Abstract
β-catenin is constantly degraded through the ubiquitin-proteasomal pathway. We here report that a different type of β-catenin degradation is causally involved in epidermal cell death. We observed that reactive oxygen species (ROS) caused β-catenin degradation in the epidermal cells through a caspase-dependent mechanism, which results in disruption of cell adhesion. Disruption of cell adhesion increased ROS and activated caspases. Upregulation of the intact β-catenin blocked ROS accumulation and caspase activation. These results indicate that a feed-forward loop consisting of ROS, caspases activation and β-catenin degradation induces epidermal cell death.
Keywords: apoptosis, β-catenin, caspase, epidermis, reactive oxygen species
Introduction
β-catenin is a component of cell adherens junction (AJ) and also functions as a transcription factor involved in cell proliferation and differentiation (Clevers, 2006; Perez-Moreno & Fuchs, 2006). At steady state, cytosolic β-catenin is rapidly degraded by a destruction complex involving APC, axin, glycogen synthase kinase 3β (GSK3β) and casein kinase (Brembeck et al, 2006; Clevers, 2006; Xu & Kimelman, 2007). β-catenin is phosphorylated by GSK3β, which targets it for proteasome degradation. β-catenin is an abundantly produced protein, however, through this homeostatic β-catenin degradation pathway, the level of β-catenin is kept constant. It has been reported that β-catenin can be cleaved by protease including caspases and calpains (Abe & Takeichi, 2007; Brancolini et al, 1997; Brancolini et al, 1998; Herren et al, 1998; Li & Iyengar, 2002; Steinhusen et al, 2000; Van de Craen et al, 1999). In neuronal cells, the calpain-dependent cleavage stabilizes and stimulates transcriptional activity of β-catenin (Abe & Takeichi, 2007). In other cell types, β-catenin cleavage has been reported as one of the consequences of apoptosis (Bannerman et al, 1998; Brancolini et al, 1997; Brancolini et al, 1998).
TAK1 is a member of mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK), and is an indispensable signaling intermediate in MAPK-AP-1 and NF-κB pathways (Ninomiya-Tsuji et al, 1999; Shim et al, 2005; Takaesu et al, 2001). AP-1 and NF-κB are major transcriptional factors of cellular antioxidant genes such as ferritin and glutamylcysteine ligase (GCL) (Go et al, 2004; Pham et al, 2004; Yang et al, 2005). Ablation of TAK1 impairs MAPK and NF-κB pathways resulting in reduced cellular antioxidant capacity (Omori et al, 2008). We have previously reported that tak1-deficient cells are hypersensitive to ROS inducers because ROS are accumulated much higher levels in tak1-deficient cells compared to wild type cells (Omori et al, 2008). In epidermal cells (keratinocytes), we have previously found that TNF is a strong inducer of ROS in tak1-deficient conditions (Omori et al, 2008). In tak1-deficient epidermis of the skin, keratinocytes undergo TNF-ROS-induced apoptosis (Omori et al, 2008). However, the underlying mechanism through which ROS kill keratinocytes has not been fully defined. In the present study, we investigate how ROS cause keratinocyte apoptosis both in cultured cells and in an in vivo setting by using TNF-induced ROS in tak1-deficient cells as model systems. We observed that ROS-induced apoptosis is accompanied by caspase-dependent degradation of β-catenin. Furthermore, we found that this non-canonical β-catenin degradation is not only the result of caspase activation but is also the cause of apoptosis.
Results and Discussion
ROS cause β-catenin degradation in the in vivo epidermis
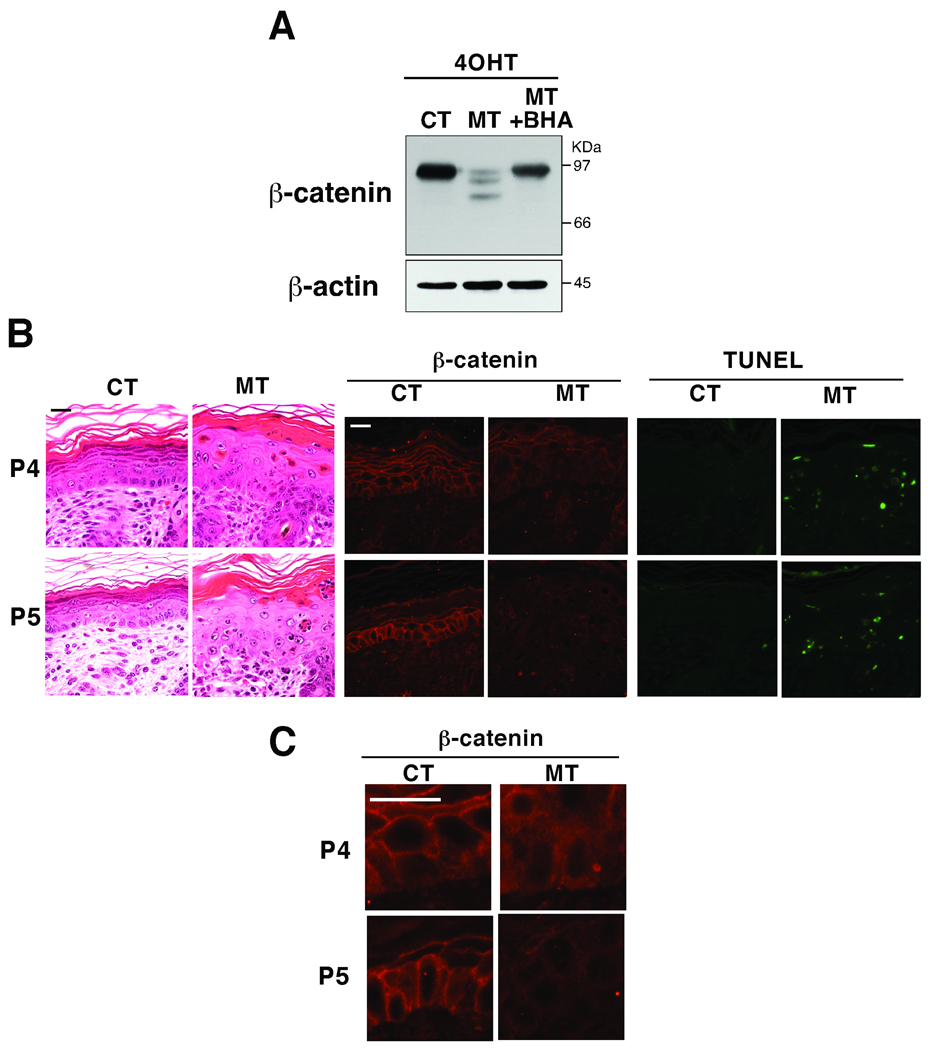
We analyzed two mouse models; one having an inducible epidermal-specific deletion of tak1 (K14-CreERT tak1 flox/flox) and the other having a constitutive epidermal-specific deletion of tak1 (K5-Cre tak1 flox/flox) mice (Fig. 1). TNF is always expressed at some levels in the normal skin, which is important for basal immunity (Pasparakis et al, 1996; Pasparakis et al, 1997). In the tak1-deficient epidermis, this TNF sufficiently induces ROS-dependent cell death (Omori et al, 2008). We treated 5-weeks old K14-CreERT tak1 flox/flox mice with 4-hydroxy tamoxifen (4-OHT) (Fig. 1A) which induced tak1 gene deletion and increased apoptotic epidermal cells as previously reported (Omori et al, 2008). We found that β-catenin was greatly decreased in the epidermis of 4-OHT-treated K14-CreERT tak1 flox/flox mice (Fig. 1A). The amount of intact β-catenin was diminished and several smaller degraded forms of β-catenin were observed. The degradation of β-catenin was blocked by feeding the mice a diet containing antioxidant butylated hydroxyanisole (BHA), suggesting that the degradation of β-catenin is associated with increased ROS (Fig. 1A). Neonatal mice with constitutive epidermal-specific deletion of tak1 (K5-Cre tak1 flox/flox) displayed severe dysregulation of epidermal homeostasis ((Omori et al, 2006) and Fig. 1B, 2nd panels from left). Apoptosis was highly induced in tak1-deficient neonatal epidermis ((Omori et al, 2006) Fig. 1B, most right panels) which was correlated with diminished membrane-bound β-catenin staining in postnatal day 4 and 5 (P4 and P5) (Fig. 1B middle panels and Fig. 1C). Impaired membrane localization of β-catenin was observed in the most areas of P4 TAK1 mutant skin, while β-catenin was largely diminished in some areas of P5 in TAK1 mutant skin.
