to plot relapse-free survival. Relapse-free survival was defined as the time from melanoma diagnosis to recurrence or death. All tests were two-sided with alpha at 0.05 level. The result was determined to be statistically significant if the p-value of the test is less than 0.05.
Results
Four hundred and thirty-nine patients were identified with stage IIB–IIIC melanoma during the designated timeframe. Of these, 52 patients (12%) underwent 3-year follow-up CT scan/MRI and met study criteria. An additional 158 (36%) patients who did not undergo 3-year imaging continued to be followed at BIDMC. The remaining patients were followed in their local communities or otherwise lost to follow-up. Characteristics of the 210 patients who were at BIDMC are displayed in Table 1 separated by whether they did or did not undergo 3-year follow-up imaging. The 52 patients with 3-year follow-up imaging contained a slightly lower proportion of patients with stage IIIB disease (19% vs 27%, P=0.28), a significantly higher proportion of patients with stage IIIA disease (35% vs 9%, P<0.0001), and significantly higher percentage of patients who received adjuvant interferon (87% vs 63%, P=0.0017), than the 158 patients who did not undergo 3-year follow-up imaging. Otherwise the two groups were very similar. Median age for the population as a whole was 54 years, 59% were male and 23% had been diagnosed with stage II disease.
Table 1.
Demographic and Pathologic Characteristics of Patients who did and did not undergo 3-year Surveillance Imaging
| Variable | Underwent 3 year scans | Did not undergo 3 year scans | Total | |||
|---|---|---|---|---|---|---|
| (n=52) | Followed at BIDMC (n=158) | (n=210) | ||||
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 32 | 62 | 91 | 58 | 123 | 59 |
| Female | 20 | 38 | 67 | 42 | 87 | 41 |
| Age, years | ||||||
| Median | 54 | 54 | 54 | |||
| Range | 16–76 | 24–86 | 16–86 | |||
| Location | ||||||
| Trunk | 15 | 29 | 65 | 41 | 80 | 38 |
| Extremity | 16 | 31 | 48 | 30 | 64 | 30.5 |
| Head/neck | 14 | 27 | 30 | 19 | 44 | 21 |
| Unknown primary | 6 | 12 | 15 | 10 | 21 | 10 |
| Prostate | 1 | 2 | 0 | 0 | 1 | 0.5 |
| Breslow Thickness (mm) | ||||||
| Median | 2.9 | 3.0 | 3.0 | |||
| Range | 1.1–55 | 0.25–34 | 0.25–55 | |||
| Ulceration | ||||||
| Present | 11 | 21 | 58 | 37 | 69 | 33 |
| Absent | 31 | 60 | 55 | 35 | 86 | 41 |
| Unknown | 4 | 8 | 30 | 19 | 34 | 16 |
| NA | 6 | 12 | 15 | 9 | 21 | 10 |
| Clark level | ||||||
| II | 0 | 0 | 2 | 1 | 2 | 1 |
| III | 2 | 4 | 7 | 4 | 9 | 4 |
| IV | 36 | 69 | 83 | 53 | 119 | 57 |
| V | 6 | 12 | 22 | 14 | 28 | 13 |
| Unknown | 2 | 4 | 20 | 13 | 22 | 11 |
| NA | 6 | 12 | 24 | 15 | 30 | 14 |
| Mitosis, mm2 | ||||||
| Median | 3 | 6 | 4 | |||
| Range | 0–60 | 0–33 | 0–60 | |||
| Stage | ||||||
| IIB | 6 | 12 | 27 | 17 | 33 | 16 |
| IIC | 4 | 8 | 11 | 6 | 14 | 7 |
| IIIA | 18 | 35 | 15 | 10 | 33 | 16 |
| IIIB | 10 | 19 | 43 | 27 | 53 | 25 |
| IIIC | 14 | 27 | 62 | 39 | 76 | 36 |
| Interferon | ||||||
| Yes | 45 | 87 | 100 | 63 | 145 | 69 |
| No | 7 | 13 | 58 | 37 | 65 | 31 |
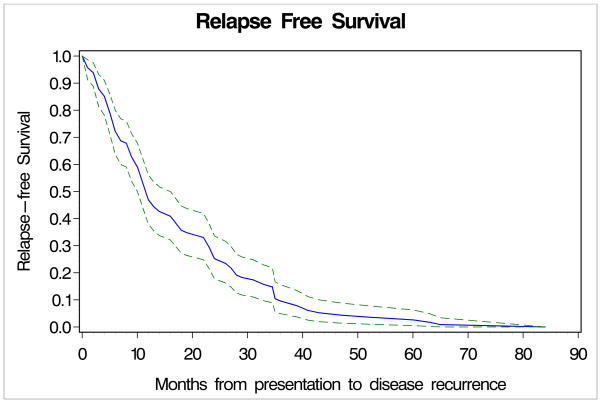
