Abstract
Background
Trauma patients present with a coagulopathy, termed early trauma induced coagulopathy (ETIC), which is associated with increased mortality. This study investigated hemostatic changes responsible for ETIC.
Methods
Case-control study of trauma patients with and without ETIC, defined as prolonged prothrombin time (PT), was performed from prospective cohort of consecutive trauma patients who presented to level I trauma center. Univariate and multivariate analyses were performed.
Results
The case control study group (n=91) was 80% male, mean age of 37 years, 17% penetrating trauma and 7% mortality rate. ETIC patients demonstrated decreased common and extrinsic pathway factor activities (factors V and VII) and decreased inhibition of the coagulation cascade (antithrombin and protein C activities) as compared to the matched control non-ETIC patients. Both cohorts had evidence of increased thrombin and fibrin generation (prothrombin fragment 1.2 levels, thrombin-antithrombin complexes, soluble fibrin monomer,), increased fibrinolysis (D-dimer levels) and increased inhibition of fibrinolysis (plasminiogen activator inhibitor-1 activity) above normal reference values. ETIC versus non-ETIC patients had increased mortality and received increased amount of blood products.
Conclusion
ETIC following injury is associated with decreased factor activities without significant differences in thrombin and fibrin generation suggesting that despite these perturbations in the coagulation cascade patients displayed a balanced hemostatic response to injury. The lower factor activities are likely secondary to increased hemodilution and coagulation factor depletion. Thus, decreasing the amount of crystalloid infused in the early phases following trauma and administration of coagulation factors may prevent the development.
Keywords: trauma induced coagulopathy, dilution, coagulopathy
INTRODUCTION
In 2006, 179,065 Americans died from injury, up from 148,209 in 2000, and unintentional injury was the leading cause of death in persons between the ages of 1 and 44 in the US.1,2 Coagulopathy is recognized as a major contributor to trauma related mortality and historically has been considered a secondary entity.3,4 This secondary coagulopathy is thought to result from an ongoing cycle of dilution and consumption of coagulation factors, hypothermia and acidosis.4 Consequently, severely injured patients have been treated preferentially with damage control measures that focus on preventing hypothermia and acidosis in order to avert the resulting coagulopathy and concomitant high mortality rate.5
The concept of early trauma induced coagulopathy (ETIC) introduces a new paradigm of coagulopathy as an early and primary event in trauma patients detected at presentation to the trauma center. Other studies have proposed alternate terms which include acute traumatic coagulopathy, early coagulopathy of trauma and acute coagulopathy of trauma-shock.6,7 This early and primary coagulopathy is supported by retrospective trials which independently identified a prolonged clotting assay upon hospital admission in 10 to 34% of trauma patients as a predictor of mortality.8 MacLeod et al analyzed a trauma registry database of 7638 patients investigating prothrombin time (PT), partial thromboplastin time (PTT), platelet count, age, Injury Severity Score (ISS), presence of head injury, admission vital signs and base deficit (BD) as predictors of mortality. The study demonstrated that a prolonged PT independently predicted a 35% increase in the likelihood of mortality and a substantially greater risk of mortality with a prolonged PTT.9 Other groups have corroborated these findings using trauma registries.10-12
The pathophysiology of ETIC has not been fully elucidated, but two proposed theories have emerged. The first hypothesis revolves around tissue factor (TF) mediated activation of extrinsic coagulation pathway, suppression of anticoagulant systems, and impaired fibrinolysis resulting in a disseminated intravascular coagulation (DIC) like picture. Alternatively, the second proposal postulates that shock and thus tissue hypoperfusion leads to activation of protein C, systemic anticoagulation, and resultant hyperfibrinolysis.