Fig. 1.
(A) tak1 flox/flox (CT) and K14-CreERT tak1 flox/flox (MT) mice from the same litter were fed with BHA-containing or regular food, and treated with 4-OHT for 5 consecutive days. β-catenin was detected by immunoblot using protein extracts from epidermis at 2 days after the completion of 4-OHT treatment. (B) The dorsal skin sections of TAK1 flox/flox (CT) and K5-Cre tak1 flox/flox (MT) mice from the same litter at postnatal day 4 and 5 were stained with hematoxylin/eosin, anti-β-catenin antibody or TUNEL. Scale bar, 20 µm. (C) Pictures with a higher magnification of (B). Scale bar, 20 µm.
ROS induce β-catenin degradation and disruption of cell adhesion
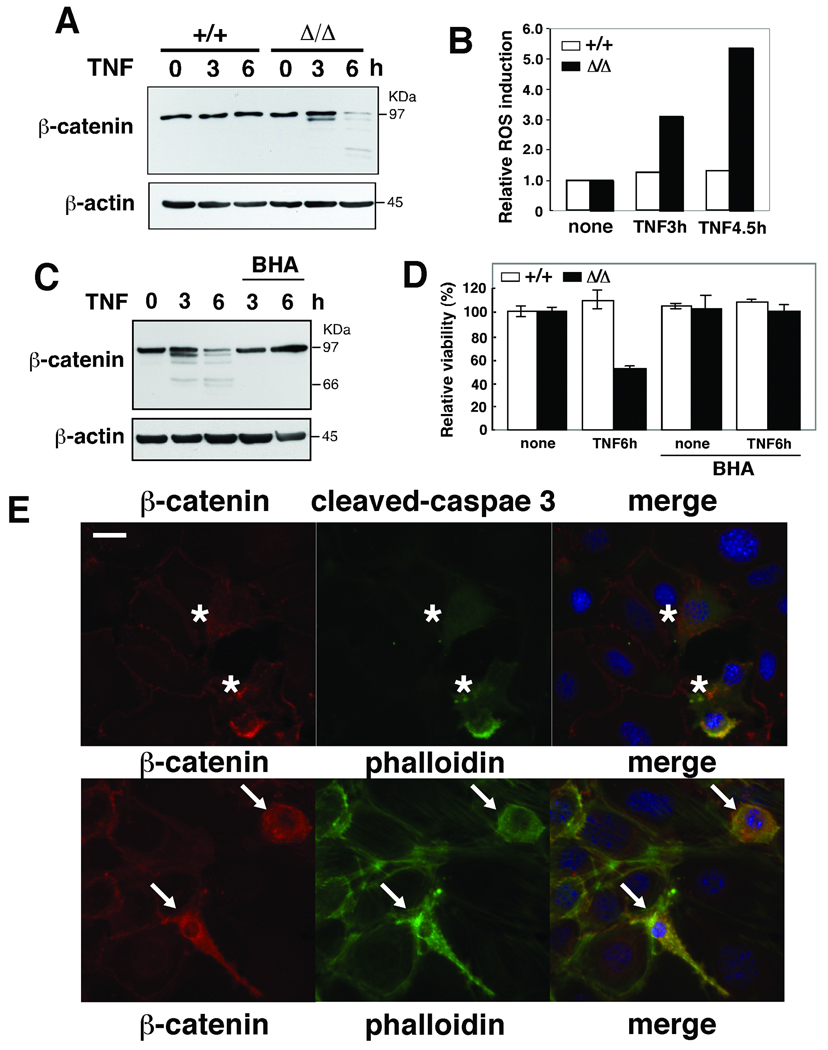
To further analyze the degradation of β-catenin, we utilized cultured tak1-deficient (Δ/Δ) keratinocytes (Omori et al, 2006). We have previously reported that TNF induce accumulation of ROS and caspase activation leading to apoptotic cell death in tak1-deficient keratinocytes, whereas no wild type keratinocyte undergo apoptosis upon TNF stimulation (Omori et al, 2008). We found that the level of β-catenin was diminished in TNF-stimulated tak1-deficient (Δ/Δ) but not in tak1 wild-type (+/+) keratinocytes (Fig. 2A), and restoration of wild type tak1 (Δ/Δ+TAK1) blocked the β-catenin degradation (supplementary Fig. S1A). Decreased β-catenin in TNF-stimulated tak1-deficient keratinocytes was correlated with accumulation of ROS and cell death (Fig. 2B, and D). The reduction of β-catenin and cell death were abolished by pretreatment of BHA (Fig. 2C and D), which is consistent with the in vivo results in mouse epidermis (Fig. 1A).
Fig. 2.
