Abstract
Risk of pancreatic cancer, the fourth deadliest cancer in the U.S., is increased by obesity. Calorie restriction (CR) prevents obesity, suppresses carcinogenesis in many models, and reduces serum levels of insulin-like growth factor (IGF)-1. In the present study, we examined the impact of CR on a model of inflammation-associated pancreatitis and pancreatic dysplasia, with a focus on the mechanistic contribution of systemic IGF-1. Administration of a 30% CR diet for 14 weeks decreased serum IGF-1 and hindered pancreatic ductal lesion formation and dysplastic severity, relative to a higher calorie control diet, in transgenic mice overexpressing cyclooxygenase (COX)-2 (BK5.COX-2). These findings in CR mice correlated with reductions in Ki-67-positive cells, vascular luminal size, vascular endothelial growth factor expression, and phosphorylation and total expression of downstream mediators of the IGF-1 pathway. Cell lines derived from BK5.COX-2 ductal lesions (JC101cells) formed pancreatic tumors in wild-type FVB mice that were significantly reduced in size by a 14-week CR regimen, relative to the control diet. To further understand the impact of circulating levels of IGF-1 on tumor growth in this model, we orthotopically injected JC101 cells into liver-specific IGF-1-deficient (LID) mice. The ~65% reduction of serum IGF-1 in LID mice resulted in significantly decreased burden of JC101 tumors, despite modestly elevated levels of circulating insulin and leptin. These data show that CR prevents development of dysplasia and growth of pancreatic cancer through alterations in IGF-1, suggesting that modulation of this pathway with dietary and/or pharmacologic interventions is a promising pancreatic cancer prevention strategy.
Keywords: Pancreatic cancer, Calorie restriction, Insulin-like growth factor-1, Energy balance, pancreatitis, BK5.COX-2
Introduction
Pancreatic cancer is the fourth deadliest malignancy in the United States, with the 5-year survival rate less than 5% (1). In 2010 an estimated 43,140 Americans were diagnosed with pancreatic cancer and more than 36,800 affected patients died from this disease (1, 2). One of the few modifiable risk and progression factors for pancreatic cancer is obesity, the incidence of which has increased dramatically in recent decades as a consequence of long-term positive energy balance (3, 4). Molecular targets and strategies for preventing pancreatic cancer are urgently needed given the strong association between pancreatic cancer risk and excess weight, the increasing prevalence of overweight/obesity across the world, and the poor prognosis of pancreatic cancer. Potential mechanistic targets for pancreatic cancer prevention include components of the insulin-like growth factor (IGF)-1 signal transduction pathway that underlie the energy balance-cancer link in other types of cancer and are affected by energy balance interventions (5).
IGF-1 is a key endocrine and paracrine regulator of tissue growth and metabolism that acts directly on cells primarily via the IGF-1 receptor (IGF-1R) or indirectly through receptor-independent processes (6). Levels of serum IGF-1 and pancreatic tissue IGF-1R are increased in patients with pancreatic cancer compared with unaffected individuals (7), and elevated serum IGF-1 levels are associated with increased risk of pancreatic cancer-related death (8). Bioavailable IGF-1 levels are increased in response to diet-induced obesity and decreased by calorie restriction (CR) (9). IGF-1 is a principal mediator of the anticancer effects of CR, a potent anti-obesity and cancer preventive intervention in several rodent models of cancer (9, 10), including carcinogen-induced pancreatic tumors in rats (11). CR also extends lifespan in rhesus monkeys by delaying many cancers (12). IGF-1 upregulates cell cycle progression primarily by activating the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signal transduction pathway downstream of IGF-1R (13, 14). Cells overexpressing IGF-1R have decreased apoptosis (15, 16), and pharmacologic inhibition of IGF-1R or mTOR diminishes tumor growth in xenograft models of human pancreatic cancer (17–19).
Being overweight or obese is associated with a state of chronic inflammation that promotes pancreatitis (20, 21), and chronic pancreatitis increases the risk of developing pancreatic cancer (22, 23). Adipose tissue releases immunomodulators, e.g., prostaglandins and cytokines that promote inflammation and tumorigenicity (24). Human pancreatitis lesions and pancreatic adenocarcinomas typically overexpress cyclooxygenase-2 (COX-2), the key enzyme in the conversion of arachidonic acid to prostaglandins, as part of an inflammatory response, suggesting that COX-2 overexpression is an early step in pancreatic carcinogenesis (25, 26). Moreover, transgenic mice with pancreatic COX-2 overexpression driven by the bovine keratin-5 promoter (BK5.COX-2 mice) develop inflamed pancreatic ductal lesions by 3 months of age and high grade pancreatic dysplasia by 6–8 months resulting from chronic pancreatitis (27). Dysplastic cells derived from this model develop anaplastic tumors when injected into mice (27). Thus, BK5.COX-2 mice provide an excellent model for evaluating strategies to prevent progression from chronic inflammation to pancreatic cancer.
Although CR is a well-established dietary intervention for preventing cancer in various preclinical models, the effects and underlying mechanisms of CR on pancreatitis and pancreatic cancer are poorly understood. We hypothesized tt CR would minimize the high-grade dysplasia in the pancreas that results from COX-2 overexpression via the IGF-1 pathway. Using the BK5.COX-2 mouse model, we assessed the effects of CR (relative to a control diet) on pancreatic pathology, IGF-1 levels, IGF-1/Akt/mTOR pathway activation, and tumor growth. By orthotopically transplanting cells derived from a BK5.COX-2 mouse, we also evaluated pancreatic tumor growth in the context of genetically reduced serum IGF-1 levels (with and without pharmacologic restoration of IGF-1 levels) to establish a causal link between circulating IGF-1 and pancreatic cancer.
Materials and Methods
Animals and treatment
Mice were maintained in a semibarrier facility at the University of Texas at Austin Animal Resource Center. The Institutional Animal Care and Use Committee of the University of Texas approved all experiments involving animals.
The Effect of Energy Balance Modulation on Spontaneous Pancreatic Lesions in BK5.COX-2 Transgenic Mice
48 male and female FVB-Tg (KRT5-Ptgs2)7Sf (BK5.COX-2) mice (27) and 48 wild-type (WT) littermates were produced at the University of Texas M.D. Anderson Cancer Center, Smithville, TX. Upon arrival at the University of Texas at Austin Animal Resource Center at 4 weeks of age, mice were singly housed and fed control diet (Research Diets, Inc., New Brunswick, NJ, #D12450B, consumed ad libitum) for a 2-week acclimation period. Mice from each genotype were randomized to receive one of two experimental diets for 14 weeks: a) control diet consumed ad libitum (n=24 per genotype) or b) CR diet (Research Diets, #D03020702), fed in daily aliquots to provide 70% of the energy and 100% of all nutrients except carbohydrates relative to the control group (n=24 per genotype). Food intake and body weights were measured weekly. At the end of the treatment period, mice were fasted for 12 hours and then anesthetized by isoflurane. Blood was collected by cardiac puncture and the mice were then killed by cervical dislocation. Blood was allowed to coagulate at room temperature (RT) for 30 minutes and then centrifuged at 9,300 × g for 5 min to obtain serum samples that were stored at −80°C. Pancreata were collected and either snap-frozen in liquid nitrogen and stored at −80°C or placed in 10% neutral buffered formalin overnight and then switched to 70% ethanol.