The first theory is supported by indirect evidence that DIC is a predictor of adult respiratory distress syndrome (ARDS), multiorgan failure (MOF) and death in trauma patients.13 The diagnosis of DIC is based on published scoring systems (International Society on Thrombosis and Haemostasis [ISTH] and Japanese Association for Acute Medicine [JAAM]) incorporating clinical symptoms (presence of bleeding and organ dysfunction) and laboratory results (elevated fibrinogen degradation products [FDP], low platelet count, low fibrinogen and prolonged PT).14 Gando et al have postulated that trauma patients have a fibrinolytic (i.e. hemorrhagic) DIC phenotype in the early stage of trauma that changes to DIC with antifibrinolytic (i.e. thrombotic) phenotype within a few days of admission, accounting for the increased incidence of venous thromboembolism. Supporting data obtained soon after trauma demonstrated decreased fibrinogen and increased FDP (i.e. fibrinolytic DIC) correlated with mortality and bleeding, which may result from tissue hypoxia leading to increased tissue plasminogen activator (tPA) activity. This is followed by antifibrinolytic DIC (i.e. thrombotic DIC; secondary to plasminiogen activator inhibitor 1 [PAI-1] mediated inhibition of fibrinolysis), associated with systemic inflammatory response syndrome (SIRS; secondary to neutrophil activation and endothelial damage), MOF and poor outcome.15
The second theory investigated by Brohi et al suggests that ETIC occurs secondary to systemic anticoagulation via inhibition of the coagulation cascade (i.e. factors Va and VIIIa) and diversion of thrombin from fibrin generation by activated protein C (APC). In addition, Brohi et al hypothesize that ETIC is exacerbated by APC consumption of PAI-1 resulting in enhanced fibrinolysis. Thus, shock is a primary driver of early coagulopathy resulting in decreased clearance of thrombin, which binds to thrombomodulin and leads to APC release and inactivation of activated clotting factors, while the later prothrombotic state results from depletion of APC.6
Therefore, a congruent understanding of the development of ETIC after injury is required. This will improve patient outcome by identifying appropriate therapies and preventative measures. The study's primary objective was to determine the hemostatic changes in patients with ETIC (i.e. patients with prolonged PT) as compared to those without a prolonged PT at presentation to level I trauma center. In addition, multivariate analysis was performed to identify significant predictors of red blood cell (RBC) transfusion, increased hospital length of stay, and 28 day all cause mortality in this cohort.
MATERIALS AND METHODS
Patients
ETIC Prospective Study
A prospective observational cohort study of all trauma admissions with trauma team activation at Grady Memorial Hospital (GMH), a level 1 trauma center, was performed from 7/16/2008-5/30/2009. Trauma team activation criteria were developed from standard criteria published by the American College of Surgeons Committee on Trauma and published in Resources for Optimal Care of the Injured Patient manual 2006 edition and modified for GMH use. All patients were treated according to standard GMH Trauma Protocols in accordance to their injury complex and clinical status as determined by the treating physicians. Patients were excluded if they were < 18 years of age, observed < 24 hours, pronounced dead prior to blood sampling, known to be taking an anticoagulant or had a personal history of coagulopathy.
Case Control Study
From this larger cohort, a case control study was performed. Patients included in this study were all patients with available frozen plasma samples who had ETIC (defined as a prolonged PT upon admission) (termed Study Cohort) and age and gender matched controls without a prolonged PT (termed Control Cohort).
This research proposal was approved by the Emory University Internal Review Board and the Grady Research Oversight Committee and was granted a waiver of informed consent. The trial was registered on ClinicalTrials.gov (identifier NCT00744003).
Data collection
Data collected included demographic data, length of time to arrival, time to initial blood draw, timing and amount of fluid and blood product administration, vital signs including Glasgow Coma Scale (GCS), results of standard trauma laboratory tests (hematocrit [Coulter LH 780, Beckman Coulter, Brea, California], base excess [BE; iSTAT System, Abbott Point of Care Inc, Princeton, New Jersey], PT [Recombiplastin 2G, Beckman Coulter, Brea, California], PTT [Synthasil, Beckman Coulter, Brea, California], and fibrinogen [HemosIL Fibrinogen-C, Beckman Coulter, Brea, California]), injury complex and patient outcome including hospital length of stay, and 28 day mortality.