(A) tak1 wild-type (+/+) and tak1-deficient (Δ/Δ) keratinocytes were stimulated with 20 ng/ml TNF for 3 or 6 h and β-catenin was detected by immunoblot. (B) Cells with TNF stimulation were subsequently incubated with 10 µM CM-H2DCFDA for 30 min, and cells were analyzed by flow cytometry. The fluorescence units relative to that of unstimulated cells are shown. (C) tak1 Δ/Δ keratinocytes were pretreated with 100 µM BHA or vehicle ethanol for 1 h and stimulated with 20 ng/ml TNF for 3 or 6 h. β-catenin was detected by immunoblot. (D) tak1 +/+ and Δ/Δ keratinocytes were pretreated with 100 µM BHA or vehicle ethanol for 1 h and stimulated with 20 ng/ml TNF for 6 h. Cell viability was measured by MTT assay. Data are the mean±S.E. of three samples. (E) tak1 Δ/Δ keratinocytes were cultured in calcium-containing media for 2 h and subsequently stimulated with 20 ng/ml TNF for 24 h. Immunostainig of β-catenin (red), cleaved caspase 3 (green) and phalloidin (green) are shown. DAPI staining is shown in blue. Scale bar, 20 µm. The cells having diffuse β-catenin, punctate phalloidin and cleaved caspase 3 staining were counted in three independent samples. 97.9% ±SD 1.3% of cells having diffuse β-catenin staining were co-stained with cleaved caspase 3. 100% of cells having punctate phalloidin staining were co-stained with cleaved caspase 3.
We observed cells having diffuse non-membrane-bound staining of β-catenin in TNF-treated tak1-deficient (Δ/Δ) (Fig. 2E, asterisks). 98% of diffuse β-catenin cells were co-stained with cleaved caspase 3, indicating that they were apoptotic. Furthermore, phalloidin staining displayed a punctate pattern and retraction of fibers, indicating that disruption of actin filament and cell adhesions (AJs) were associated with the loss of membrane-bound β-catenin (Fig. 2E, arrows). All the cells showing the disruption of actin filament were co-stained with cleaved caspase 3. These results suggest that β-catenin degradation is associated with disruption of AJs. We also observed decreased amounts of other AJ components, E-cadherin and p120 catenin in TNF-stimulated tak1-deficient keratinocytes, and the reduction of those proteins were blocked by the antioxidant BHA treatment (supplementary Fig. S1B). These results suggest that AJs were disrupted via ROS in TNF-treated tak1-deficient keratinocytes.
Caspases degrade β-catenin
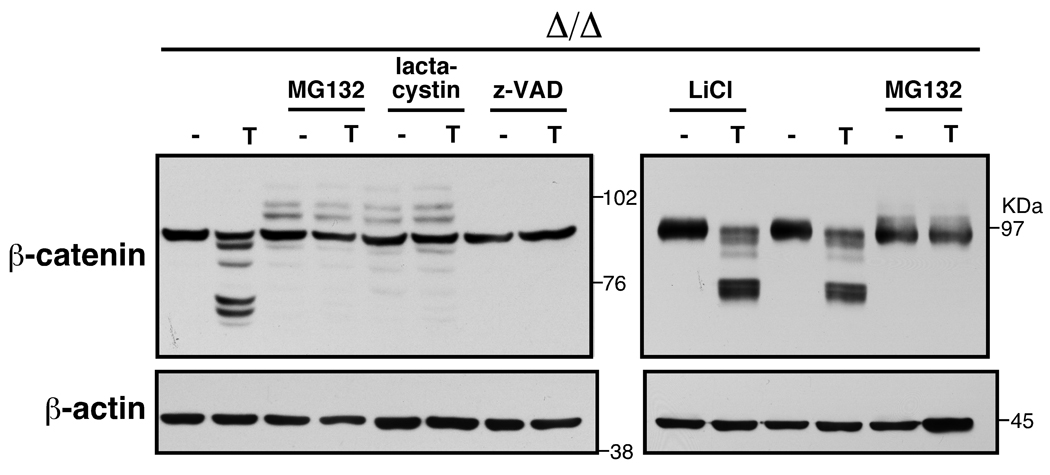
We next examined whether the reduction of β-catenin is mediated through the GSK3β-mediated proteasomal degradation pathway. We pretreated tak1-deficient keratinocytes with several types of inhibitors including proteasomal inhibitors (MG132 and lactacystin) and a GSK3β inhibitor (LiCl), and then stimulated the cells with TNF. Degradation of β-catenin was inhibited by pretreatment of a proteasomal inhibitor, MG132 or lactacystin (Fig. 3), but inhibition of GSK3β had almost no effect on TNF-induced β-catenin degradation. We found that a caspase inhibitor, Z-VAD-fmk (Z-VAD) abolished degradation of β-catenin and a calpain inhibitor N-acetyl-Leu-Leu-methional (ALLM) less effectively inhibited degradation of β-catenin (Fig. 3 and supplementary Fig. S1C). Therefore, it is likely that TNF-induced ROS do not degrade β-catenin through the homeostatic GSK3β pathway, but it may be through a non-canonical pathway which involves caspases.
Fig. 3.
tak1 Δ/Δ keratinocytes were pretreated with the indicated inhibitors for 0.5 h (LiCl) or 1.0 h (other inhibitors) and stimulated with 20 ng/ml TNF for 4 h (left panels) or 4.5 h (right panels). Cell lysates were immunoblotted with anti-β-catenin. 10 µM MG132, 5 µM lactacystin, 20 µM Z-VAD-fmk (Z-VAD), and 10 mM LiCl were used.