JC101 Pancreatic Tumor Transplant Studies
Our studies in BK5.COX-2 transgenic mice revealed that energy balance modulates pathologies associated with chronic, unresolved pancreatic inflammation. In order to extend these findings to dietary effects on pancreatic tumor burden, transplant studies were performed using JC101 mouse pancreatic cancer cell lines which were derived from spontaneous pancreatic ductal lesions in a BK5.COX-2 transgenic mouse (27). Prior to transplantation, JC101 cells were maintained under an atmosphere of 5% CO2 in a 37°C incubator with Dulbecco’s Minimum Essential Media (HyClone, ThermoScientific, Waltham, MA) supplemented with 10% fetal bovine serum (HyClone), penicillin/streptomycin, glutamine, sodium pyruvate, and HEPES. Cells were lightly trypsinized, washed and resuspended in Hanks’ Buffered Saline Solution for injection. Species identification and karyotyping were performed on the JC101 cell line at the T.C. Shu Molecular Cytogenetics Core at The University of Texas M.D. Anderson Cancer Center (Houston, TX).
Male WT FVB mice (4 weeks of age; Jackson Laboratories) were singly housed, and fed chow diet for one week. Between 6 and 8 weeks of age, 18 mice were randomized to receive either control diet (same as described above) or the CR regimen for 11 weeks. Food intake and body weights were measured weekly. After 7 weeks on these diet treatments, JC101 cells were subcutaneously transplanted (2×105 cells) into the right flank of each mouse and allowed to grow for 4 weeks. Tumors were palpated weekly. Mice were fasted for 12 hours then anesthetized with isoflurane for cardiac puncture and killed by cervical dislocation. Serum and tumors were harvested and stored as described above.
To determine the impact of circulating IGF-1 on tumor burden, another transplant study was performed using 15 male liver-specific IGF-1-deficient (loxP+/+ Cre+/−; LID) mice and 15 male floxed IGF-1 littermate control (loxP+/+ Cre−/−; LC) mice purchased from Taconic Laboratories (Rockville, MD). These were bred and genotyped as previously described (28). All of these mice were fed a chow diet for the duration of the study. JC101 mouse pancreatic cancer cells were orthotopically transplanted (2.5×105 cells) in LID and LC mice between 6 and 8 weeks of age. After 28 days, mice were fasted for 12 hours then anesthetized with isoflurane for cardiac puncture and killed by cervical dislocation. Serum and tumors were harvested and stored as described above. One LC mouse died prior to study endpoint, resulting in a final sample size of 14 LC mice and 15 LID mice.
Serum marker analyses
For the BK5.COX-2 and LID mouse studies, serum levels of IGF-1 were determined by RIA according to manufacturer’s instructions (DSL-2900 kit, DSL/Beckman Coulter Laboratories, Webster, TX). For the study assessing energy balance effects on transplanted pancreatic tumors, serum levels of IGF-1, insulin and leptin were measured using Lincoplex® bead-based assays (Millipore Corporation, Billerica, MA) on a BioRad Bioplex® analyzer (BioRad, Hercules, CA) according to manufacturer’s directions. All analyses were performed on serum samples collected at study termination on mice that had been fasted for 12 hours. Sample size for each assay was dictated by serum availability at the time assay was performed.
Histopathologic Analyses
Formalin-fixed pancreata from WT and BK5.COX-2 mice were embedded in paraffin and then cut into a 4-µm thick section and processed for hematoxylin and eosin (H&E) staining. The effect of diet on development of pancreatic ductal lesions in the BK5.COX-2 pancreata was assessed by determining the percentage of the entire pancreatic section comprised of regions of atypical ductal lesions relative to unaffected areas. This was performed for each mouse under low-power magnification and averaged over the diet group. Metaplastic changes in ducts (metaplasia), cellular and nuclear atypia (atypia), degree of inflammatory cell infiltration (inflammation), and relative size of the stroma and level of fibrosis (fibrosis) were scored in a blinded manner by a certified veterinary pathologist (M.J.M.) according to the following criteria: 0=normal pancreas, 1=minimal (5–10% affected), 2=mild (10–25% affected), 3=moderate (25–50% affected), 4=marked (>50% affected). These indices of pathology were also combined to give a composite score of severity for each mouse and averaged for each diet group.
Immunohistochemical Analyses
Formalin-fixed tissues were embedded in paraffin, cut into 4-µm thick sections, and processed for immunohistochemistry at the Histology Core Laboratory at The U.T. M.D. Anderson Cancer Center, Science Park Research Division (Smithville, TX). Antibodies used for immunohistochemistry were optimized by core personnel using positive and negative controls for each analysis. Slides were deparaffinized in xylene and sequentially rehydrated in ethanol to water. Antigen retrieval required microwaving slides with 10mM citrate buffer, except CD31 which required protease treatment. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 minutes. Nonspecific binding was blocked by treating sections with Biocare blocking reagent (Biocare Medical, Concord, CA) for 30 min at RT, followed by incubation with primary antibody diluted in blocking buffer overnight at 4°C, unless otherwise specified. The following primary antibodies and dilutions were used: Ki-67 (Dako, Carpenteria, CA; 1:200); CD31 (Pharmingen, San Jose, CA; 1:400); VEGF (Santa Cruz Biotechnology; 1:100, 1hr at RT); phospho-IGF1RY1135/1136 (Cell Signaling, Beverly, MA; 1:100); IGF1R (Cell Signaling; 1:50); phospho-AktS473 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:50, 1 hr at RT); and Akt (Cell Signaling; 1:100). Slides were washed twice in PBS, incubated for 30 min with secondary antibody, washed 3 times with PBS, stained with diaminobenzidine and counterstained with hematoxylin. Images were captured using a light microscope equipped with a Leica digital color camera (Leica Camera Inc, Allendale, NJ). Ki-67-positive cells were counted in 3- 6 nonoverlapping fields (depending on size of tumor section) with 20X objective in WT and BK5.COX-2 pancreatic sections, and the 40X objective in the transplant study, and averaged per group (5 mice per diet group per genotype).
Western blotting
Pancreatic tissues from the WT and BK5.COX-2 mice were homogenized and lysed in RIPA buffer (Sigma, St. Louis, MO) with protease inhibitor tablet (Roche Applied Sciences, Indianapolis, IN) and phosphatase inhibitor cocktails I and II (Sigma). Protein lysates (100µg) were resolved by SDS-PAGE using 8, 12, or 15% gels and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked using LI-COR Blocking Buffer for 1 hour at RT (LI-COR Biotechnologies, Lincoln, NE) then incubated with primary antibody (all from Cell Signaling) diluted in blocking buffer overnight at 4°C. The primary antibodies (and their dilutions) used were against: phospho-AktS473 (1:500), Akt (1:1000), phospho-mTORS2448 (1:500), mTOR (1:1000), phospho-p70/S6KT389 (1:500), p70/S6K (1:1000), phospho-S6S235/236 (1:1000), and S6 (1:1000). After 3 washes (5 min each) in 0.1% Tween-20/PBS (PBS-T), membranes were incubated for 1 hour at RT in species-specific secondary antibody (LI-COR Biotechnologies) diluted in LI-COR blocking buffer (1:5000). Following 3 washes in PBS-T, membranes were scanned using the Odyssey Infrared Fluorescent Imaging System (LI-COR Biotechnologies). Blots shown are representative of 3 randomly selected mice per diet group.