Sample analysis
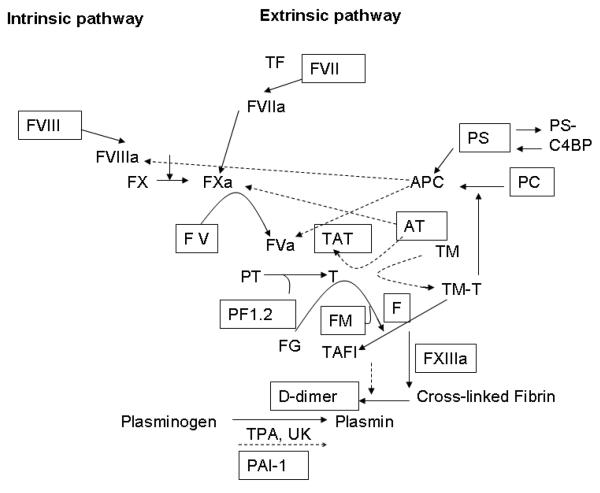
Residual plasma remaining after routine coagulation testing obtained at the time of admission was frozen within 4 hours of collection for future coagulation testing. Laboratory assays performed included factor V activity (r2 Diagnostics Inc, South Bend, Indiana), factor VII activity (r2 Diagnostics Inc, South Bend, Indiana), factor VIII activity (r2 Diagnostics Inc, South Bend, Indiana), factor XIIIa (Berichrom FXIII, Dade Behring, Marburg, Germany), protein C activity (Protein C Reagent, Dade Behring, Marburg, Germany), protein S activity (Protein S Ac, Dade Behring, Marburg, Germany), antithrombin (AT; Berichrom Antithrombin III, Dade Behring, Marburg, Germany), prothrombin fragment 1.2 (PF1.2; Enzygnost F1.2, Dade Behring, Marburg, Germany), thrombin-antithrombin complexes (TAT; Enzygnost TAT Micro, Dade Behring, Marburg, Germany), soluble fibrin monomer (FM; STA Liatest FM, Diagnostica Stago, Parsippany, New Jersey), D-dimer (STA Liatest D-DI, Diagnostica Stago, Parsippany, New Jersey), and PAI-1 (StachromR PA1, Diagnostica Stago, Parsippany, New Jersey) (figure 1).
Figure 1.
Coagation pathway: Intrinsic and extrinsic pathways with the primary activators and inhibitors are diagramed. Activators are demonstrated with solid arrows and inhibitors with dashed arrows. Factors measured in the study are in boxes.
Abbreviations: TF (tissue factor), FVII (factor VII), FVIIa (activated factor VII), FVIII (factor VIII), FVIIIa (activated factor VIII), FX (factor X), FXa (activated factor X), FV (factor V), FVa (activated factor V), TAT (thrombin-antithrombin), AT (antithrombin), PT (prothrombin), T (thrombin), FG (fibrinogen), FM (fibrin monomer), F (fibrin), TM (thrombomodulin), TM-T (thrombomodulinthrombin), PS (protein S), PC (protein C), PS-C4BP (protein S- x), TAFI (tissue activatable fibrinolysis inhibitor), TPA (tissue plasminogen activator), UK (urokinase), PAI-1 (plasminogen activator inhibitor- 1),
Statistical Methods
All data were entered into an Excel database (Microsoft Corp, Redmond, WA). Data analyses were performed by SPSS Statistics 17 (SPSS Inc, Chicago, IL). Quantitative variables were reported as means with standard deviations for normally distributed data. All group comparisons for quantitative data were done using Student's T-test for means. All categorical data was summarized using frequencies and all group comparisons were performed using X2 test and a Fisher's exact test for any comparisons where one group had a sample size that is less than 5. Correlation analyses were performed using Pearson correlation with 2-tailed significance. Multivariable analyses were performed using variables that were significant on univariate analysis. Significant differences were defined as those <0.05.
RESULTS
ETIC Prospective Study
During the study period, a total of 1,143 trauma team activations occurred at GMH with 808 activations meeting inclusion criteria. 545 trauma team activations were captured during the study period, of which 383 (70%) were included in the study. Reasons for exclusion included less than 24 hour admission (26%), non-trauma service admission (1%), pronounced dead upon arrival (2%), and patients with a personal history of cirrhosis or history of recent anti-coagulant administration (2%). Of the patients who met inclusion criteria, 371 had an admission PT (97%), of which 67 (18%) were prolonged (defined as greater than the normal range), and 354 had an admission PTT (92%), of which 11 (3%) were prolonged. Two of the eleven patients with elevated PTT had normal PT values.