β-catenin degradation is causally involved in apoptosis
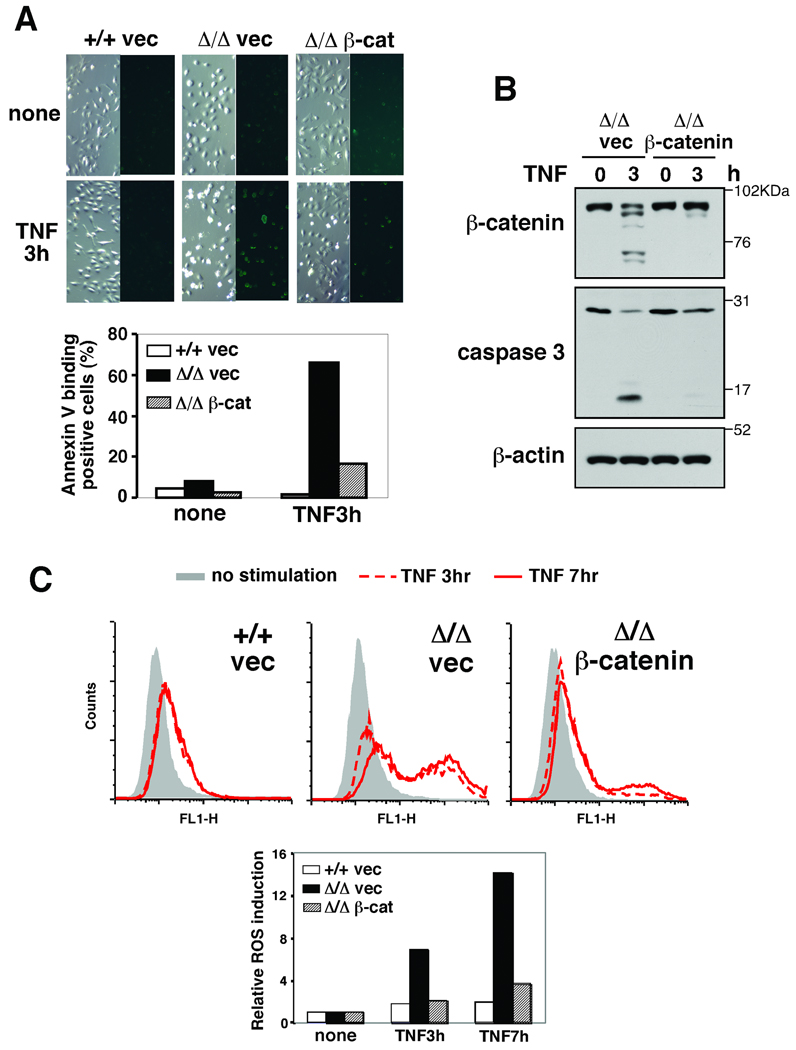
Epidermis is characterized by tightly connected cells (Perez-Moreno & Fuchs, 2006). AJs are important for maintenance of this tight connection, and disruption of cell-cell connection is known to be associated with apoptosis (Perez-Moreno & Fuchs, 2006; Steinhusen et al, 2000). Therefore, we postulated that β-catenin degradation-associated AJ disruption might not only be the result of caspase activation but also be causally involved in apoptosis in keratinocytes. To test this hypothesis, we attempted to prevent the reduction of the level of β-catenin, and examined TNF-induced apoptosis. We stably introduced a vector expressing β-catenin into tak1-deficient keratinocytes by using the retrovirus system. However, we noted that the level of β-catenin was not noticeably increased by the β-catenin vector infection in unstimulated keratinocytes. We postulated that β-catenin degradation through the GSK3β-proteasomal pathway might mask the increased β-catenin expression. To verify this, we used S33Y-β-catenin, which is not phosphorylated by GSK3β or degraded through the canonical pathway (supplementary Fig. S2). The expression level of S33Y-β-catenin was higher than that in the wild type β-catenin or control vector infected unstimulated keratinocytes (supplementary Fig. S2B), which is consistent with our hypothesis. We found that the stable introduction of even the wild type β-catenin vector could maintain intact β-catenin at nearly control levels following TNF stimulation (Fig. 4B and supplementary Fig. S2B). Exogenous expression of either wild type or S33Y-β-catenin reduced the number of TNF-induced morphologically apoptotic (shrink) cells and annexin V-binding positive cells (Fig. 4A and supplementary Fig. S2A). Activation of caspase 3 was found to be blocked by β-catenin overexpression (Fig. 4B). Furthermore, β-catenin overexpression could effectively block ROS accumulation (Fig. 4C).
Fig. 4.
(A) tak1 +/+ and Δ/Δ keratinocytes expressing vector or β-catenin were stimulated with 20 ng/ml TNF for 3 h and apoptotic cells were stained by Annexin V-Alexa Fluor 488. (B) tak1 Δ/Δ keratinocytes expressing vector or β-catenin were stimulated with 20 ng/ml TNF for 3 h and cell lysates were immunoblotted with the indicated antibodies. (C) tak1 +/+ and Δ/Δ keratinocytes expressing vector or β-catenin were stimulated with 20 ng/ml TNF for 3 or 7 h. Cells were subsequently incubated with 10 µM CM-H2DCFDA for 30 min and analyzed by flow cytometry. The fluorescence units relative to that of unstimulated cells are also shown.