Statistical analyses
All values shown are mean ± standard deviation, except serum hormone levels and Ki-67 quantification which are shown as mean ± standard error of the mean. Statistical analyses were conducted using SPSS (Apache Software Foundation, Wilmington, DE). Differences among diet groups with respect to body weight over time were determined using repeated measures analysis, and differences in final body weights were determined by independent t-tests. Diet-dependent differences in the percent pancreatic lesion measurements and pathology scores were determined using a non-parametric Wilcoxon test. Differences between diet groups in serum levels of IGF-1 and insulin as well as Ki-67-positive cells were determined by one-way ANOVA followed by either a Tukey’s or Games-Howell post-hoc test of significance. In the transplant studies, tumor burden and serum hormone levels were compared between diet groups/genotypes using independent t-tests. Effect of diet on Ki-67-positivity was determined by independent t-test. Results were considered significant if P < 0.05.
Results
CR reduced body weight and circulating IGF-1 without altering serum insulin
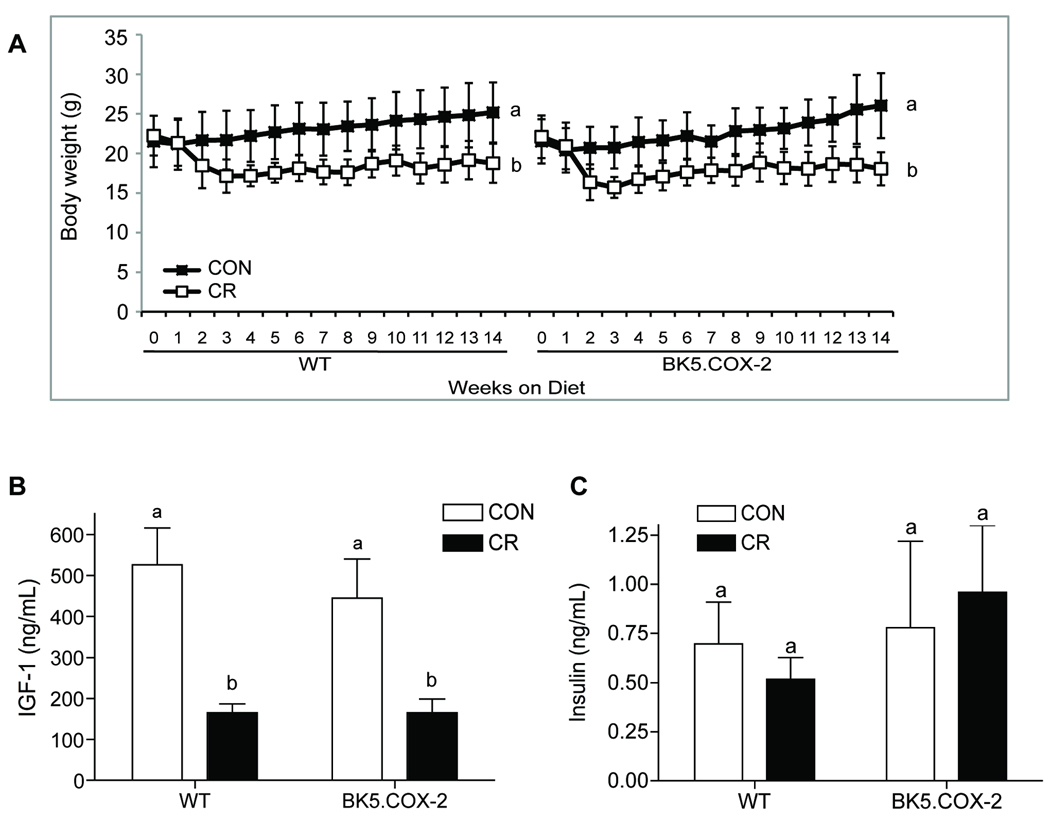
We compared the effects of a control diet consumed ad libitum, which is a positive energy balance regimen that results in overweight mice (9), to a 14-week CR regimen (70% of control group’s energy intake) in 48 male and female BK5.COX-2 mice (n=12 per sex and diet group) and 48 WT littermates (n=12 per sex and diet group). Because there were no observed differences in serum markers or pathological parameters between male and female BK5.COX-2 mice (data not shown), all results are reported as the combined data from both sexes. CR, relative to control, significantly reduced body weights (n=24 per diet group and genotype, P < 0.0001 each genotype; Fig. 1A) and fasting serum IGF-1 concentrations (WT, n=12 per diet group, P < 0.0001; BK5.COX-2, n=12 for control and n=7 for CR group, P < 0.05; Fig. 1B), regardless of genotype. No diet-dependent differences were detected in fasting serum insulin levels in either genotype (Fig. 1C).
Figure 1.
Effect of diet on body weight, IGF-1, and insulin serum levels in WT and BK5.COX-2 mice. A, weekly average body weights of control and CR WT mice (n=24 per diet group) and control and CR BK5.COX-2 mice (n=24 per diet group) through 14 weeks of dietary regimens. Error bars represent SD. Fasting serum IGF-1 levels (B) and insulin levels (C) at week 14 for control and CR WT mice (IGF-1, n=12 per diet group; insulin, n=10 per diet group) and control BK5.COX-2 (IGF-1, n=12 and insulin, n=10) and CR BK5.COX-2 mice (IGF-1, n=7; insulin, n=10). Differences in sample size were dictated by serum availability at the time assay was performed. Error bars represent SEM. Significant differences are denoted by different letters.