The imaging studies and disease outcomes of the 210 patients followed within the BIDMC COP are displayed in Table 2. Of these 210 patients, 115 (55 %) experienced disease recurrence (101 recurred before 3 years and 14 at or after 3 years). Of the 101 patients whose disease recurred before 3 years, 70 (69%) recurrences were identified by symptoms, 9 (8%) by physical exam, 3 by laboratory testing and 5 by routine chest X-ray. The majority of the patients recurring with symptoms noted skin or subcutaneous nodules or lymphadenopathy (75%). The reminder had neurologic symptoms (9%), pain (6%), pathologic fracture, hemoptysis, ascites, lymphedema, systemic symptoms or pharyngitis from a metastatic deposit. Fifteen patients’ (15%) recurrences were first identified on more sophisticated imaging (13 CT scans, 1 liver ultrasound and 1 PET-CT). Given that routine imaging other than chest x-ray was not a standard procedure in the COP, the reason for imaging was documented. Eight patients were on clinical trials which required CT scans, 2 had CT scans by physicians outside of BIDMC for restaging, 1 had liver ultrasound following up another medical issue that incidentally identified recurrence, and 3 underwent scans at BIDMC for restaging for unspecified reasons. Of the patients recurring before 3 years, 70 (69%) have died of melanoma, 1 died of colon cancer and one died of unknown causes. The median time to recurrence was 12 months (Figure 1), and the median time from recurrence to death was 13 months.
Table 2.
Methods for Recurrence Detection
| No. of patients | % patients | |
|---|---|---|
| Total number of patients | 210 | |
| Total number recurred | 115 | 55% |
| Recurred before 3 years | 101 | 48 % |
| Symptoms | 70 | 69 % |
| Physical exam | 9 | 8 % |
| Imaging | 15 | 15 % |
| CT | 13 | |
| MRI | 0 | |
| PET | 1 | |
| Ultrasound | 1 | |
| CXR | 5 | 5 % |
| Labs | 3 | 3 % |
| Died of melanoma | 70 | 69 % |
| Died of other causes | 1 | 1 % |
| Died of unknown cause | 1 | 1 % |
| Alive | 29 | 29 % |
| Unknown | 1 | 1 % |
| Recurred after 3 years | 12 | 6 % |
| Symptoms | 9 | 75 % |
| Physical exam | 2 | 17 % |
| Imaging | 0 | |
| CXR | 1 | 8 % |
| Died of melanoma | 4 | 33 % |
| Alive | 8 | 67 % |
| Recurred on 3-year imaging | 2 | 1 % |
| Died of melanoma | 2 | 100 % |
| Did not recur | 95 | 45 % |
| Alive | 86 | 91 % |
| Died of other causes | 8 | 8 % |
| Died of unknown cause | 1 | 1 % |
| Total (followed at BIDMC) | 210 | |
| Alive | 123 | 59 % |
| Died of melanoma | 76 | 36 % |
| Died of other causes | 9 | 4 % |
| Died of unknown cause | 2 | 1 % |
Figure 1.
Fifty-seven patients who remained asymptomatic and recurrence free at the 3-year time point did not undergo the proposed restaging imaging. This largely reflected incomplete initial uptake of the new staging policy as 49% of these patients crossed their 3-year follow-up threshold by January 2006 (2 years after the policy was first proposed). In this group of patients, 9 eventually developed recurrence following the 3-year time point, with 7 (78%) being detected based on symptoms with the remaining 2 (22%) being identified based on exam or chest x-ray. Of these 9 patients, 3 (33%) have died of melanoma, while the remaining 6 patients are alive at a median follow-up of 23 months from diagnosis of stage IV. The median months to recurrence in this group of 9 patients was 53 months, and the median number of months from recurrence to death for the 3 patients who have died was 34 months.