In the ETIC Prospective Study, the mean age of the included patients was 39 ± 16.4 years with 74% (n=282) males. Most patients experienced blunt trauma (75%, n=284) with an ISS of 9 (interquartile range 5-16). The average time from injury to arrival at GMH was 50 minutes (min) (interquartile range 36-66 min) and time from arrival to blood draw was 8 min (interquartile range 5-13 min); there was no statistical difference in modes of arrival, time to arrival, or time to blood draw between patients with ETIC and those without ETIC (data not shown). The overall mortality rate was 7.9%; patients with ETIC had a 2.5 fold increased risk of 28 day mortality with a mortality rate of 20.2% as compared to 4.7% in patients without ETIC (p<0.001).
Case Control Study
91 patients were selected for additional coagulation testing. Of these, 38 samples from patients with prolonged PT (Study Cohort) were paired with age and sex matched controls with normal PT (Control Cohort). In univariate analysis, Study Cohort patient's compared to Control Cohort, had more severe injuries, lower EMS diastolic blood pressures, and received more fluid resuscitation in transit and while in the ECC (table 1). Sixteen patients had temperature taken either pre-admission, upon admission or prior to blood sampling (average 36.4C, standard deviation 0.7C): only one patient had a temperature less than 35C, who also had low blood pressure, prolonged PT, low platelet count, and low hematocrit. RBC transfusion prior to blood sampling for laboratory tests occurred in two patients (both in the Study Cohort); no patients received plasma, platelets, or cryoprecipitate before plasma samples for coagulation testing were obtained. The base excess did not significantly differ significantly between the two groups. The Study Cohort compared to the Control Cohort had lower, but not statistically significant, survival rates, longer hospital length of stay, and increased likelihood of blood transfusion (table 1).
Table 1.
Demographic data of Control Cohort (patients without ETIC) and Study Cohort (patients with ETIC)
| Control Cohort |
Study Cohort |
p value | |
|---|---|---|---|
| n=53 | n=38 | ||
| Age | 38±16 | 38±20 | 0.989 |
| Male (%) | 81% | 79% | 0.796 |
| Blunt (%) | 89% | 77% | 0.154 |
| ISS | 9±7 | 14±13 | 0.014 |
| Positive head CT (%) | 35.7% | 45.8% | 0.444 |
| Head AIS | 1±2 | 1±2 | 0.980 |
| EMS Data | |||
| Crystalloid (ml) | 452±552 | 852±1166 | 0.040 |
| Systolic BP (mmHg) | 135±24 | 128±28 | 0.329 |
| Diastolic BP (mmHg) | 80±20 | 65±24 | 0.009 |
| Respiratory rate | 18±8 | 19±5 | 0.672 |
| Heart Rate (bpm) | 96±26 | 104±29 | 0.226 |
| GCS | 13±4 | 13±4 | 0.696 |
| ECC Data | |||
| Crystalloid (ml) | 96±183 | 350±1042 | 0.091 |
| Systolic BP (mmHg) | 136±26 | 134±35 | 0.771 |
| Diastolic BP (mmHg) | 78±18 | 77±20 | 0.898 |
| Respiratory rate | 17±8 | 19±8 | 0.281 |
| Heart Rate (bpm) | 97±21 | 97±26 | 0.482 |
| GCS | 14±3 | 13±4 | 0.331 |
| Laboratory Data | |||
| Platelet count (103/μl) | 273±60 | 212±88 | <0.001 |
| PT (sec) | 12.0±0.7 | 15.1±1.9 | <0.001 |
| PTT (sec) | 26.9±3.7 | 32.2±25.3 | 0.138 |
| Fibrinogen (mg/dl) | 312±110 | 250±95 | 0.027 |
| Hematocrit (%) | 41.5±4.2 | 36.1±7.1 | <0.001 |
| Base excess | −3.9±3.2 | −3.4±5.4 | 0.740 |
| Outcome Data | |||
| Hospital LOS (days) | 9±13 | 15±24 | 0.120 |
| 28 d Mortality | 3.7% | 8.1% | 0.646 |
| 24 Hour Blood Utilization Data | |||
| RBC units transfused | 0±0 | 2±4 | 0.002 |
| RBC patients transfused (%) | 1.8% | 32.4% | <0.001 |
| Plasma units transfused | 0±0 | 1±3 | 0.002 |
| Plasma patients transfused (%) | 0.0% | 29.7% | <0.001 |
| Platelet units transfused | 0±0 | 2±4 | 0.010 |
| Platelets patients transfused (%) | 0.0% | 13.5% | 0.011 |
| Cryoprecipitate units transfused | 0±0 | 1±3 | 0.100 |
| Cryoprecipitate patients transfused (%) | 0.0% | 5.4% | 0.172 |
Coagulation factor measurements demonstrated decreased activity of factors V and VII in Study versus Control Cohorts, but factor VIII levels were not significantly different, most likely attributable to an acute phase response following trauma (table 2). Both Cohorts had evidence of increased activation of the coagulation system with increased thrombin generation and fibrin formation as evidenced by increased production of PF1.2, TAT, and FM. Inhibition of the coagulation cascade was demonstrated by decreased AT and protein C activity in Study and Control Cohorts but no difference in protein S activity. Both Cohorts displayed increased fibrinolysis as evident by similar D-dimer levels. Inhibition of fibrinolysis as measured by PAI-1 activity was not significantly different between the Cohorts and levels were increased above normal reference values in both Cohorts.