CR suppresses spontaneous pancreatic ductal lesions in BK5.COX-2 mice
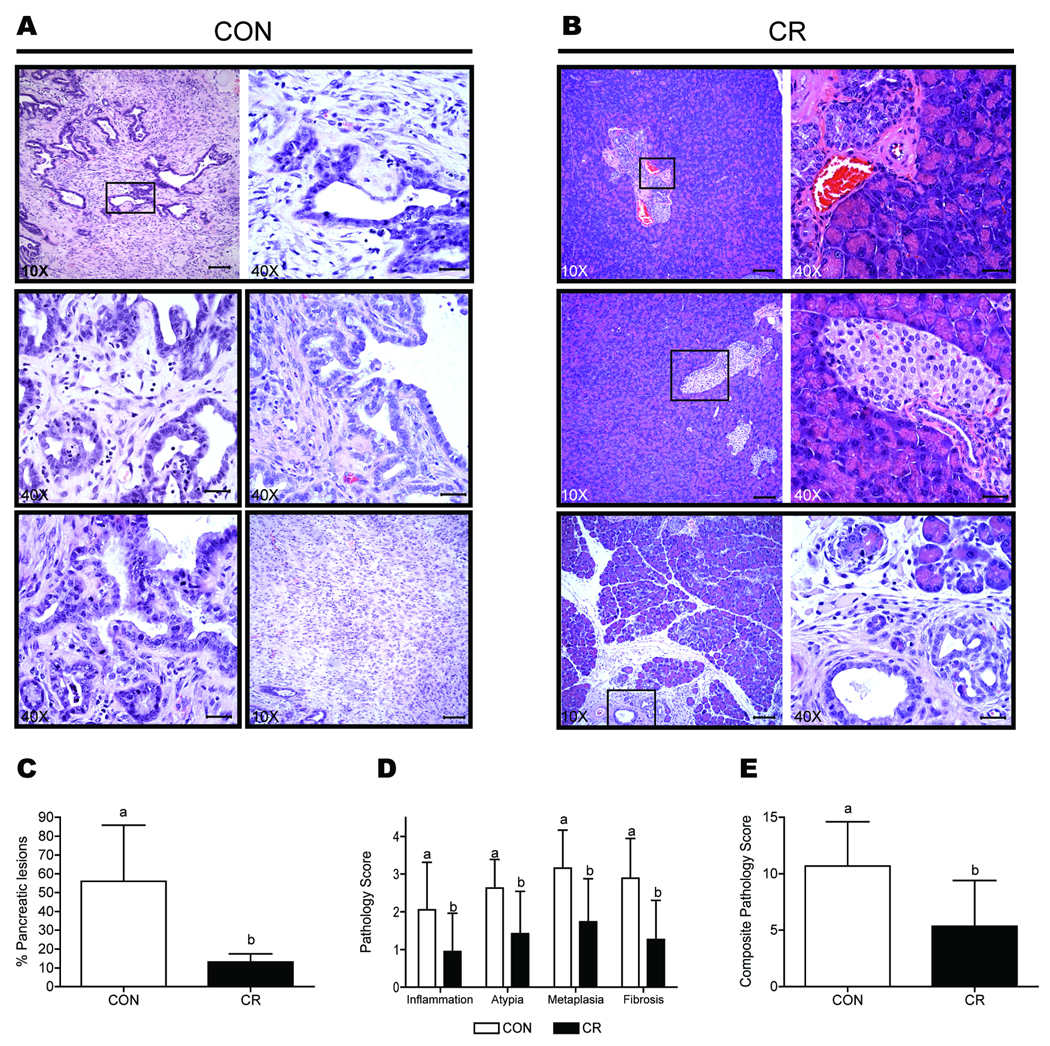
Pancreatic tissue from the control BK5.COX-2 mice consisted of enlarged ductal lesions with a disorganized growth pattern and associated fibrosis, which are indicators of pancreatitis (Fig. 2A). Moreover, pancreata exposed to this diet displayed substantial changes associated with neoplasia including anisokaryosis, pleomorphism, mitosis, fibroplasia, and disruption of the basement membrane. In contrast, the majority of pancreata from CR BK5.COX-2 mice retained typical pancreatic morphology including normal lobar and lobular arrangement and minimal to no changes in acinar, ductular or islet cells (Fig. 2B). On average, 56% ± 29% of each pancreas in control BK5.COX-2 mice consisted of enlarged ductal lesions whereas only 13% ± 18% of each pancreas in CR BK5.COX-2 mice consisted of ductal lesions (P < 0.0001 versus control; n=19 per diet group; Fig. 2C).
Figure 2.
Pathological assessment of spontaneous pancreatic ductal lesions in the BK5.COX-2 transgenic mouse model. A, H&E-stained sections of pancreatic tissue from control BK5.COX-2 mice show changes in pancreatic structure (10×) and neoplastic features (40×) such as fibrosis, anisokaryosis, and pleomorphism. B, normal pancreatic architecture is largely preserved in pancreatic sections in CR BK5.COX-2 mice (10×) with fewer ductal structures evident. Minimal metaplastic change occurred in the ductal structures present (40×). C, the effect of diet on the average percentage of pancreatic sections from BK5.COX-2 mice comprised of ductal lesions, based on histopathologic assessment of H&E-stained pancreata (n=19 mice per diet group). Scale bars for 10X magnification, 100-µm; scale bars for 40X magnification, 30-µm. D, the effect of diet on the average pathology scores (on a 0 to 4 scale) for inflammation, nuclear atypia, metaplasia, and fibrosis in pancreatic sections of each pancreas from BK5.COX-2 mice. E, the effect of diet on average composite pathology scores (on a 0–16 scale) of BK5.COX-2 pancreatic ductal lesions. Error bars represent SD. Significant differences are denoted by different letters.
Sections of pancreatic tissue from each BK5.COX-2 mouse were scored in a blinded fashion for severity of four pathological indices of dysplasia (and their composite): inflammatory cell infiltration, metaplasia, nuclear/cellular atypia, and fibrosis. Pancreatic lesions present in the CR group consistently had lower indices of pathology compared to the control group, which mainly consisted of high-grade dysplastic lesions (P < 0.01; n=19 per diet group; Fig. 2D). The CR group, relative to control, also had significantly (P < 0.01) lower composite pathology scores (Fig. 2E). All of the control BK5.COX-2 pancreata that were scored displayed pathological ductal lesions. On the other hand, 21% of the CR mice displayed a complete absence of pathological pancreatic lesion development.
CR reduces proliferation and hinders vascular changes in BK5.COX-2 pancreatic tissue
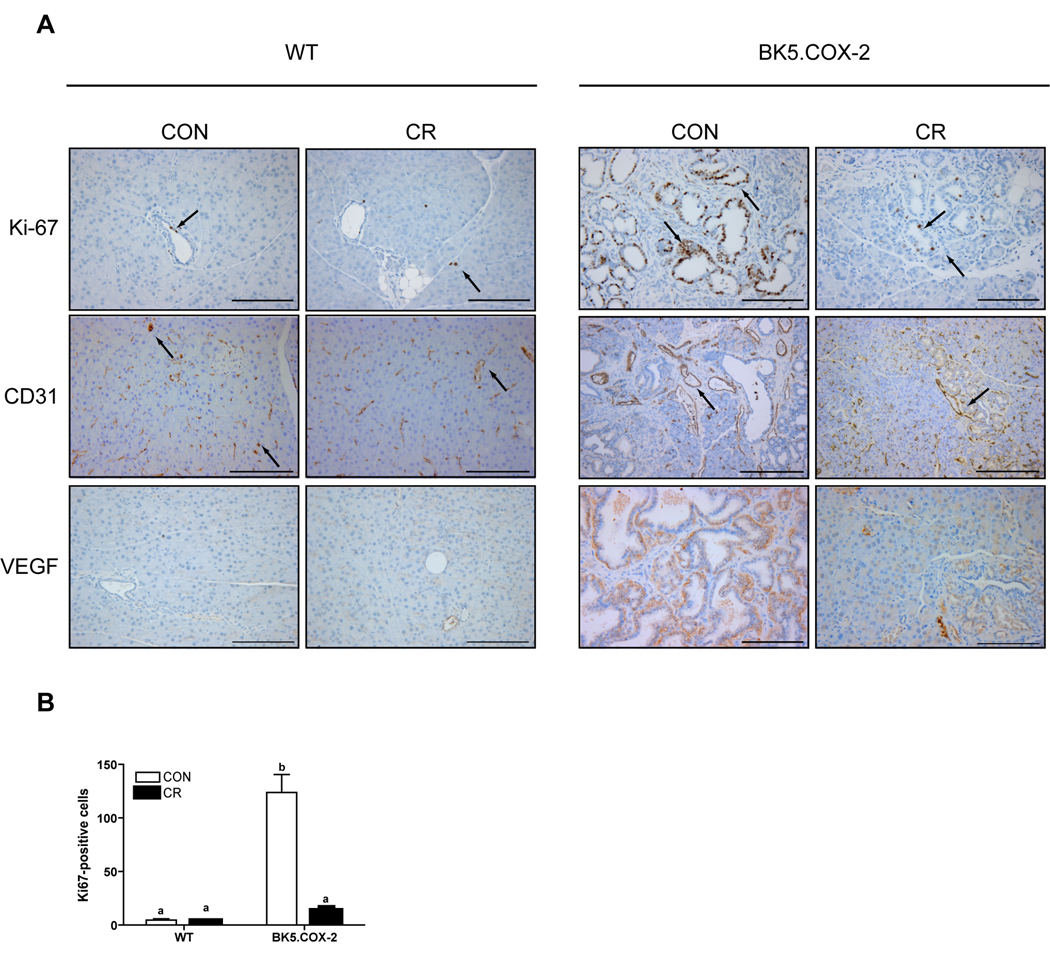
Based on immunohistochemical analysis of Ki-67, a marker of cellular proliferation, WT pancreata exhibited very low levels of proliferation that were unaffected by diet treatment (control, 4.6 ± 1.2 Ki-67-positive cells per field versus CR, 3.7 ± 0.6; n=5 mice per diet group; Fig. 3A and B). Conversely, control BK5.COX-2 mice showed marked increases in ductal cell proliferation relative to either group of WT mice (123.9 ± 16.7 Ki-67-positive cells; P < 0.0001; n=5 mice; Fig. 3A and B). Proliferation in the pancreata of CR BK5.COX-2 mice was significantly lower than control BK5.COX-2 mice (13.2 ± 3.6 Ki-67-positive cells; P < 0.01; n=5 mice; Fig. 3A and B).