Of the 52 patients undergoing imaging at the 3-year time point, there was 1 false positive and no true positive head CT/MRIs and 3 false positive and 2 true positive torso CTs (Table 3). The total cost including 3-year follow-up scans, additional imaging (1 repeat head CT, 2 additional chest CTs, 1 repeat CT torso, 1 abdominal MRI) and diagnostic procedures (1 CT-guided lung biopsy and one pelvic lymph node biopsy) was $625,980 (Table 4). Thus, the cost per diagnosis of melanoma recurrence was $312,990.
Table 3.
Results of three-year imaging
| Radiologic Studies (No. patients=52) | No. of abnormal scans | False positive | True positive | False Positive rate | True positive rate |
|---|---|---|---|---|---|
| Head CT (n=25) | 1 | 1 | 0 | 4 % | NA |
| Head MRI (n=27) | 0 | 0 | 0 | NA | NA |
| All Head Imaging (n=52) | 1 | 1 | 0 | 2 % | NA |
| Torso CT (n=52) | 5 | 3 | 2 | 6% | 4 % |
| Total | 6 | 4 | 2 | 8 % | 4 % |
Table 4.
Cost of Imaging
| 3 year imaging | No. of scans | Cost of scans | No. of scans in follow up of abnormal findings | Cost of follow up imaging | Total cost |
|---|---|---|---|---|---|
| CT torso | 52 | $7,592.00 | 1 | $7,592.00 | $402,376.00 |
| CT head | 25 | $2,734.00 | 1 | $2,734.00 | $71,084.00 |
| MRI head | 27 | $5,195.00 | 0 | 0 | $140,265.00 |
| Other follow up imaging/procedures | |||||
| CT chest | $2,306.00 | 2 | $4612.00 | $4612.00 | |
| Abdominal MRI | $6,052.00 | 1 | $6,052.00 | $6,052.00 | |
| CT-guided lung biopsy | $779.00 | 1 | $779.00 | $779.00 | |
| CT-guided pelvic LN biopsy | $812.00 | 1 | $812.00 | $812.00 | |
| Total cost | $625, 980.00 |
Both patients with true positive scans developed symptomatic recurrence within 1 week of positive imaging and died at 1 and 6.5 months after the diagnosis of distant metastatic disease (Table 5). In the remaining 50 patients, at a median follow up of 23 months after the 3-year follow-up imaging, 2 patients have developed symptomatic distant metastatic melanoma with one patient succumbing to this disease and 1 patient has developed a local/regional recurrence that was detected on physical exam and surgically resected.
Table 5.
Characteristics and outcomes in patients who recurred on 3 year imaging
| Patient No. | Age | Sex | Location of primary | Pathology | Location of metastatic disease | No. of months after 3year scans until symptoms | Additional therapy | No. of months after recurrence until death |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | F | Lower extremity | 1.9 mm, 3 mit/mm2, CL IV, NU, Stage IIIA | Retroperitoneal, inguinal, pelvic LN | 0.25 | HD IL-2 Dacarbazine | 6.5 |
| 2 | 70 | F | Head and neck | 1.33 mm, 15 mit/mm2, CL IV, NU, Stage IIIB | Lung, hemidiaphragm | 0 | None | 1 |
Mit=mitoses, CL=Clark level, NU=non-ulcerated, LN=lymph nodes
Discussion
Currently, there are no standardized guidelines regarding routine surveillance imaging in patients with high-risk melanoma. Three single-institution studies evaluated the role of CT imaging at the time of diagnosis in patients with documented local-regional metastases and with a normal chest radiograph and LDH [8,9,13]. These series demonstrated a low true positive rate (4–16%) and a higher false positive rate (8–22%) [8,9,13]. Despite the low yield in detecting distant metastases in patients with stage III disease, the percentage of true positive findings is not insignificant, making further imaging reasonable in this patient population, especially in patients with inguinal nodal disease who are being considered for inguinal and possibly pelvic node dissection. The utility of subsequent imaging in such patients has not been fully examined.