We showed above that a proteasomal inhibitor MG132 reduced β-catenin degradation (Fig. 3). Therefore, we also examined whether MG132-mediated upregulation of β-catenin could modulate caspase, ROS and cell survival (supplementary Fig. S3). MG132 completely abolished TNF-induced caspase 3 activation and ROS accumulation. Collectively, maintenance of intact β-catenin level can block caspase 3 activation and ROS accumulation. These results indicate that degradation of β-catenin is not a consequence of apoptosis but causes ROS accumulation and apoptosis.
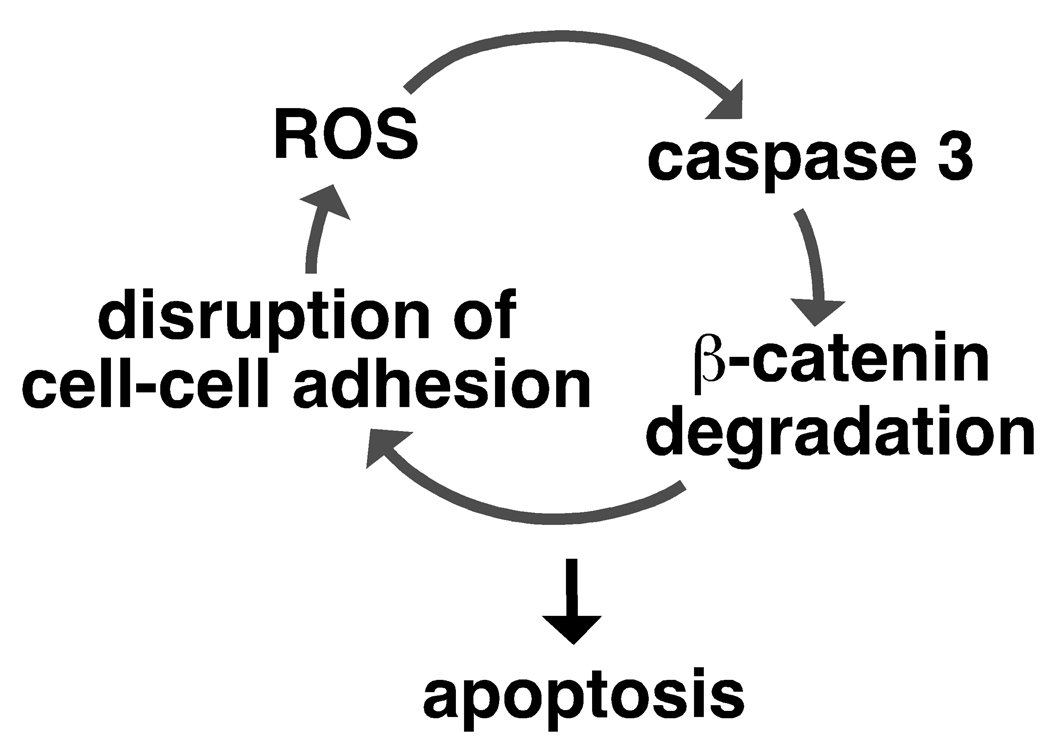
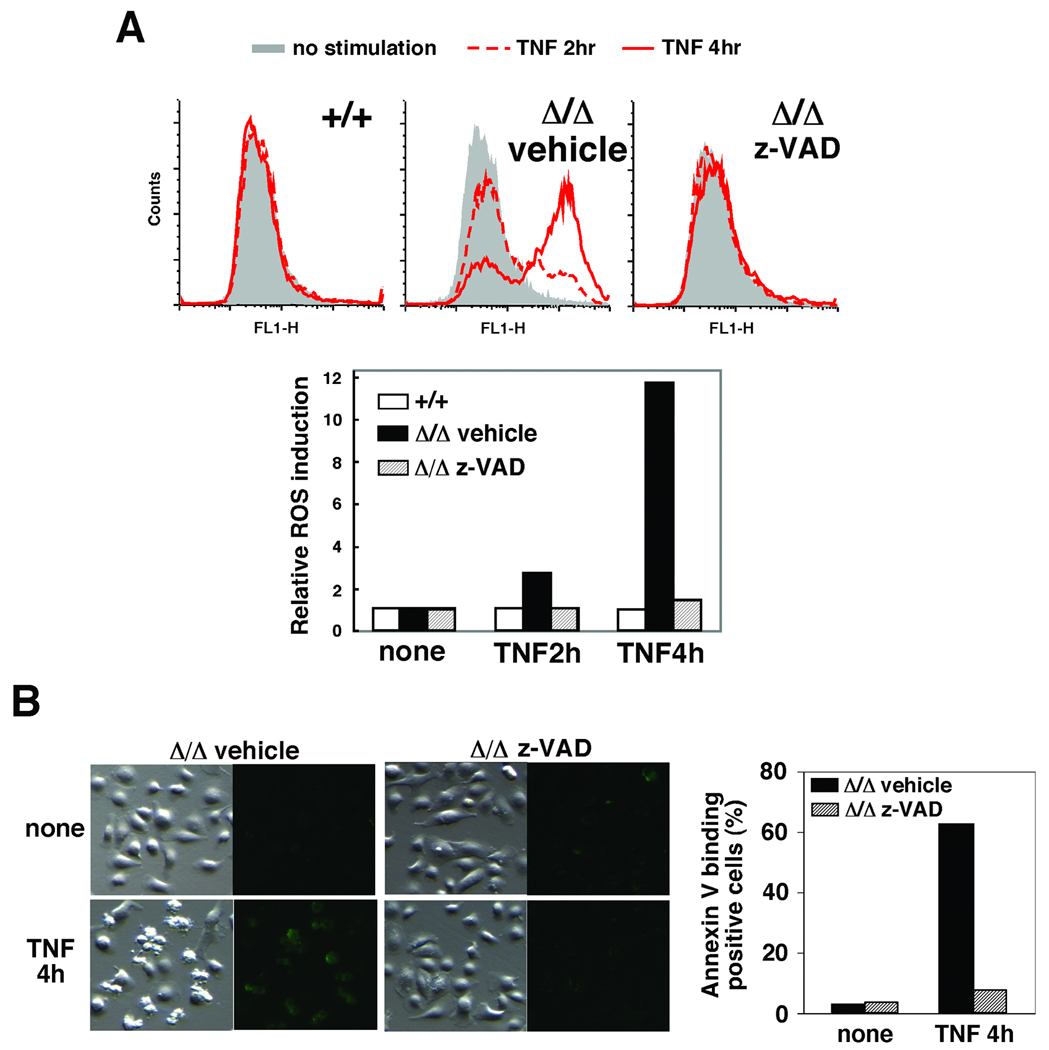
These results led us to hypothesize that ROS, caspase activation and β-catenin degradation form a feed-forward loop leading to keratinocyte apoptosis (see Fig. 7). If our hypothesis is correct, disrupting any of them should block all other events. Indeed, we showed above that reduction of ROS by the antioxidant BHA blocked caspase activation and β-catenin degradation (Fig. 1 and 2), and that inhibition of caspases blocked β-catenin degradation (Fig. 3). We next examined whether inhibition of caspases could block ROS accumulation. We treated tak1-deficient keratinocytes with Z-VAD and determined the levels of TNF-induced ROS (Fig. 5A). Inhibition of caspases completely abolished the accumulation of ROS (Fig. 5A) and reduced the number of shrinking cells and annexin V-binding positive cells (Fig. 5B) in tak1-deficient keratinocytes following TNF stimulation. These results are consistent with our killing loop hypothesis.
Fig. 7.
Model
Fig. 5.
(A) tak1 +/+ and Δ/Δ keratinocytes were pretreated with or without 20 mM Z-VAD-fmk or DMSO for 1 h and stimulated with 20 ng/ml TNF for 2 or 4 h. Cells were subsequently incubated with 10 µM CM-H2DCFDA for 30 min and analyzed by flow cytometry. The fluorescence units relative to that of unstimulated cells are also shown. (B) tak1 Δ/Δ keratinocytes were pretreated with 20 mM Z-VAD-fmk or DMSO for 1 h and stimulated with 20 ng/ml TNF for 4 h and apoptotic cells were stained by Annexin V-Alexa Fluor 488.