Figure 3.
Effect of diet on proliferation and vascular markers in WT and BK5.COX-2 pancreatic tissue. A, representative images of pancreatic sections stained with antibodies to Ki-67, CD31, and VEGF. Scale bars, 150-µm, except CD31, 300-µm. B, Ki-67-positive cells were quantified at 20X magnification in pancreatic sections from WT and BK5.COX-2 mice (5 mice per diet group). Ki-67- and CD31-positive cells indicated with arrows. Error bars represent SEM. Significant differences are denoted by different letters.
To assess diet-induced vascular changes, we identified vessels in pancreatic sections by immunohistochemical staining for CD31, an endothelial cell marker, and vascular endothelial growth factor (VEGF), a principal marker of blood vessel development. Although there was no apparent effect of diet on the vasculature of pancreata from the WT mice, control BK5.COX-2 mice displayed enlarged vessels with increased luminal size, particularly in and around areas of ductal lesions (Fig. 3A). The majority of the blood vessels in the pancreata of CR BK5.COX-2 mice were morphologically similar to vessels in WT mice. Only near the few areas of ductal lesions and fibrosis did vessels from the CR BK5.COX-2 mice show enhanced luminal size. Similar to morphological changes detected with CD31 staining, a diet-dependent effect on VEGF expression was apparent in the ductal-rich regions of pancreata from the BK5.COX-2 mice, but not the WT pancreata (Fig. 3A). VEGF expression in pancreatic tissue of the control BK5.COX-2 mice was qualitatively increased relative to WT, and CR reduced this elevated expression (Fig. 3A).
CR decreases phosphorylation and expression of components of the IGF-1 signaling pathway
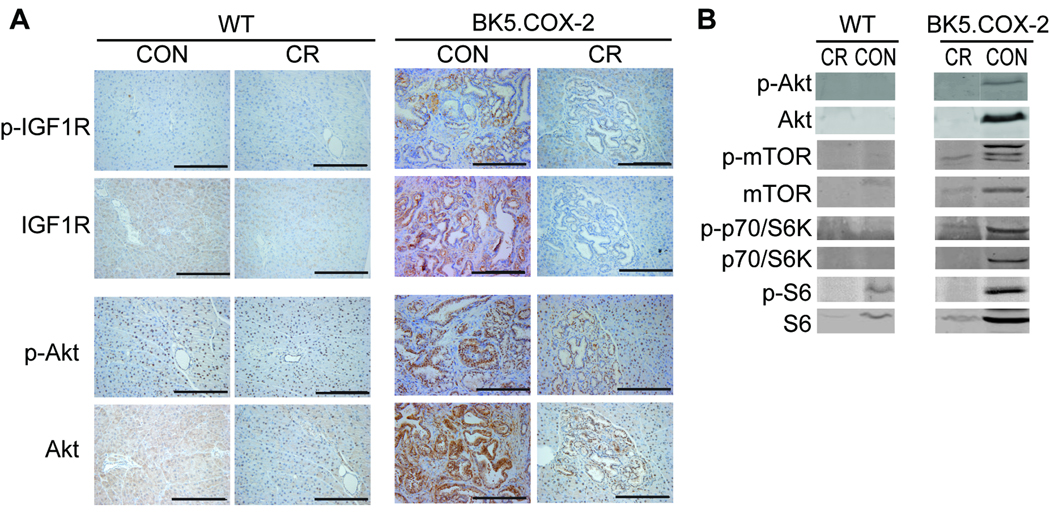
To determine if CR suppressed components of the IGF-1 pathway, pancreatic lesions were analyzed for protein phosphorylation and protein expression changes in IGF-1R, Akt, mTOR, p70/S6K, and S6 ribosomal protein. Immunohistochemical analyses of ductal lesions from BK5.COX-2 mice revealed reduced phosphorylated and total protein expression of IGF-1R and Akt in the CR group compared to the control group (Fig. 4A). Immunoblotting for intermediates of the IGF-1 pathway showed increases in both phosphorylated and total levels of the downstream signaling components Akt, mTOR, p70/S6K, and S6 in control BK5.COX-2 pancreata relative to WT pancreata. However, CR BK5.COX-2 exhibited pancreata with reduced expression of these proteins, similar to levels seen in WT groups (Fig. 4B).
Figure 4.
Dietary modulation of the IGF-1 signaling pathway in WT and BK5.COX-2 pancreatic tissue. A, pancreatic sections from WT and BK5.COX-2 mice on either control or CR diets were stained with antibodies to p-IGF-1R, IGF-1R, p-Akt and Akt. Images are representative of 3 mice per group and were captured at 20X magnification. Scale bars, 200-µm B, whole pancreatic lysates from WT or BK5.COX-2 mice on either control or CR diets were resolved by western blot analyses. Figures shown are representative of 3 mice per group.