Relapse free survival data in patients with stage IIB–IIIC melanoma suggest that 30–80% of patients will experience a tumor recurrence with 50–80% of those recurrences happening within 3 years of initial presentation [3,14]. Consequently, many programs recommend more intensive patients follow-up in the first 3 years following surgical management in patients with high-risk melanoma with subsequent follow-up being reduced to every 6 months. Given that most disease recurrences are detected via careful history and physical exam and that these would be less frequent following the 3-year milestone, we sought to determine whether restaging head and torso imaging at the 3-year follow-up time point, prior to lengthening the routine follow-up interval, could identify sufficient numbers of patients with then asymptomatic disease for whom such extended interval follow-up would be inappropriate. Having incorporated routine restaging head CT or head MRI and torso CT scans for patients remaining asymptomatic 3 years following initial diagnosis, we sought to determine not only the yield of this approach, but its cost effectiveness.
Of the 439 patient charts reviewed, 229 patients (52%) were followed elsewhere and 210 (48%) were followed at BIDMC. Of those followed at BIDMC 115 patients (55%) developed disease recurrence with a median follow-up of close to 5 years. While these figures are consistent with the published recurrence risks for the stage IIB–IIIC patient population, it is likely that higher risk patients chose to maintain their follow-up at BIDMC and furthermore, other patients returned to BIDMC when they developed symptoms or physical findings possibly reflecting recurrence. Therefore, this recurrence percentage most likely overstates the recurrence risk for the 439 patients as a whole.
Of note, 101 (88%) of these recurrences happened before the 3 year time point (median 12 months, 95% CI: 10–16 months). This median relapse free survival and percent relapse at 3 years are also consistent with the published literature for this high risk patient population. In two randomized controlled trials Eastern Cooperative Oncology Group (ECOG) E1684 and Intergroup E1690 comparing high dose IFN-α 2b versus observation in patients with resected stage IIB–III melanoma demonstrated a statistically significant improvement in relapse free survival but little to no benefit in overall survival [14–15]. Given that 87% of patients in our study received adjuvant interferon treatment, which has been reported to prolong median relapse free survival by around 8–9 months [14], this median time to recurrence of 12 months in our cohort is surprisingly short. This further suggests a potential bias toward those patients experiencing recurrence being more likely to be followed at BIDMC. Nonetheless, these data also suggest that our general screening approach of omitting CT imaging did not result in a significant delay in diagnosis of melanoma recurrence relative to the published literature.
Retrospective studies have shown that 80–90% of recurrences are discovered by history and physical exam, rather than by imaging or laboratory tests [16–18,19]. A prospective study was performed at the Sydney Melanoma Unit of patients with stage I, II or III melanoma to determine the value of surveillance studies [10]. Of all recurrences, 73 % were detected by the patient. There was no statistically significant difference in survival between patients who discovered the recurrence themselves versus those discovered by physicians [10]. These findings were consistent with our findings that 69 % (79/115) of patients who recurred presented with symptoms. However, given our more regimented follow-up schedule the majority of recurrences in our population were detected by the physician either by history or physical exam.
Three-year follow-up imaging performed in the 52 patients in our study revealed 6 abnormal scans; of which 4 were false positive. Given that 85% of recurrences happen before the 3-year time point and 80% of those recurrences are detected by history or physical examination, it is not surprising that routine imaging at the 3-year time point did not detect many recurrences. Furthermore, the fact that the 2 patients documented to have true disease recurrence at the 3-year time point developed symptomatic disease within 1 week of their imaging, further undercuts the value of such delayed “landmark” imaging as a clinical tool. The cost of 3-year follow-up imaging and related diagnostic testing for the 52 patients in this analysis was estimated to be $625,980. Thus, the cost per diagnosis of metastatic disease was $312,990. While undoubtedly the imaging provided some comfort to those patients who received clean reports, this unquantifiable benefit must be balanced against the likely emotional distress and physical and perhaps economic consequences associated with additional testing that was experienced by the 4 patients (8%) with false positive scans. Thus, in this time of increasing cost-consciousness, it is hard to justify the performance of such routine landmark based imaging.
Furthermore, given the low risk of recurrence beyond 3 years, it is likely that subsequent routine imaging would have similarly low utility. A recent study of stage III melanoma patients suggests that routine imaging after 2 years in stage IIIC patients and after 3 years in stages IIIA and IIIB would likely detect only a small number of recurrences [11]. The current follow-up schedule utilized by the BIDMC Cutaneous Oncology Program. Whether such routine imaging may have value within the first 12–18 months, when the majority of recurrences are likely to occur, remains to be assessed. However, given that the vast majority of recurrences are picked up by symptoms and physical exam and the lack of current evidence that earlier detection is associated with improved outcome, documentation of such a role for earlier imaging seems unlikely. The BIDMC COP follow-up schedule, omitting the three year imaging, is sufficient in identifying the majority of recurrences and therefore represents a practical and effective follow-up strategy.