Table 2.
Coagulation data of Control Cohort (patients without ETIC) and Study Cohort (patients with ETIC)
| Coagulation factor (normal range) | Control Cohort |
Study Cohort |
p value |
|---|---|---|---|
| n=53 | n=38 | ||
| Factor V (50-150%) | 114±29 | 77±29 | <0.001 |
| Factor VII (50-150%) | 99±25 | 63±20 | <0.001 |
| Factor VIII (50-150%) | 157±50 | 152±42 | 0.703 |
| Factor XIIIa (50-130%) | 117±29 | 94±36 | 0.007 |
| Antithrombin (80-125%) | 100±17 | 80±21 | <0.001 |
| Protein C (70-140%) | 83±22 | 64±21 | <0.001 |
| Protein S (75-130%) | 55±20 | 51±18 | 0.372 |
| PF1.2 (65-288 pmol/l) | 769±394 | 716±469 | 0.592 |
| TAT (1-4.1 (μ/l) | 34±28 | 32±23 | 0.873 |
| Fibrin Monomer (<7 μg/ ml) | 70±60 | 89±62 | 0.141 |
| D-dimer (<260 ng/ml) | 3545±4960 | 5080±6334 | 0.211 |
| PAI-1 (<15 U/ml) | 14±11 | 15±20 | 0.673 |
Using data from the Case Control Study, correlations of PT, PTT, and protein C activity with other coagulation factors and outcome were also performed.16 PT did correlate with D-dimer levels (p=0.025), factor VII activity (p<0.001), and AT activity (p<0.001), but did not correlate with BE (p=0.595), PF1.2 (p=0.520), and PAI-1 activity (p=0.521). PTT did correlate with PT (p<0.001), and PF1.2 levels (p=0.048), but did not correlate with BE (p=0.596), D-dimer levels (p=0.319), PAI-1 activity (p=0.683), and AT activity (P=0.967). Protein C activity correlated with PT (p<0.001), D-dimer levels (p=0.011), and PF1.2 (p=0.004), but did not correlate with PAI-1 activity (p=0.787), PTT (p=0.678), and BE (p=0.789). In addition, protein C activity was not correlated with 28-day mortality (p=0.132).
Correlations of the presence of head injury with coagulation values were performed. PT, PTT, and protein C did not correlate with Glasgow Coma Score (PT, p=0.683; PTT, p=0.791; and protein C, p=0.210), head AIS score (PT, p=0.582; PTT, p=0.641; and protein C, 0.163), and positive head CT (PT, p=0.824; PTT, p=0.496; and protein C, p=0.062).
Multiple regression analysis was performed on three models: length of hospital stay, RBC utilization, and mortality (table 3). RBC utilization (R = 0.846), length of hospital stay (R = 0.665) and mortality (R = 0.577) models showed the positive correlations: ISS, EMS crystalloid, PT and AT activity were significantly associated with length of hospital stay; ISS and PT were significantly associated with RBC transfusion; and ISS and PT were significantly associated with mortality. In addition, using a linear regression model ISS was significantly correlated with EMS crystalloid administration (p=0.012), but not with hematocrit (p=0.196), PT (p=0.269), and factor VII (p=0.050).
Table 3.