CR significantly reduced growth of JC101 pancreatic tumors
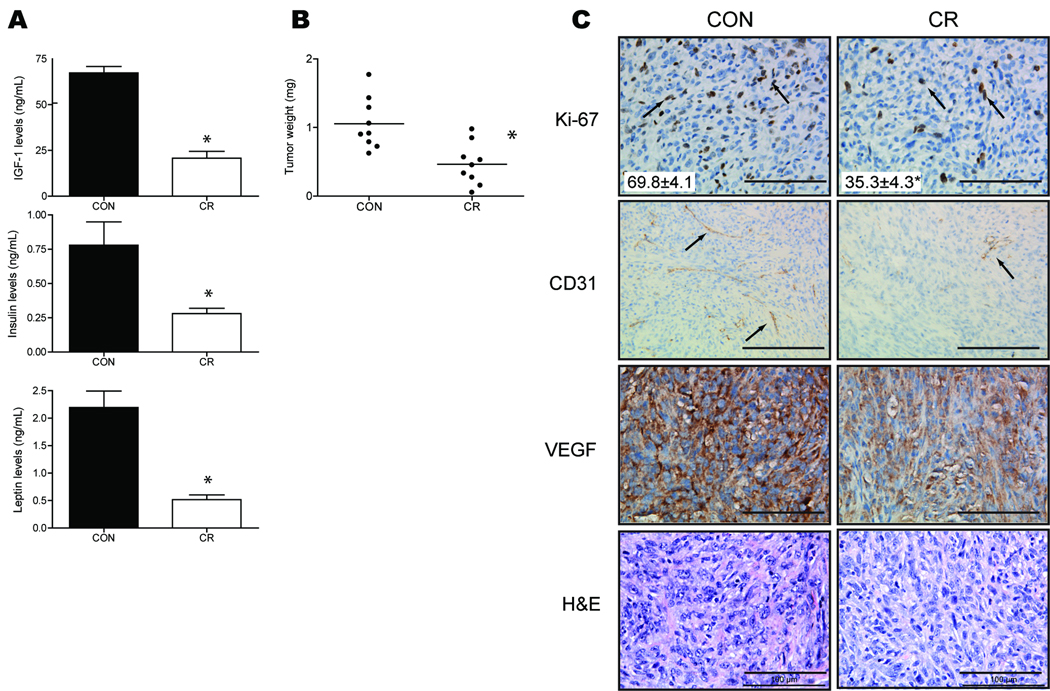
Pancreatic ductal lesions in the BK5.COX-2 mice displayed high-grade dysplasia but did not progress to ductal adenocarcinoma. However, JC101 cell lines derived from spontaneous pancreatic ductal lesions of a BK5.COX-2 mouse (27) form invasive tumors when injected into mice. In order to identify the effects of diet on pancreatic tumor growth, we fed WT FVB mice either a control or CR diet for seven weeks, subcutaneously injected them with JC101 cells, then allowed tumors to grow for four more weeks. CR resulted in significantly reduced fasting serum levels of IGF-1, insulin and leptin (IGF-1 and leptin each at P < 0.0001 and insulin P < 0.01; n=9 mice per diet group; Fig. 5A). Decreased levels of circulating hormones displayed by the CR group were associated with significantly reduced tumor burden (0.47g ± 0.30g, P < 0.05; n=9 mice per diet group; Fig. 5B) relative to the mice fed the higher calorie, control diet (1.05g ± 0.38g). Immunohistochemical analysis of Ki-67 revealed that this reduction in tumor burden was associated with significantly decreased proliferation (69.8 ± 4.1 Ki-67-positive cells, P < 0.01; n=5 mice per diet group; Fig. 5C) compared to the control diet (35.3 ± 4.3 Ki-67-positive cells). Unlike the pancreatic lesions of the BK5.COX-2 transgenic mice, JC101 control tumors were relatively avascular (Fig. 5C). However, the CR tumors exhibited less CD31-positive blood vessels than did the control tumors. Along with a diminished presence of tumor vasculature, there were qualitative reductions in expression of VEGF in the CR tumors relative to the control tumors (Fig. 5C). Subcutaneous injection of JC101 cells resulted in growth of tumors with mild to moderate differentiation, cellular and nuclear atypia, and fibrosis similar to that seen in the BK5.COX-2 pancreata (Fig. 5C).
Figure 5.
Effects of CR on JC101 pancreatic tumor growth and vasculature. A, mean fasting serum IGF-1, insulin and leptin levels (n=9 per group). Errors bars represent SEM. B, pancreatic tumor weight in FVB mice fed either a control or CR diet for 11 weeks (n=9 per group). JC101 cells were injected after 7 weeks on diet and allowed to grow for 4 weeks. Error bars represent SD. Significant differences are denoted with an asterisk. C, representative images of JC101 tumor sections stained with antibodies against Ki-67, CD31, and VEGF captured with 40X objective; except CD31, captured with 20×. Ki-67- and CD31-positive cells indicated with arrows. H&E-stained tumor sections captured with 40X objective. Scale bars, 100-µm; except CD31, 200-µm. Ki-67-positive cells were quantified and mean ± sem is embedded within image (5 mice per diet group). Significant difference denoted by asterisk.
Genetic reduction of circulating IGF-1 reduces pancreatic tumor growth
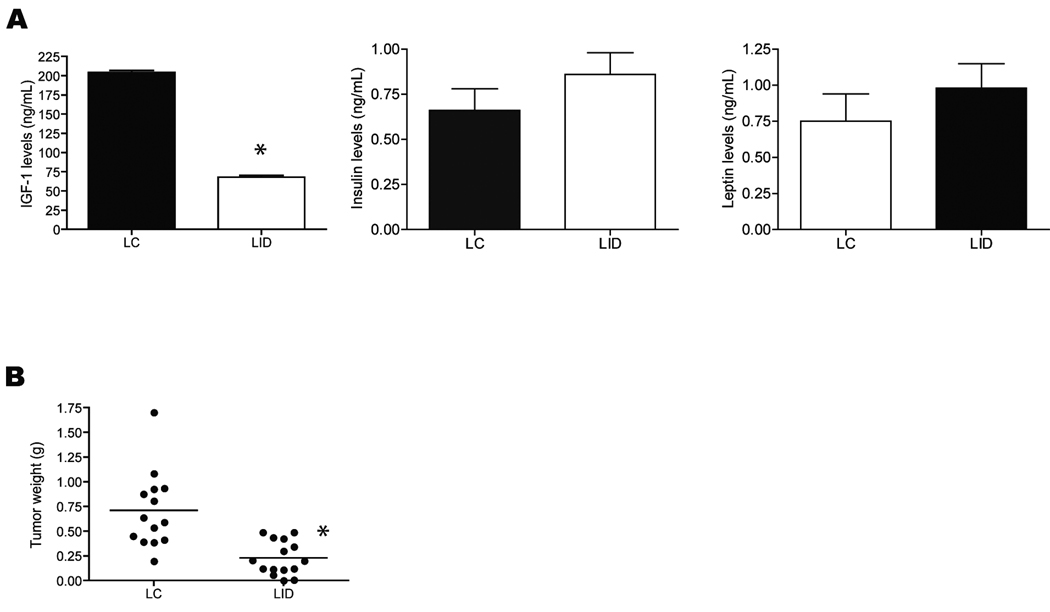
To evaluate the effect of genetic reduction of circulating IGF-1 on pancreatic cancer, we assessed tumor growth and serum hormone levels in LID and LC mice orthotopically injected with JC101 pancreatic cancer cells. Serum IGF-1 levels were significantly reduced by ~65% in LID mice (P < 0.0001; n=10 per genotype), relative to LC mice, at study termination (day 28; Fig. 6A). Although insulin and leptin levels were slightly elevated in LID mice, this did not reach significance (n=10 per genotype; Fig. 6A). After 28 days of growth, tumors from LID mice (0.23g ± 0.17g, n=14) were significantly smaller (P < 0.0001) than tumors from LC mice (0.71g ± 0.38g n=14; Fig. 6B).
Figure 6.