The ROS-caspase-β-catenin degradation loop kills epidermal cells
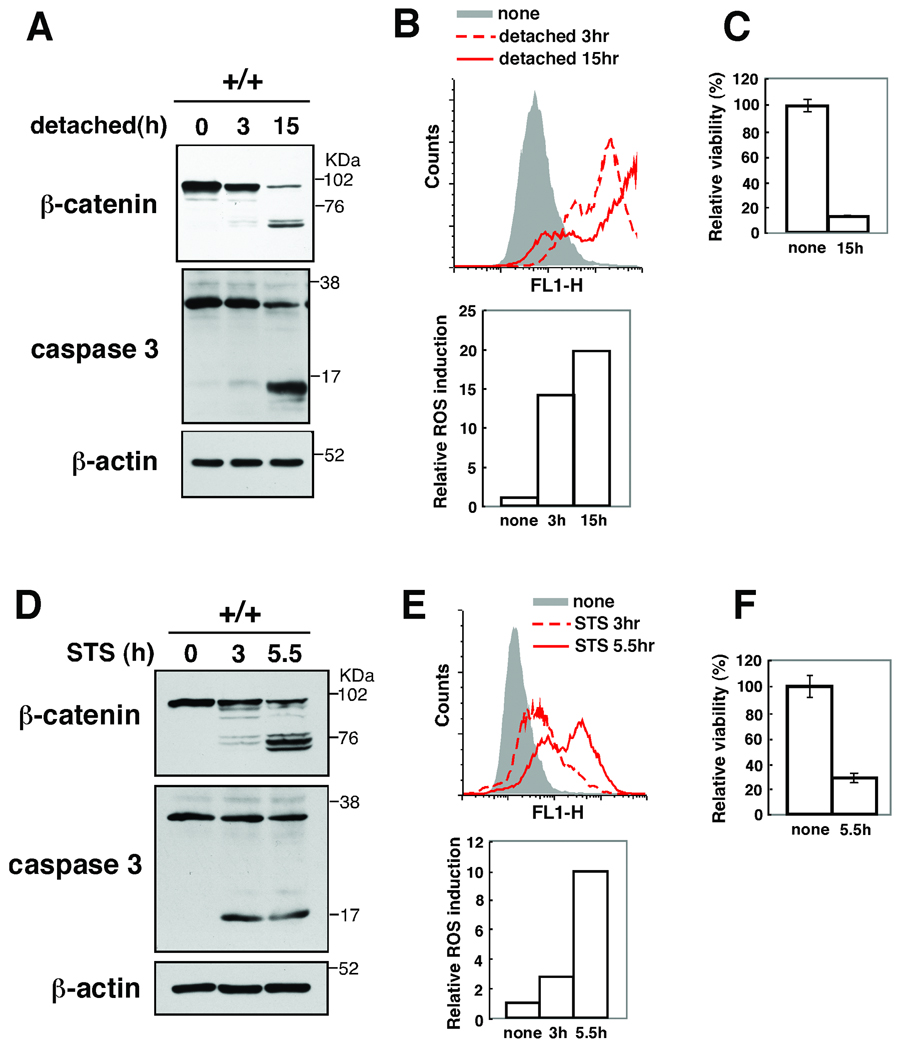
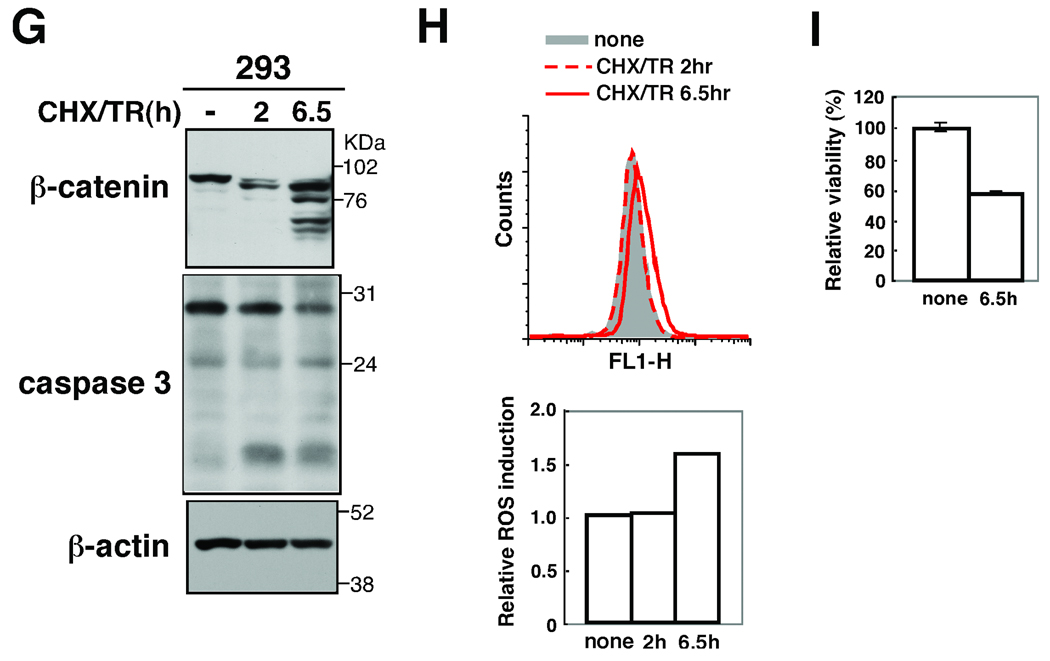
If there is such a feed-forward loop shown in Fig. 7, any of the events, including ROS accumulation, caspase activation and β-catenin degradation, should be capable of initiating the loop leading to keratinocyte death. We attempted to trigger the ROS-caspase-β-catenin degradation loop by stimuli other than the TNF-induced ROS. We mimicked β-catenin-induced AJ disruption by trypsin treatment of cells. Trypsin-treated keratinocytes exhibited β-catenin degradation (Fig. 6A). Concomitantly, ROS was highly increased (Fig. 6B), caspase 3 was activated (Fig. 6A) and cells died (Fig. 6C). These results indicate that disruption of cell adhesion can initiate the ROS-caspase-β-catenin degradation killing loop. Staurosporine, a non-specific kinase inhibitor, impairs mitochondrial function and is commonly used as an inducer of apoptosis (Tafani et al, 2002; Tafani et al, 2001). We treated wild type keratinocytes with staurosporine and observed that it induced β-catenin degradation, caspase activation, ROS accumulation and cell death (Fig. 6D–F). Staurosporine-induced β-catenin degradation and caspase activation was blocked by BHA treatment (supplementary Fig. S4). Thus, staurosporin can activate the ROS-caspase-β-catenin degradation loop. To determine whether this loop mediates cell killing in other epithelial cells, we analyzed human embryonic kidney 293 cells stimulated with combination of TNF-related apoptosis inducing ligand (TRAIL) and cycloheximide, which is an inducer of apoptosis in 293 cells. We detected cleavage of β-catenins and observed a moderate increase of caspases 3 activation and ROS accumulation (Fig. 6G–I). Collectively, these results support the idea that there is a ROS-caspase-β-catenin degradation loop, which functions to kill epithelial cells (Fig. 7). ROS are known to damage mitochondria and promote caspase activation (Lin & Beal, 2006). When ROS are accumulated and damage mitochondria or when cell adhesion is disrupted, the feed-forward loop consisting of ROS, caspases and β-catenin degradation, is activated and irreversibly kills the damaged epidermal cells. This killing loop is likely to be important for securely eliminating damaged epithelial cells, promoting repair, and preventing potential carcinogenesis in the epidermis.
Fig. 6.