Regression analysis for variables predicting hospital length of stay, RBC transfusion and mortality
| Hospital Length of Stay | B | SE | β | p value |
|---|---|---|---|---|
| ISS | 1.88 | 0.61 | 0.59 | < 0.01 |
| EMS blood pressure, diastolic | 0.02 | 0.20 | 0.02 | 0.92 |
| EMS blood pressure, systolic | 0.30 | 0.19 | 0.33 | 0.12 |
| EMS crystalloid | −0.01 | 0.00 | −0.41 | 0.03 |
| PT | 7.44 | 3.32 | 0.49 | 0.03 |
| PTT | 0.48 | 1.22 | 0.07 | 0.70 |
| Platelet count | 0.03 | 0.05 | 0.09 | 0.59 |
| Fibrinogen | −0.05 | 0.04 | −0.25 | 0.24 |
| Protein C | 0.47 | 0.25 | 0.40 | 0.06 |
| Antithrombin | −0.01 | 0.27 | 0.00 | 0.98 |
| RBC Transfusion | ||||
| ISS | 0.13 | 0.04 | 0.51 | <0.01 |
| EMS blood pressure, diastolic | 0.00 | 0.01 | −0.05 | 0.82 |
| EMS blood pressure, systolic | −0.01 | 0.01 | −0.10 | 0.62 |
| PT | 0.55 | 0.20 | 0.51 | 0.01 |
| PTT | 0.28 | 0.08 | 0.60 | <0.01 |
| Platelet count | 0.00 | 0.00 | 0.12 | 0.41 |
| Protein C | 0.02 | 0.02 | 0.20 | 0.34 |
| Fibrin monomer | 0.00 | 0.00 | 0.16 | 0.23 |
| PAI-1 | 0.04 | 0.02 | 0.34 | 0.05 |
| Mortality | ||||
| ISS | 0.01 | 0.00 | 0.42 | <0.01 |
| EMS blood pressure, diastolic | 0.00 | 0.00 | −0.10 | 0.15 |
| EMS blood pressure, systolic | 0.00 | 0.00 | 0.18 | 0.01 |
| EMS crystalloid | 0.00 | 0.00 | 0.07 | 0.23 |
| PT | 0.03 | 0.01 | 0.26 | <0.01 |
| PTT | 0.00 | 0.00 | −0.03 | 0.67 |
| Hematocrit | 0.00 | 0.00 | 0.08 | 0.21 |
| Platelet Count | 0.00 | 0.01 | −0.01 | 0.84 |
B- Un-standardized coefficient, SE – sampling error, β – standardized regression coefficient
DISCUSSION
The data support more severe injury, as demonstrated by increased ISS scores and resultant hypotension, results in increased amounts of crystalloid administered in transit to the emergency department. This subsequent hemodilution results in a lower hematocrit and lower factor activities, most importantly factor VII, leading to the prolonged PT which defines ETIC. The difference in factor VII activity between the two Cohorts is primarily responsible for the differences in PT. Moreover, the presence versus absence of ETIC was associated with decreased factor activities without significant impairment in thrombin and fibrin generation; both cohorts had evidence of increased thrombin and fibrin generation, as measured by increased PF1.2, TAT and FM levels. In addition, both cohorts demonstrated evidence of significant fibrinolysis, as verified by elevated D-dimer levels, without an increase in the antifibrinolytic activity of PAI-1. Activities of anticoagulant pathways, AT and protein C, were also decreased, likely as a result of depletion and/or dilution..17 Thus, it appears at the initial hospital admission time point, patients with ETIC have multiple derangements in the coagulation profiles; however, when evaluating the relationship between thrombin generation and fibrinolysis appear to have a balanced physiologic response to trauma.
The data presented here is in contrast to two alternate proposed theories: 1) TF mediated activation of extrinsic coagulation pathway, suppression of anticoagulant systems, and impaired fibrinolysis leading to a DIC like picture; and 2) tissue hypoperfusion leading to activation of protein C and systemic anticoagulation.
Gando et al have postulated that trauma patients have a fibrinolytic (hemorrhagic) DIC phenotype in the early stage of trauma.15 Supporting data demonstrated early following trauma decreased fibrinogen and increased FDP correlated with mortality and bleeding (fibrinolytic DIC), which may result from tissue hypoxia leading to increased tPA activity. Although the fibrinogen levels are lower in the Study Cohort versus Control Cohort (which is likely secondary to hemodilution and/or depletion), the D-dimer levels are not statistically different between Cohorts in the current study. In addition, neither fibrinogen nor D-dimer levels were significant predictors of RBC transfusion (as a marker for bleeding) or mortality in multivariate analysis. Gando et al's study included patients with an ISS ≥ 9 (ISS 24± 13 in survivor group and 32±13 in the nonsurvivor group) while patients in this study had an average ISS of 12±10.15 Thus, injury severity may result in differences seen in coagulation factor levels and their correlation with outcome between these studies.