Effect of genetically reduced circulating IGF-1 on pancreatic tumor burden and serum levels of IGF-1 and insulin. (A) mean serum IGF-1, insulin, and leptin levels in LC and LID mice (n=10 per genotype) 28 days after orthotopic injection of JC101 cells. Errors bars represent SEM. (B) mean pancreatic tumor weight in LC mice (n=14) and LID mice (n=15) 28 days after orthotopic injection of JC101 cells. Significant differences are denoted by an asterisk.
Discussion
Our findings show for the first time that a reduced calorie diet regimen suppresses pancreatitis, pancreatic dysplasia, and tumor growth in a mouse model of COX-2-driven pancreatic cancer. Specifically, we show in BK5.COX-2 mice that a chronic CR regimen, relative to a control (positive energy balance) diet, significantly reduced body weight and both the presence and pathological severity of pancreatic ductal lesions. In addition, we found that CR reduced tumor growth in a transplant model using cell lines derived from a BK5.COX-2 mouse. To our knowledge, only two published studies have described preventive effects of CR on pancreatic tumorigenesis, and both were performed on rats initiated with the chemical carcinogen azaserine (11, 29). Neither of these studies identified possible mechanisms underlying the anticancer effects of CR. One additional study found no effect of CR on N-nitrosobis(2-oxopropyl)amine-induced pancreatic tumors in Syrian hamsters (30). We report here that CR significantly decreased circulating levels of IGF-1, consistent with data published in earlier studies showing that CR decreases serum IGF-1 in multiple mouse strains (9, 10, 31). We also found that the growth of orthotopically injected JC101 pancreatic tumor cells was suppressed by genetic reduction of circulating IGF-1 in LID mice. Thus, reduction of IGF-1 by either CR or genetic manipulation abrogated pancreatic cancer progression. Therefore, our results implicate IGF-1 as a key mediator linking dietary energy intake and pancreatic cancer progression.
The enhanced presence of inflammatory cells and fibrosis noted in the pancreatic microenvironment of the BK5.COX-2 mice mimics the intense desmoplasia associated with human pancreatic cancers. These desmoplastic regions have been shown to release protumorigenic cytokines, growth factors, and angiogenic factors (32, 33). In fact, the extensive fibrosis observed in the pancreata of control BK5.COX-2 mice was adjacent to ductal epithelial cells showing marked increases in proliferation. These areas were also associated with striking changes in both vascular luminal size and VEGF expression. Interestingly, the CR diet significantly diminished the impact of COX-2 overexpression on proliferation and vascularity coincident with reduced inflammatory cell infiltration and fibrosis. The observed relationship between amplified inflammatory cell infiltration and alteration in vascular morphology is supported by others who have shown that pancreatic cancer cells and inflammatory cell infiltrate (predominately macrophages and mast cells) contribute to elevated expression of pro-angiogenic factors such as VEGF (34). Moreover, Abdollahi et al., showed that human pancreatic tissues exhibit increases in expression of an angiogenic gene signature in a progressive manner from normal pancreatic tissue to pancreatic cancer, with pancreatitis being intermediate (35). Our data suggest that the changes in proliferation, vascularity, and VEGF expression in the ductal regions of BK5.COX-2 mice pancreata exposed to the higher calorie diet contribute to the transition from benign to neoplastic lesions.
In the BK5.COX-2 mice, CR relative to the control diet significantly reduced the extent of cellular and nuclear atypia, fibrosis, metaplasia, and inflammatory cell infiltrate. The inhibitory effects of CR on pancreatic pathology in BK5.COX-2 mice were associated with concomitant reductions in serum levels of IGF-1, as well as expression and activation of downstream effectors of the IGF-1 pathway. Although elevation of circulating insulin has been associated with poor cancer outcomes (24, 36), we found that the protumorigenic environment associated with the higher calorie control diet in the BK5.COX-2 mice occurred independently of fasting insulin levels. Furthermore, we offer evidence of a causal role for IGF-1 by demonstrating a dramatic reduction in pancreatic tumor growth when pancreatic cancer cells derived from a BK5.COX-2 mouse were transplanted into LID mice. In addition to having lower circulating IGF-1 levels, LID mice are hyperinsulinemic (37) further suggesting that IGF-1 may be more influential on pancreatic tumor progression than insulin in this model.
We have previously used the LID mouse model to examine the effect of reduced circulating IGF-1 on carcinogen-induced skin and colon tumor development (31, 38). In both of these studies, reduced circulating IGF-1 levels had a significant inhibitory effect on tumor development. Furthermore, the reduced circulating IGF-1 levels in LID mice were associated with inhibition of growth and metastasis of mouse colon 38 adenocarcinoma cells following orthotopic transplantation (39) and carcinogen-induced and spontaneous mammary tumors (40). Findings from these studies suggest an important role for IGF-1 in mediating development and growth of multiple types of epithelial cancers. Our studies expand this conclusion to include the impact of systemic IGF-1 on pancreatic cancer.
Alterations in the IGF-1 pathway in response to overexpression of IGF-1R have been associated with enhanced pancreatic tumor progression, angiogenesis, and resistance to chemotherapies (18, 41–44). In addition, pharmacologic inhibitors of IGF-1R can diminish pancreatic tumor growth in several models of pancreatic cancer (17, 18). Our data extend these findings by identifying a link between dietary energy balance, circulating levels of IGF-1, and pancreatic cancer. Taken together, these findings suggest that targeting components of the IGF-1 pathway may be an efficacious strategy for preventing or controlling pancreatic cancer, particularly in obese individuals.
Unfortunately, the development of effective IGF-1R inhibitors has been a challenge because of the significant homology between the insulin receptor and IGF-1R (45). Promising IGF-1R inhibitors have recently been developed that exhibit specificity for IGF-1R and show significant antitumor activity in both in vitro and in vivo models of pancreatic cancer (17, 46), although a moderate degree of hyperglycemia remains in approximately 25% of all patients undergoing IGF-1R inhibition (45, 47). The management of hyperglycemia in phase I and II clinical trials of IGF-1R inhibitors typically includes administration of anti-diabetic drugs. The anti-diabetic drug metformin is itself associated with a 62% reduction in pancreatic cancer risk in type II diabetics (48) and protects hamsters from developing carcinogen-induced pancreatic lesions enhanced by a high-fat diet (49). Metformin has been considered a CR mimetic based on findings that an 8-week metformin treatment in mice resulted in a hepatic gene expression profile similar to a long-term CR regimen (50). Studies in our model are underway to determine effective combinations of lifestyle interventions (such as CR and exercise) and pharmacological agents (such as metformin and IGF-1R inhbitiors) for pancreatic cancer prevention and control.
In conclusion, our findings show that CR protects against the protumorigenic nature of chronic pancreatitis, diminishes progression to high-grade dysplastic ductal lesions, and decreases tumor growth in a COX-2-driven mouse model of pancreatic neoplasia. Some of the anticancer effects of CR in the pancreas include diminished proliferation, altered vasculature, and reduced IGF-1/Akt/mTOR signaling. Specifically, CR prevented the metaplasia and fibrosis caused by a chronic state of inflammation, suggesting that dietary modulation could produce effects similar to those seen with the selective COX-2 inhibitor celecoxib (27). Findings from our LID mouse study suggest that reduced circulating levels of IGF-1 substantially contribute to the reduced tumor growth conferred by CR. Exploiting the apparent sensitivity of pancreatic cancer to dietary intervention and circulating IGF-1 modulation offers a viable approach to pancreatic cancer prevention strategies, including CR mimetics and IGF-1 targeted therapies.