References
- 1.National Cancer Institute. Surveillance Epidemiology and End Results. 2010. [Google Scholar]
- 2.Balch CM, Soong SJ, Atkins MB, Buzaid AC, Cascinelli A, Coit DG, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–49. doi: 10.3322/canjclin.54.3.131. quiz 182-4. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Houghton A, Jr, Sober A, Soong SJ, Atkins MB, Thompson JF. Missouri: Quality Medical Publishing, Inc. 5. 2009. Cutaneous Melanoma; pp. 565–572. [Google Scholar]
- 4.Aloia TA, Gershenwald JE, Andtbacka RH, Johnson MM, Schacherer CW, Ng CS, et al. Utility of computed tomography and magnetic resonance imaging staging before completion lymphadenectomy in patients with sentinel lymph node-positive melanoma. J Clin Oncol. 2006;24:2858–65. doi: 10.1200/JCO.2006.05.6176. [DOI] [PubMed] [Google Scholar]
- 5.Miranda EP, Gertner M, Wall J, Grace E, Kashani-Sabet M, Allen R, et al. Routine imaging of asymptomatic melanoma patients with metastasis to sentinel lymph nodes rarely identifies systemic disease. Arch Surg. 2004;139:831–6. doi: 10.1001/archsurg.139.8.831. discussion 836-7. [DOI] [PubMed] [Google Scholar]
- 6.Gold JS, Jaques DP, Busam KJ, Brady MS, Coit DG, et al. Yield and predictors of radiologic studies for identifying distant metastases in melanoma patients with a positive sentinel lymph node biopsy. Ann Surg Oncol. 2007;14:2133–40. doi: 10.1245/s10434-007-9399-3. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D, et al. Primary staging and follow-up in melanoma patients--monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87:151–7. doi: 10.1038/sj.bjc.6600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzaid AC, Tinoco L, Ross MI, Legha SS, Benjamin RS, et al. Role of computed tomography in the staging of patients with local-regional metastases of melanoma. J Clin Oncol. 1995;13:2104–8. doi: 10.1200/JCO.1995.13.8.2104. [DOI] [PubMed] [Google Scholar]
- 9.Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol. 1997;4:252–8. doi: 10.1007/BF02306618. [DOI] [PubMed] [Google Scholar]
- 10.Francken AB, Shaw HM, Accortt NA, Soong SJ, Hoekstra HJ, Thompson JF. Detection of first relapse in cutaneous melanoma patients: implications for the formulation of evidence-based follow-up guidelines. Ann Surg Oncol. 2007;14:1924–33. doi: 10.1245/s10434-007-9347-2. [DOI] [PubMed] [Google Scholar]
- 11.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and Timing of First Relapse in Stage III Melanoma Patients: Implications for Follow-Up Guidelines. J of Clin Oncol. 2010;28:3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) guidelines. 2010. [Google Scholar]
- 13.Johnson TM, Fader DJ, Chang AE, Yahanda A, Smith JW, 2nd, Hamlet KR, et al. Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Ann Surg Oncol. 1997;4:396–402. doi: 10.1007/BF02305552. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–58. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 16.Francken AB, Accortt NA, Shaw HM, Colman MH, Wiener M, Soong SJ, et al. Follow-up schedules after treatment for malignant melanoma. Br J Surg. 2008;95:1401–7. doi: 10.1002/bjs.6347. [DOI] [PubMed] [Google Scholar]
- 17.Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance. Patient or physician based? Ann Surg. 1995;221:566–9. doi: 10.1097/00000658-199505000-00014. discussion 569-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss M, Loprinzi CL, Creagan ET, Dalton RJ, Novotny P, O’Fallon JR. Utility of follow-up tests for detecting recurrent disease in patients with malignant melanomas. JAMA. 1995;274:1703–5. [PubMed] [Google Scholar]
- 19.Meyers MO, Yeh JJ, Frank J, Long P, Deal AM, Amos KD, et al. Method of detection of initial recurrence of stage II/III cutaneous melanoma: analysis of the utility of follow-up staging. Ann Surg Oncol. 2009;16:941–7. doi: 10.1245/s10434-008-0238-y. [DOI] [PubMed] [Google Scholar]