(A–C) tak1 +/+ keratinocytes were trypsinized and cultured in calcium free media for 3 or 15 h. Cell lysates were immunoblotted with anti-β-catenin and anti-caspase 3 (A). Cells were incubated with 10 µM CM-H2DCFDA for 30 min and analyzed by flow cytometry (B). The fluorescence units relative to that of unstimulated cells are also shown (A, bottom). Cell viability was measured by MTT assay. Data are the mean±S.E. of three samples (C).
(D–F) tak1 +/+ keratinocytes were stimulated with 0.5 µM staurosporine (STS) for 3 or 5.5 h. Cells were analyzed as described above. (G–I) HEK293 cells were prestimulated with 100 µg/ml cycloheximide (CHX) for 1 h and stimulated with 100 ng/ml TRAIL (TR) for 2 or 6.5 h. Cells were analyzed as described above.
Materials and Methods
Mice
tak1-floxed (tak1 flox/flox) mice were from Dr. Akira, Osaka University (Sato et al, 2005). Mice harboring an epidermal-specific constitutive tak1 deletion (K5-Cre tak1 flox/flox) were generated using K5-Cre mice (mixed background of C57LBL/6 and DBA/2J)2. K14-CreERT mice were obtained from the Jackson Labs (Vasioukhin et al, 1999). Mice harboring an epidermal-specific inducible tak1 deletion (K14-CreERT tak1 flox/flox) were generated and at 5 weeks of age were topically treated on the dorsal skin once a day for 5 consecutive days with 4-hydroxy tamoxifen (4-OHT) (1 mg/ mouse) (Omori et al, 2008). Some mice were fed with food containing 0.7 % BHA starting at 3 days prior to the tamoxifen treatment. tak1 gene deletion was examined by PCR described previously. Littermate controls were used in all experiments. All animal experiments were done with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Cell culture
tak1 +/+ and tak1 Δ/Δ keratinocytes were isolated from tak1 flox/flox, K5-Cre tak1 flox/flox mice described previously (Omori et al, 2006). Spontaneously immortalized keratinocytes derived from the skin of postnatal day 0–2 mice were cultured in Ca2+-free minimal essential medium (Lonza) supplemented with 4% Chelex-treated bovine growth serum (Hyclone), 10 ng/ml of human or murine epidermal growth factor (Peprotech), 0.05 mM calcium chloride, and penicillin-streptomycin at 33°C in 8% CO2.
Reagents
Reagents used were TNF-α (Peprotech), butylated hydroxyanisole (BHA), lithium chloride, 4-hydroxy tamoxifen (Sigma), MG132, Z-VAD-fmk, Staurosporine ALLM (Calbiochem), clasto-lactacystin β-lactone (Cayman), TRAIL (Peprotech) and cycloheximide (Merck). Polyclonal antibodies were caspase 3 and cleaved caspase 3 (Cell Signaling). Monoclonal antibodies were β-catenin , p120-catenin (BD), β-actin (Sigma) and E-cadherin (ZYMED). Alexa Fluor 488 phalloidin (Invitrogen) was used.
Immunoblotting
Keratinocytes were washed once with ice-cold phosphate-buffered saline and whole cell extracts were prepared using a lysis buffer (20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 µM aprotinin, 0.5% Triton X-100). Cell extracts were resolved on SDS-PAGE and transferred to Hybond-P membranes (GE Healthcare). The membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse IgG using the ECL or ECL advance Western blotting detection kit (GE Healthcare).
Immunohistochemistry
Bouin-fixed paraffin sections were heated in 10 mM citrate buffer for 10 min and β-catenin immunofluorescence staining was performed. dUTP nick-end labeling (TUNEL) assay was performed on Bouin-fixed paraffin sections using an apoptotic cell death detection kit (Promega) according to the manufacturer’s instructions. Sections were counterstained with DAPI.
Cell viability assay
To determine the number of viable cells, a modified version of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium Bromide (MTT) assay, MTS assay, was performed according to the manufacture's instructions (Promega).
Retroviral infection
Retroviral vectors for TAK1 (pMX-puro-TAK1) was described previously (Kim et al, 2008). β-catenins (pMX-puro-β-catenin WT and S33Y) were generated by inserting β-catenin cDNA into the retroviral vector which was generated from pMX-puro (Kitamura, 1998). β-catenin cDNAs (Kolligs et al, 1999) were gifts from Dr. Eric R. Fearon, The University of Michigan Health System. EcoPack293 cells (BD) were transiently transfected with the various β-catenin vectors. After 48 h culture, growth medium containing retrovirus was collected and filtered with 0.45 µm cellulose acetate membrane to remove packaging cells. Keratinocytes were incubated with the collected virus-containing medium with 8 µg/ml polybrene for 24 h. Uninfected cells were removed by puromycin selection.
ROS measurement
Cells were stimulated, and then incubated with 10 µM CM-H2DCFDA (Invitrogen) for 30 min at 37°C. Harvested cells were analyzed by flow cytometry and FlowJo software (Tree Star Inc). One representative data from 2–3 similar results were shown in each experiment.
Trypsin treatment
Keratinocytes were trypsinized and incubated in Ca2+-free minimal essential medium with no calcium on plates coated with poly-2-hydroxyethyl methacrylate (10 mg/ml, Sigma).
Annexin V-binding assay
To determine apoptotic cells, Annexin V-Alexa Fluor 488 binding was performed according to the manufacturer's protocol (Invitrogen), and fluorescence was detected with fluorescent microscope (Olympus). 5–6 randomly selected areas were photographed with the same exposure time. The images were processed using the same fixed threshold in all samples by Photoshop software, and representative images are shown. The annexin positive cells were counted in total at least 1000 cells in each treatment, and one representative data with 2–3 similar results are shown.
Supplementary Material
(A) tak1-deficient (Δ/Δ) keratinocytes were infected with control vector (vec) or TAK1 retrovirus. Cells were stimulated with 20 ng/ml TNF (T) for 2.5 h and β-catenin was detected by immunoblot. Exogenously expressed TAK1 was detected as two bands, and the faster migrating band is thought to be degraded TAK1. (B) tak1-deficient (Δ/Δ) keratinocytes were pretreated with 100 µM BHA or vehicle ethanol for 1 h and stimulated with 20 ng/ml TNF for 3 or 6 h. E-cadherin and p120 catenin were detected by immunoblot. The samples were isolated in the same experiment shown in Fig. 2C. β-actin blot is the same as that in Fig. 2C. (C) tak1 Δ/Δ keratinocytes were pretreated with the indicated inhibitors for 1 h and stimulated with 20 ng/ml TNF for 4 h. Cell lysates were immunoblotted with anti-β-catenin. 45 µM ALLM and 20 mM LiCl were used.