Multiple publications from Brohi et al suggest that ETIC occurs secondary to systemic anticoagulation via inhibition of the coagulation cascade (factors Va and VIIIa) and diversion of thrombin from fibrin generation by APC.8 In addition, Brohi et al hypothesize that ETIC is exacerbated by APC consumption of PAI-1 resulting in enhanced fibrinolysis. Brohi et al propose shock as a primary driver of early coagulopathy resulting in decreased clearance of thrombin, which binds thrombomodulin leading to formation of APC resultant inactivation of activated clotting factors (factor Va and VIIIa) and shunting of the hemostatic response from fibrin generation.6 In this current study, protein C activity was decreased in the Control Cohort, in addition, to activity of factor V; however, factor VIII or PAI-1 were not decreased as would be expected if APC was the primary perturbation in the coagulation cascade. Moreover, the Study Cohort despite lower protein C activity had evidence of thrombin generation and fibrin formation that was not significantly different from Control Cohort. In addition, decreased activity of protein C would not account for the prolonged PT that defines ETIC. In multivariate analysis, protein C was not a significant predictor of increased mortality, hospital length of stay, or increased risk of bleeding as gauged by RBC transfusion requirements. In addition, BE as a marker tissue hypoperfusion did not correlate with mortality or bleeding, presence of ETIC, or protein C levels. Thus, these results differ from Brohi's findings where decreased protein C activity correlated with base deficit, PAI-1 and mortality.16 In contrast to the study above the inclusion criteria for Brohi et al's study was similar to this study and the patients' ISS scores were similar (average 17, range 9-26). 16
In the ETIC prospective study, which included all patients who met trauma team activation criteria and trauma registry inclusion, 19% of patients had ETIC and patients with versus without ETIC had increased mortality independent of ISS. These findings are consistent with other studies investigating ETIC.9-12
One limitation to this study is the ETIC Case Control Study patient selection depended on obtaining an adequate plasma sample upon hospital admission. Patients with severe injury frequently go directly to the operating room for definitive care and thus do not have samples obtained. In addition patients with mild injury are typically admitted less than 24 hours and were not included in this study. As a result, this study focused on the hemostatic changes in moderately injured patients. A second limitation of this study includes the inability to accurately determine hemodilution. The study used markers of hemodilution including hematocrit (which is 100% intravascular and the majority of patients did not receive RBC transfusion) and fibrinogen (which is 80% intravascular). In future studies, alternate markers such as α2-macroglobulin (which is 100% intravascular) may be used to further demonstrate the impact of hemodilution on coagulation factor levels. Third, this study included a single time point for coagulation factor activities. Given data that trauma patients are initially hypocoagulable and become hypercoagulable during their admission, these additional time points would be beneficial to study to see at what point these changes occur and what are the important factors which contribute to these changes.6,15 Fourth, the PT reagent used in this study was sensitive to factor VII levels, thus mildly decreased factor VII levels resulted in prolongation of PT(this was noted during method validation (data not shown)).
Lastly, patient outcome data was limited to mortality and length of stay, and because of the difficulty obtaining plasma samples from those more severely injured who went directly to the operating room this group and thus those patients less likely to survive may be under-represented.
In conclusion, the results of the current study support the presence of ETIC in trauma patients is associated with more severe injury, hemodilution with resultant coagulation factor depletion, increased risk of bleeding and mortality. In addition, Study and Control Cohorts (i.e. those with and without ETIC) had comparable increases in markers of thrombin generation and fibrinolysis. Thus, ETIC is likely at one end of the continuum of the normal response to trauma and may not be a unique pathophysiological mechanism. While a prolonged PT remains a strong predictor of mortality and correlates with injury severity, it may not fully explain the mechanism of coagulopathy. The prevention and/or treatment of ETIC, based on these data, includes conservative intravenous fluid administration as well as adequate factor replacement through plasma transfusion or other factor containing concentrates. Future studies should address the limitations of this study as well as investigate the effects of decreased crystalloid use and increased plasma use on ETIC and its associated outcomes.