Acknowledgements
The authors thank Dr. Glen Otto for assistance in experimental design and analysis, Dr. Craig Logsdon for critical review of this manuscript and Dr. Sanaz Khanbolooki for helpful advice.
Grant Support: R01 CA135386 and NIEHS P30ES007784 (S Hursting and S Fischer); R25T CA57730 and T32 CA135386 (L Lashinger).
Footnotes
No potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul–Aug;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep–Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007 May;132(6):2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Hursting SD, Lashinger LM, Colbert LH, Rogers CJ, Wheatley KW, Nunez NP, et al. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr Cancer Drug Targets. 2007 Aug;7(5):484–491. doi: 10.2174/156800907781386623. [DOI] [PubMed] [Google Scholar]
- 6.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003 Jun 10;195(2):127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 7.Karna E, Surazynski A, Orlowski K, Laszkiewicz J, Puchalski Z, Nawrat P, et al. Serum and tissue level of insulin-like growth factor-I (IGF-I) and IGF-I binding proteins as an index of pancreatitis and pancreatic cancer. Int J Exp Pathol. 2002 Oct;83(5):239–245. doi: 10.1046/j.1365-2613.2002.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Tamakoshi A, Kikuchi S, Yagyu K, Obata Y, Ishibashi T, et al. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the risk of pancreatic cancer death. Int J Cancer. 2004 Jul 1;110(4):584–588. doi: 10.1002/ijc.20147. [DOI] [PubMed] [Google Scholar]
- 9.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 10.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997 Nov 1;57(21):4667–4672. [PubMed] [Google Scholar]
- 11.Roebuck BD, Baumgartner KJ, MacMillan DL. Caloric restriction and intervention in pancreatic carcinogenesis in the rat. Cancer Res. 1993 Jan 1;53(1):46–52. [PubMed] [Google Scholar]
- 12.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009 Jul 10;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994 Dec 16;79(6):927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 14.Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990 Jul;70(3):591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- 15.Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, et al. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998 Aug 1;58(15):3353–3361. [PubMed] [Google Scholar]
- 16.Resnicoff M, Abraham D, Yutanawiboonchai W, Rotman HL, Kajstura J, Rubin R, et al. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995 Jun 1;55(11):2463–2469. [PubMed] [Google Scholar]
- 17.Moser C, Schachtschneider P, Lang SA, Gaumann A, Mori A, Zimmermann J, et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur J Cancer. 2008 Apr 27; doi: 10.1016/j.ejca.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, et al. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003 Sep;163(3):1001–1011. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito D, Fujimoto K, Mori T, Kami K, Koizumi M, Toyoda E, et al. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer. 2006 May 1;118(9):2337–2343. doi: 10.1002/ijc.21532. [DOI] [PubMed] [Google Scholar]
- 20.Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6(4):279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 21.Martinez J, Johnson CD, Sanchez-Paya J, de Madaria E, Robles-Diaz G, Perez-Mateo M. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology. 2006;6(3):206–209. doi: 10.1159/000092104. [DOI] [PubMed] [Google Scholar]
- 22.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993 May 20;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 23.Whitcomb DC, Applebaum S, Martin SP. Hereditary pancreatitis and pancreatic carcinoma. Ann N Y Acad Sci. 1999 Jun 30;880:201–209. doi: 10.1111/j.1749-6632.1999.tb09524.x. [DOI] [PubMed] [Google Scholar]
- 24.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004 Aug;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 25.Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001 Jan 15;91(2):333–338. doi: 10.1002/1097-0142(20010115)91:2<333::aid-cncr1006>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999 Mar 1;59(5):987–990. [PubMed] [Google Scholar]
- 27.Colby JK, Klein RD, McArthur MJ, Conti CJ, Kiguchi K, Kawamoto T, et al. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia. 2008 Aug;10(8):782–796. doi: 10.1593/neo.08330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roebuck BD, Yager JD, Jr, Longnecker DS. Dietary modulation of azaserine-induced pancreatic carcinogenesis in the rat. Cancer Res. 1981 Mar;41(3):888–893. [PubMed] [Google Scholar]
- 30.Birt DF, Pour PM, Nagel DL, Barnett T, Blackwood D, Duysen E. Dietary energy restriction does not inhibit pancreatic carcinogenesis by N-nitrosobis-2-(oxopropyl)amine in the Syrian hamster. Carcinogenesis. 1997 Nov;18(11):2107–2111. doi: 10.1093/carcin/18.11.2107. [DOI] [PubMed] [Google Scholar]
- 31.Moore T, Carbajal S, Beltran L, Perkins SN, Yakar S, Leroith D, et al. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res. 2008 May 15;68(10):3680–3688. doi: 10.1158/0008-5472.CAN-07-6271. [DOI] [PubMed] [Google Scholar]
- 32.McCawley LJ, Matrisian LM. Tumor progression: defining the soil round the tumor seed. Curr Biol. 2001 Jan 9;11(1):R25–R27. doi: 10.1016/s0960-9822(00)00038-5. [DOI] [PubMed] [Google Scholar]
- 33.Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res. 2001 Mar 1;61(5):1833–1838. [PubMed] [Google Scholar]
- 34.Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004 Jun;57(6):630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdollahi A, Schwager C, Kleeff J, Esposito I, Domhan S, Peschke P, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci U S A. 2007 Jul 31;104(31):12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008 Feb;114(1):71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 37.Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001 May;50(5):1110–1118. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- 38.Olivo-Marston SE, Hursting SD, Lavigne J, Perkins SN, Maarouf RS, Yakar S, et al. Genetic reduction of circulating insulin-like growth factor-1 inhibits azoxymethane-induced colon tumorigenesis in mice. Mol Carcinog. 2009 Sep 16;48(12):1071–1076. doi: 10.1002/mc.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002 Feb 15;62(4):1030–1035. [PubMed] [Google Scholar]
- 40.Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003 Aug 1;63(15):4384–4388. [PubMed] [Google Scholar]
- 41.Freeman JW, Mattingly CA, Strodel WE. Increased tumorigenicity in the human pancreatic cell line MIA PaCa-2 is associated with an aberrant regulation of an IGF-1 autocrine loop and lack of expression of the TGF-beta type RII receptor. J Cell Physiol. 1995 Oct;165(1):155–163. doi: 10.1002/jcp.1041650118. [DOI] [PubMed] [Google Scholar]
- 42.Nair PN, De Armond DT, Adamo ML, Strodel WE, Freeman JW. Aberrant expression and activation of insulin-like growth factor-1 receptor (IGF-1R) are mediated by an induction of IGF-1R promoter activity and stabilization of IGF-1R mRNA and contributes to growth factor independence and increased survival of the pancreatic cancer cell line MIA PaCa-2. Oncogene. 2001 Dec 13;20(57):8203–8214. doi: 10.1038/sj.onc.1205044. [DOI] [PubMed] [Google Scholar]
- 43.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer research. 1995 May 15;55(10):2007–2011. [PubMed] [Google Scholar]
- 44.Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001 Jan 15;61(2):589–593. [PubMed] [Google Scholar]
- 45.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008 Sep;7(9):2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009 Apr 14; doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 47.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009 Aug 27;28(34):3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 48.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009 Aug;137(2):482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001 Apr;120(5):1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- 50.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005 Nov 17;23(3):343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]