(A) tak1 Δ/Δ keratinocytes expressing vector, β-catenin wild type or S33Y were stimulated with 20 ng/ml TNF for 3 h. Apoptotic cells were stained by Annexin V-Alexa Fluor 488. (B) tak1 Δ/Δ keratinocytes expressing vector, β-catenin wild type or S33Y were stimulated with 20 ng/ml TNF for 3 or 6 h. Cell lysates were immunoblotted with the indicated antibodies.
(A) tak1 Δ/Δ keratinocytes were pretreated with 10 µM MG132 for 1.0 h and stimulated with 20 ng/ml TNF for 4 h. Cell lysates were immunoblotted with the indicated antibodies. (B) tak1 Δ/Δ keratinocytes were pretreated with 10 µM MG132 or DMSO for 1 h and stimulated with 20 ng/ml TNF for 2 or 4 h. Cells were incubated with 10 µM CM-H2DCFDA for 30 min and analyzed by flow cytometry. The fluorescence units relative to that of unstimulated cells are shown. This experiment was performed together with Fig. 5A. The result of DMSO-treated tak1 Δ/Δ keratinocytes is same as Fig. 5A.
tak1 +/+ keratinocytes were pretreated with 100 µM BHA or vehicle ethanol for 1 h and stimulated with 0.5 µM staurosporine (STS) for 2 or 5 h. β-catenin and caspase 3 were detected by immunoblot.
Acknowledgements
We thank S. Akira for tak1-floxed mice, E.R. Fearon for β-catenin cDNAs, J. Dow, B.J. Welker and M. Mattmuler for support. This work was supported by National Institutes of Health Grant GM068812 and GM084406 (to J. N-T.).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated β-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998a;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- Brancolini C, Lazarevic D, Rodriguez J, Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of β-catenin. J Cell Biol. 1997;139:759–771. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C, Sgorbissa A, Schneider C. Proteolytic processing of the adherens junctions components β-catenin and gamma-catenin/plakoglobin during apoptosis. Cell Death Differ. 1998;5:1042–1050. doi: 10.1038/sj.cdd.4400443. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Go Y-M, Gipp JJ, Mulcahy RT, Jones DP. H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem. 2004;279:5837–5845. doi: 10.1074/jbc.M307547200. [DOI] [PubMed] [Google Scholar]
- Herren B, Levkau B, Raines EW, Ross R. Cleavage of β-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell. 1998;9:1589–1601. doi: 10.1091/mbc.9.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-Y, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008;283:137–144. doi: 10.1074/jbc.M704746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T. New experimental approaches in retrovirus-mediated expression screening. Int J Hematol. 1998;67:351–359. doi: 10.1016/s0925-5710(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant β-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Iyengar R. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/β-catenin-regulated cell proliferation. Proc Natl Acad Sci U S A. 2002;99:13254–13259. doi: 10.1073/pnas.202355799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF α-deficient mice: a critical requirement for TNF α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bluethmann H, Kollias G. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc Natl Acad Sci U S A. 1997;94:6319–6323. doi: 10.1073/pnas.94.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhusen U, Badock V, Bauer A, Behrens J, Wittman-Liebold B, Dorken B, Bommert K. Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J Biol Chem. 2000;275:16345–16353. doi: 10.1074/jbc.M001458200. [DOI] [PubMed] [Google Scholar]
- Tafani M, Cohn JA, Karpinich NO, Rothman RJ, Russo MA, Farber JL. Regulation of intracellular pH mediates Bax activation in HeLa cells treated with staurosporine or tumor necrosis factor-alpha. J Biol Chem. 2002;277:49569–49576. doi: 10.1074/jbc.M208915200. [DOI] [PubMed] [Google Scholar]
- Tafani M, Minchenko DA, Serroni A, Farber JL. Induction of the mitochondrial permeability transition mediates the killing of HeLa cells by staurosporine. Cancer Res. 2001;61:2459–2466. [PubMed] [Google Scholar]
- Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark GR, Matsumoto K. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol Cell Biol. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Craen M, Berx G, Van den Brande I, Fiers W, Declercq W, Vandenabeele P. Proteolytic cleavage of β-catenin by caspases: an in vitro analysis. FEBS Lett. 1999;458:167–170. doi: 10.1016/s0014-5793(99)01153-9. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kimelman D. Mechanistic insights from structural studies of β-catenin and its binding partners. J Cell Sci. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Yang H, Magilnick N, Ou X, Lu SC. Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor κB and activator protein-1. Biochem J. 2005;391:399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) tak1-deficient (Δ/Δ) keratinocytes were infected with control vector (vec) or TAK1 retrovirus. Cells were stimulated with 20 ng/ml TNF (T) for 2.5 h and β-catenin was detected by immunoblot. Exogenously expressed TAK1 was detected as two bands, and the faster migrating band is thought to be degraded TAK1. (B) tak1-deficient (Δ/Δ) keratinocytes were pretreated with 100 µM BHA or vehicle ethanol for 1 h and stimulated with 20 ng/ml TNF for 3 or 6 h. E-cadherin and p120 catenin were detected by immunoblot. The samples were isolated in the same experiment shown in Fig. 2C. β-actin blot is the same as that in Fig. 2C. (C) tak1 Δ/Δ keratinocytes were pretreated with the indicated inhibitors for 1 h and stimulated with 20 ng/ml TNF for 4 h. Cell lysates were immunoblotted with anti-β-catenin. 45 µM ALLM and 20 mM LiCl were used.
(A) tak1 Δ/Δ keratinocytes expressing vector, β-catenin wild type or S33Y were stimulated with 20 ng/ml TNF for 3 h. Apoptotic cells were stained by Annexin V-Alexa Fluor 488. (B) tak1 Δ/Δ keratinocytes expressing vector, β-catenin wild type or S33Y were stimulated with 20 ng/ml TNF for 3 or 6 h. Cell lysates were immunoblotted with the indicated antibodies.
(A) tak1 Δ/Δ keratinocytes were pretreated with 10 µM MG132 for 1.0 h and stimulated with 20 ng/ml TNF for 4 h. Cell lysates were immunoblotted with the indicated antibodies. (B) tak1 Δ/Δ keratinocytes were pretreated with 10 µM MG132 or DMSO for 1 h and stimulated with 20 ng/ml TNF for 2 or 4 h. Cells were incubated with 10 µM CM-H2DCFDA for 30 min and analyzed by flow cytometry. The fluorescence units relative to that of unstimulated cells are shown. This experiment was performed together with Fig. 5A. The result of DMSO-treated tak1 Δ/Δ keratinocytes is same as Fig. 5A.
tak1 +/+ keratinocytes were pretreated with 100 µM BHA or vehicle ethanol for 1 h and stimulated with 0.5 µM staurosporine (STS) for 2 or 5 h. β-catenin and caspase 3 were detected by immunoblot.