ACKNOWLDEDGMENTS
We acknowledge Andrew Young, MD, PhD, and James Zimring, MD, PhD, in their assistance with coagulation laboratory testing. We acknowledge the following individuals for their assistance in data collection: Mikhail Akbashey, Dominique Allan, Lauren Arnold, E. Christopher Casstevens, TaSheena Cole, David Conalson, Sara DiCamillo, Peter Flueckiger, Samuel Galgano, Britt Gayle, Lucas Golub, Joshua Hammel, Matthew Hardy, Micheal Holdsworth, Layla Jaffree, Jordan Kaylor, Jane Keating, Ira Leeds, Anna Lipowska, Sara McClintock, Cameron McCoy, Patrick Mulligan, Ayo Ogunbameru, Oyin Olowokere, Justine Phifer, R. Harry Powers, Emily Rousso, Pearl Ryder, Ellen Schenk, Colleen Smith, Praneetha Thulasi, Matthew Tice, Sarah Wallace, Michael Woodall, and Denise Umpierrez.
Funding: Supported in part by PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resource and Emory Medical Care Foundation
Footnotes
Reprints: available upon request by corresponding author
Conflict of interest: There are no conflicts of interest for all authors.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Beth H Shaz, New York Blood Center, New York, NY, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA, bshaz@nybloodcenter.org.
Anne M Winkler, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA, AWINKL2@emory.edu.
Adelbert B James, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA;abjames@emory.edu.
Christopher D Hillyer, New York Blood Center, New York, NY, Department of Medicine, Weill Cornell Medical College, New York, NY, chillyer@nybloodcenter.org.
Jana B MacLeod, Department of Surgery, Faculty of Health Sciences College, Aga Khan University Hospital, Nairobi, Kenya, jm7072003@yahoo.com.
References
- 1.National Vital Statistics System, National Center for Health Statistics, Centers for Disease Control and Prevention . 10 leading causes of death by age group, United States - 2006. Office of Statistics and Programing, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; Atlanta: [PubMed] [Google Scholar]
- 2.Office of Statistics and Programming, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention . WISQARS Injury Mortality Reports. Atlanta: 1999. 2006. [Google Scholar]
- 3.McNamara JJ, Burran EL, Stremple JF, et al. Coagulopathy after major combat injury: occurrence, management, and pathophysiology. Ann Surg. 1972;176:243–246. doi: 10.1097/00000658-197208000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddy VA, Morris JA, Cullinane DC. Hypothermia, coagulopathy, and acidosis. Surg Clin North Am. 2000;80:845–854. doi: 10.1016/s0039-6109(05)70099-2. [DOI] [PubMed] [Google Scholar]
- 5.Becher J, Brimert T, Jeppesen JO, et al. Epidemiology of major trauma and trauma deaths in Los Angeles County. J Am Coll Surg. 1998;187:373–383. doi: 10.1016/s1072-7515(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 6.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod JB. Trauma and coagulopathy: a new paradigm to consider. Arch Surg. 2008;143:797–801. doi: 10.1001/archsurg.143.8.797. [DOI] [PubMed] [Google Scholar]
- 8.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 10.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 11.Hess JR, Lindell AL, Stansbury LG, et al. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion. 2009;49:34–39. doi: 10.1111/j.1537-2995.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 12.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Gando S, Nanzaki S, Morimoto Y, et al. Tissue factor pathway inhibitor response does not correlate with tissue factor-induced disseminated intravascular coagulation and multiple organ dysfunction syndrome in trauma patients. Crit Care Med. 2001;29:262–266. doi: 10.1097/00003246-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost. 2001;27:585–592. doi: 10.1055/s-2001-18864. [DOI] [PubMed] [Google Scholar]
- 15.Sawamura A, Hayakawa M, Gando S, et al. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res. 2009;124:608–613. doi: 10.1016/j.thromres.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams GL, Manson RJ, Turner I, et al. The balance of thrombosis and hemorrhage in surgery. Hematol Oncol Clin North Am. 2007;21:13–24. doi: 10.1016/j.hoc.2006.11.013. [DOI] [PubMed] [Google Scholar]